Institute of Oceanology, Chinese Academy of Sciences
Article Information
- KAWEE-AI Arthitaya, KIM Aaron Taehwan, KIM Sang Moo
- Inhibitory activities of microalgal fucoxanthin against α-amylase, α-glucosidase, and glucose oxidase in 3T3-L1 cells linked to type 2 diabetes
- Journal of Oceanology and Limnology, 37(3): 928-937
- http://dx.doi.org/10.1007/s00343-019-8098-9
Article History
- Received May. 6, 2018
- accepted in principle Jun. 14, 2018
- accepted for publication Aug. 15, 2018
2 Department of Biotechnology, Chiang Mai University, Chiang Mai 50200, Thailand;
3 Department of Food Science and Biotechnology, Kyung Hee University, Yongin 17104, Republic of Korea
Type 2 diabetes is a complex metabolic disorder. The underlying mechanism responsible for this disease and its complication remains unclear even with an apparent connection between diabetic hyperglycemia and complications of diabetes (Giugliano et al., 1996). Based on the link to the generation of free radicals, many possible biochemical mechanisms for an excess glucose to cause tissue damage have been found to include non-enzymatic glycation, oxidative stress, polyol pathway, inhibition of carbohydrate digestive enzymes and glucose uptake (Giugliano et al., 1996; King et al., 1996). Postprandial hyperglycemia performs a crucial role in the advancement of type 2 diabetes and complications like microvascular and macrovascular diseases (Baron, 1998). Therefore, the management of postprandial blood glucose status is critical for the treatment of diabetes and reduction of chronic vascular complications (Baron, 1998). One of the approaches to medicate diabetes mellitus is to inhibit the carbohydrate digestion by hindering activity of enzymes like α-amylase and α-glucosidase in the digestive system, thereby reducing intestinal glucose intake and postprandial blood glucose level. Several drugs (acarbose, voglibose, and nojirimycin) for type 2 diabetes exist today, but they have many side effects such as liver toxicity and an increase of heart disease possibility, making them inappropriate be used as a food supplement (Tewari et al., 2003). In order to reduce the side effect of current medications, and to provide more choices of drugs, it is imperative to study new inhibitors for potential remedy development (Lam et al., 2008).
Marine source exhibits an abundant chemical variation that can be utilized to discover and establish new probable therapeutic solution (Molinski et al., 2009). Fucoxanthin is a xanthophyll, non-provitamin A carotenoid consisting of allenic, conjugated carbonyl, epoxide, and acetyl groups in its molecule, and widely distributed in brown algae and diatoms (Haugan et al., 1992). Studies reported that macroalgal (seaweed) fucoxanthin suppressed the development of white adipose tissue that leads to obesity and obesity-related disorders, such as diabetes mellitus, hypertension, and cardiovascular disease in obese/ diabetic KK-Ay mice (Maeda et al., 2005; Maeda et al., 2007). Macroalgal fucoxanthin also modulated the blood glucose and insulin levels by suppressing monocyte chemoattractant protein-1 (MCP-1) and promoting β3-adrenergic receptor (Adrb3) and glucose transporter 4 (GLUT4) expression in a murine model (Maeda et al., 2009). It also suppressed lipid accumulation and decreased lipid peroxidation in hepatocytes through regulated Sirt1/AMPK signaling pathway (Chang et al., 2018). In addition, fucoxanthinol, a metabolite of fucoxanthin, improved obesity-induced inflammation in adipocyte cells (Maeda et al., 2015). Recent commercial-purpose fucoxanthin is produced mainly from macroalgae, but microalgal fuxoxanthin is starting to gain attention due to its higher concentration of fucoxanthin (Petrushkina et al., 2017). Regardless of variety and richness of fucoxanthin only a few microalgal species and their potential applications have been researched (Kim et al., 2012; Xia et al., 2013; Kawee-Ai and Kim, 2014) However, there is no report to utilize the microalgal fucoxanthin for the treatment of diabetes mellitus or obesity via the inhibition of carbohydratedigesting enzymes, such as α-amylase and α-glucosidase. In our previous study (Kawee-Ai et al., 2013), the microalgal fucoxanthin resulted in three main peaks consisted of the trans form along with two isomers, which was similar to the structure of brown seaweed fucoxanthin, but the ratio of isomers was different. Furthermore, the ratio of trans-to cis-form of fucoxanthin resulted in very strong antioxidant activity. In this study, the inhibitory effects of microalgal fucoxanthin on pancreatic α-amylase and rat-intestinal α-glucosidase, glucose-6- phosphate dehydrogenase, and glucose oxidase and lipid release in adipocyte (3T3-L1) cell linked to type 2 diabetes were determined to utilize microalgal fucoxanthin as a dietary supplement or food ingredient.
2 MATERIAL AND METHOD 2.1 MaterialPorcine pancreatic α-amylase (EC 3.2.1.1, type VI), potato starch, rat-intestinal acetone power, p-nitrophenyl α-D-glucopyranoside, β-nicotinamide adenine dinucleotide phosphate hydrate (NADP+), glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides (G6PD), 1-methoxy-5- methylphenylphenazinium methyl sulfate (1-methoxy PMS), epigallocatechin gallate (EGCG), Oil Red-O, and fucoxanthin from brown alga with >95% purity of all-trans form were purchased from Sigma-Aldrich (St. Louis, MO, USA). 3T3-L1 preadipocyte cell was purchased from the American Type Culture Collection (Rockville, MD, USA). Fetal Bovine Serum (FBS) and Dulbecco's Modified Eagle's Medium (DMEM) were purchased from Lonza (Walkersville, MD, USA). All other chemicals that were used in this study were of analytical grade.
2.2 Preparation of microalgal fucoxanthinFucoxanthin was extracted from Phaeodactylum tricornutum according to the method of Kawee-Ai et al. (2013). Phaeodactylum was obtained from the Korea Marine Microalgae Culture Center (KMMCC, Seoul, Korea). The previous study has found the hot soaking extraction was the most efficient extraction method to yield 17.84±0.21 mg/g (KaweeAi et al., 2013). Four milliliters of acetone was added to 1.0 g of the freeze-dried sample in a test tube (15 mm×150 mm) and then incubated at 45℃ for 2 h, followed by centrifugation at 10 000×g for 10 min to obtain the supernatant containing fucoxanthin. The extracts were pooled and filtered using Whatman No. 2 filter paper to remove the cell and cell debris. The crude extracts were dried using a rotary evaporator under vacuum at 40℃. The dry extract (60 mg) dissolved in 100 mL of methanol was partitioned using water (10 mL) and hexane (100 mL) in the ratio of methanol/water/hexane (10:1:10, v/v/v). The lower phase (methanol and water) was washed using hexane several times to the point of colorless. The methanol/ water phase (110 mL) was concentrated using a rotary evaporator under vacuum, packed into silica gel column (2.0 cm×30.0 cm), and was then eluted using 100 mL of hexane/acetone (2:1, v/v) to obtain fucoxanthin. The purity of microalgal fucoxanthin was more than 99% by HPLC analysis equipped with ODS column.
2.3 α-amylase inhibitory activityThe α-amylase inhibitory activity was determined using the chromogenic method of Bernfeld (1955). Porcine pancreatic α-amylase was dissolved in a 4℃ water to make up to a concentration of 4 U/mL. Potato starch (0.5%, w/v) dissolved in 20 mmol/L phosphate buffer (pH 6.9) with 6.7 mmol/L sodium chloride played as the substrate. Forty microliters of the P. tricornutum extracts, 400 μL of starch solution, and 160 μL of distilled water were conjoined in a test tube. An amount of 200 μL the enzyme solution (4 U/ mL) was added to start the reaction and the mixture was then incubated for 3 min at 25℃. This mixture (200 μL) was added into a separating tube consist of 100 μL of DNS solution (96 mmol/L 3, 5-dinitrosalicylic acid, 5.31 mol/L sodium potassium tartrate in 2 mol/L NaOH) and was placed into in a water bath for 15 min at 85℃, and was then diluted with 900 μL of distilled water. The absorbance at 540 nm was used to determine the activity of α-amylase. Acarbose, the positive control, was dissolved in distilled water. The α-amylase inhibitory activ7ity (%) was calculated as follows:
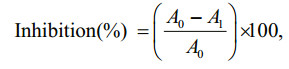
where A0 and A1 are the absorbance of the control and the sample, respectively.
2.4 α-glucosidase inhibitory activityThe α-glucosidase inhibitory activity was determined according to the method of Kurihara et al. (1999). One gram of rat-intestinal acetone power was mixed in 10 mL 0.9% saline using sonication for 30 s at 4℃ three times, followed by centrifugation (1 000×g, 30 min). The supernatant was diluted 4 times with distilled water. An amount of 50 μL sample solution was incubated with 100 μL of rat-intestinal α-glucosidase for 10 min at 37℃. Afterwards, 50 μL of 5 mmol/L p-nitrophenyl α-D-glucopyranoside in 0.01 mol/L phosphate buffer (pH 7) was added, followed by incubation for 15 min at 37℃. The absorbance at 540 nm was measured using a microplate reader (Bio-Tek Instruments Inc., Winooski, VT, USA). The α-glucosidase inhibitory activity (%) was calculated as the follows:
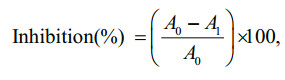
where A0 and A1 are the absorbance of the control and the sample, respectively.
2.5 Glucose-6-phosphate dehydrogenase inhibitory activityThe glucose-6-phosphate dehydrogenase inhibitory activity was determined using the colorimetric method of Olive et al. (1971) with slight modification. The reaction mixture contained 675 μL of 135 mmol/L Tris-HCl buffer (pH 7.8), 100 μL of 30 mmol/L glucose 6-phosphate, 100 μL of 3 mmol/L NADP+, 100 μL of 20 mmol/L magnesium chloride, and 15 μL of the sample. The mixture reaction was initiated by adding 10 μL of 0.035 U/mL G6PD and was then incubated at 25℃ for 15 min. The reaction was terminated by adding 1 mL of saturated aqueous sodium chloride. For the determination of produced NADPH, each 400 μL of 0.05 WST-1 and 0.025 mmol/L 1-methoxy PMS was added to 400 μL of the mixture. The absorbance at 438 nm was measured. EGCG was used as the positive control.
2.6 Kinetic studies of α-amylase and α-glucosidaseinhibition The enzyme inhibition reaction was performed according to the method described above at different inhibitor concentrations. The plots of Dixon (1953), and Lineweaver and Burk (1934) were used to determine the inhibition constants (Ki) and the type of inhibition, respectively.
2.7 3T3-L1 preadipocyte cultureThe 3T3-L1 pre-adipocyte cells were cultured in DMEM with 10% FBS and 1% penicillin (100 U/mL)- streptomycin (100 μg/mL) at 37℃ in 95% air and 5% CO2 atmosphere. The 3T3-L1 pre-adipocytes (5×103 cells/well) were seeded into a 96-well plate and were cultured in the initiation medium containing 10 μg/mL insulin, 0.5 mmol/L isobutylmethyxanthine and 0.1 μmol/L dexanmethazone in DMEM supplemented with 10% FBS for 2 days. The medium was then replaced with progression medium containing DMEM supplemented with 10% FBS, 5 μg/mL insulin, and fucoxanthin every other day.
2.8 Oil Red-O staining for lipid accumulationOn the 5th days of cultivation, the 3T3-L1 cells were washed twice with PBS (pH 7.4) and fixed in a fresh 10% formalin-containing PBS solution for 1 h at 4℃. The settled cells were rinsed twice using distilled water, stained with 0.3% Oil Red-O for 15 min at room temperature, and then washed with distilled water again to remove excess oil. Stained oil droplets in the 3T3-L1 cells were extracted with 100% isopropanol and the absorbance at 490 nm was measured to determine lipid accumulation. Additionally, 3T3-L1 cells with no staining were incubated with fucoxanthin and the absorbance at 490 nm was measured. The difference of the absorbances between the extracts from cells with and without Oil Red-O dye was determined and was displayed as a proportionate percentage of differentiated 3T3-L1 cells without fucoxanthin treatment (control).
2.9 Glucose consumption assayGlucose consumption assay was performed according to the modified method of Li et al. (2007). Briefly, the 3T3-L1 adipocyte was starved for 12 h in DMEM containing 0.1% FBS. Afterwards, the cells were treated with fucoxanthin and 1 nmol/L insulin for 24 h. Then, glucose (GO) assay kit was used to measure the concentration of glucose in 0.5 mL of the medium. The glucose consumption ratio was calculated as the follows:
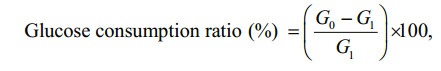
where G0 represents glucose concentration of medium without cell and G1 represents glucose concentration of medium with the cells.
2.10 Cell viabilityThe effect of fucoxanthin on the viability of 3T3- L1 cells was determined using EZ-cyTox Cell Viability Assay Kit (Daeil Lab Service, Seoul, Korea). The 3T3-L1 cells were placed into a 96-well plate at a density of 1×104 cells/well, incubated at 37℃ for 24 h, and treated with fucoxanthin for 72 h. An amount of 20-μL WST-1 solution was added to the wells, and the solution was further incubated at 37℃ for 4 h. The optical density at 450 nm was measured using a microplate reader (Bio-Tek Instruments Inc.).
2.11 Statistical analysisAll experiments were carried out in triplet. The results were displayed as the mean±standard deviation. The differences among the bioactivities of the samples observed in the different assays were analyzed using an ANOVA, followed by Duncan's multiple comparison tests, with a P < 0.05 set as the level of significance using the SPSS software package (Version 16.0; SPSS Inc., Chicago, IL, USA).
3 RESULT AND DISCUSSIONIn our previous study (Kawee-Ai et al., 2013), the purified compound of microalga P. tricornutum was identified as fucoxanthin via the analyses of LC-MS at 450 nm and NMR (Fig. 1). P. tricornutum fucoxanthin (purity ~99%) consisted of trans (90.1%) and two cis isomers (2.78% and 6.99%). The ratio of trans-fucoxanthin to two cis isomers was 100:3:8. The microalgal fucoxanthin content is influenced by the culture conditions, mainly the residence time in the photobioreactor and the external irradiance intensity, which can be artificially controlled. The fucoxanthin content of P. tricornutum was 17.5–18.0 mg/g dry weight (Kawee-Ai et al., 2013), which was much higher than those of the macroalgal brown seaweeds Undaria pinnatifida (2.7 mg/g) (Mori et al., 2004), Sargassum fusiforme (1.1 mg/g), S. confusum (1.6 mg/g), Leathesia difformis (0.3 mg/g), Sphaerotrichia divaricata (0.2 mg/g), and Desmarestia viridis (0.1 mg/g) (Terasaki et al., 2009).
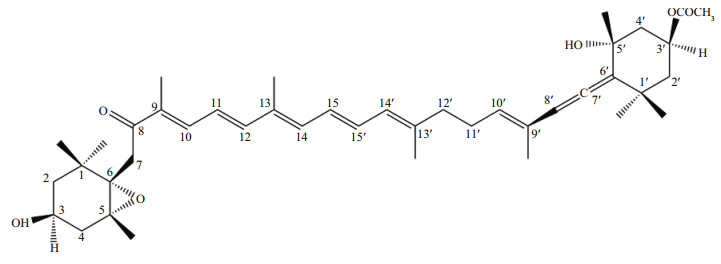
|
| Fig.1 The structure of fucoxanthin extracted from Phaeodactylum tricornutum (Kawee-ai et al., 2013) |
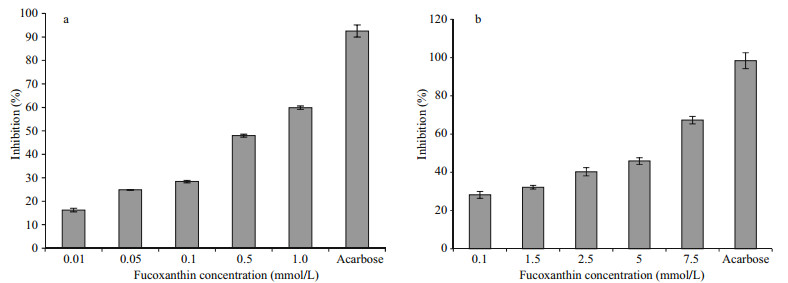
Small intestinal α-glucosidase and pancreatic α-amylase are decisive enzymes of the human carbohydrate digestion system. Related inhibitors may be effective in retarding carbohydrate digestion and glucose absorption to suppress postprandial hyperglycemia hyperglycemia (Tadera et al., 2006). Some antidiabetic drugs act through the inhibition of complex carbohydrate digestion and absorption in the gastrointestinal tract (Guyton and Hall, 2005). The P. tricornutum extract was tested to determine its inhibitory effect on α-amylase and α-glucosidase, which are well known key enzymes in the carbohydrate metabolism. The crude extract of the P. tricornutum exerted strong inhibitory activity against rat-intestinal α-glucosidase and pancreatic α-amylase. The inhibitory activities of the P. tricornutum crude extract against α-amylase at 0.1, 0.5, and 1.0 mmol/L were 28.38%, 47.98%, and 59.92% while 25.93%, 28.96%, and 32.18% on rat-intestinal α-glucosidase, respectively.
Several macroalgae (brown seaweed) have been reported to contain α-amylase and α-glucosidase inhibitors, such as Esienia bicylis (Okada et al., 2004), Graeloupia elliptica (Kim et al., 2008), Ishige okamurae (Heo et al., 2009), and Sargassum patens (Kawamura-Konishi et al., 2012). However, most of these inhibitors were identified as bromophenols and bromophenol derivatives (Kim et al., 2008), diphlorethohydroxycarmalol (Heo et al., 2009), eckol, dieckol and phlorotannins (Okada et al., 2004), and phologlucinol derivative (Kawamura-Konishi et al., 2012). In our previous study (Kawee-Ai et al., 2013), the fucoxanthin with the ratio of trans-fucoxanthin to its two isomers of 100:3:8 was purified from the P. tricornutum extract, which was slightly different from 100:2:3 of the edible brown seaweed Hijikia fusiformis (Yan et al., 1999). Hence, the inhibitory activities of microalgal fucoxanthin against carbohydrases were further investigated in this study. The inhibitory activities of fucoxanthin against α-amylase at 0.1, 0.5, and 1.0 mmol/L were 28.38%, 47.98%, and 59.92%, respectively, while 25.93%, 28.96%, and 32.18% on α-glucosidase (Table 1), in which the inhibitory activity was stronger against α-amylase than α-glucosidase. Most α-glucosidase inhibitors identified from marine algae were phenolic compounds or polyphenol derivatives (Okada et al., 2004; Kim et al., 2008; Heo et al., 2009; KawamuraKonishi et al., 2012). In this study, fucoxanthin had weak inhibitory activity against α-glucosidase, while strong inhibitory activity against α-amylase (Fig. 2a) in a dose-dependent manner, with an IC50 value of 0.68 mmol/L (Fig. 2a), which was almost the same as those of diphlorethohydroxycarmalol (Heo et al., 2009) and flavonoids (Tadera et al., 2006). On the other hand, the IC50 value, 4.75 mmol/L, of microalgal fucoxanthin against rat-intestinal α-glucosidase (Fig. 2b) was also almost the same as that of flavonoids (Tadera et al., 2006). On the contrary, Jung et al. (2012) reported that fucoxanthin had no inhibitory activities against yeast α-glucosidase at the concentration less than 200 μmol/L. Meanwhile, acarbose, a commercial drug, had strong inhibitory activities against both two enzymes, in which its IC50 values against pancreatic α-amylase and rat-intestinal α-glucosidase were 0.64 and 1.11 mmol/L, respectively. Both fucoxanthin and acarbose exhibited the similar inhibitory activities against amylase, but acarbose expressed stronger inhibitory activity on α-glucosidase. However, this drug has side effects caused by the abnormal bacterial fermentation of undigested carbohydrates due to an excessive inhibition of α-amylase (Horii et al., 1986). α-Amylase belongs to the family of glycoside hydrolase that breaks down starch into glucose by acting on α-1, 4 glycosidic bonds. Amylase inhibitors are also called starch blockers because of their activity to degrade enzymes like amylase and to decrease the starch conversion rate (Marshall and Lauda, 1975). Lo Piparo et al. (2008) proposed that the relationship between the inhibitory activity and the interaction of flavonoids against human salivary α-amylase depends on the formation of hydrogen bonds between OH groups of flavonoids and the carboxylate groups of Glu233 and Asp197 in the active site of human salivary α-amylase. Furthermore, both human salivary and pancreatic α-amylase composed of single polypeptide chains with 496 amino acids (Gumucio et al., 1988). Therefore, the OH groups of fucoxanthin may play the similar part in the association with the side chains of the active site. Hence, fucoxanthin may act as a starch blocker in the process of carbohydrate digestion in human because fucoxanthin had strong inhibitory activity against α-amylase.
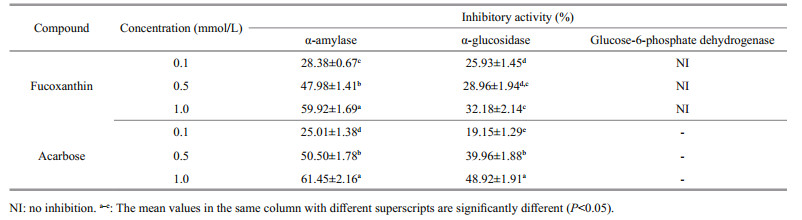
|
|
| Fig.2 The inhibitory activities of fucoxanthin against pancreatic α-amylase (a) and rat-intestinal α-glucosidase (b) Table 2 The kinetic studies of fucoxanthin against α-amylase and α-glucosidase |
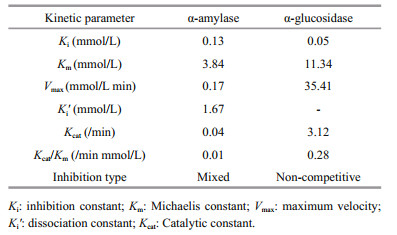
A kinetic study for the inhibition effect of fucoxanthin on α-amylase and α-glucosidase were performed using potato starch and p-nitrophenyl α-Dglucopyranoside as the substrate. Fucoxanthin exhibited a mixed type of inhibition against pancreatic α-amylase with Ki and Ki' values of 0.13 and 1.67 mmol/L, respectively (Fig. 3a, b, Table 2), which was characterized by a combination of competitive and non-competitive inhibition (Li et al., 2006). This type of inhibition against amylase was also reported in the phenolics of finger millet malt (Chethan et al., 2008) and wheat inhibitors of Rhyzopertha dominica, with the Ki values of 0.013 and 0.018 μmol/L, respectively (Priya et al., 2010). In the contrast, phlorotannins showed the competitive inhibition, with a Ki value of 1.8 μg/mL (Kawamura-Konishi et al., 2012). Fucoxanthin inhibited α-glucosidase in a noncompetitive inhibition, with a Ki value of 0.05 mmol/L (Fig. 3c, d). According to the above result, the velocity of the reaction catalyzed by α-glucosidase was slowed down as the concentration of fucoxanthin increased. The kinetic values of α-amylase and α-glucosidase are presented in Table 2. The similar result was also reported for millet seed coat phenolics, with the Km and Vmax of 8.0 mmol/L and 83.3 mmol/L/min, respectively (Shobana et al., 2009). The Kcat values, turnover number, and Kcat/Km, catalytic efficiency, of α-amylase and α-glucosidase were 0.04 and 3.12/min, and 0.01 and 0.28/min mmol/L, respectively.
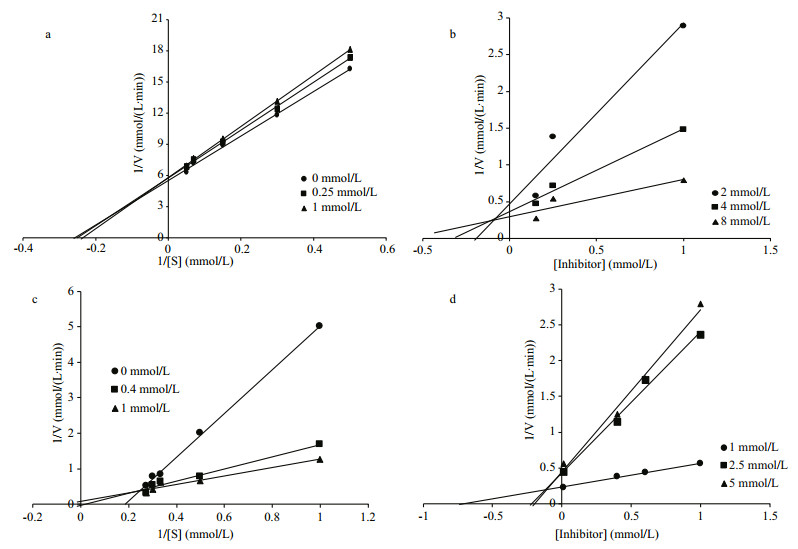
|
| Fig.3 Lineweaver-burk (a, c) and Dixon plot (b, d) for the inhibition of fucoxanthin against α-amylase (a, b) and α-glocosidase (c, d) |
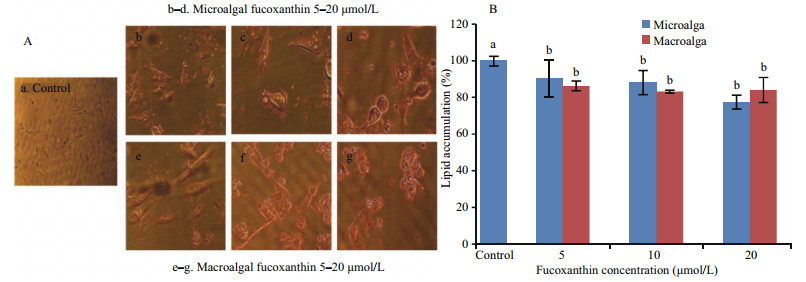
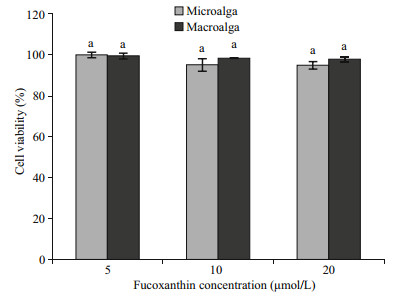
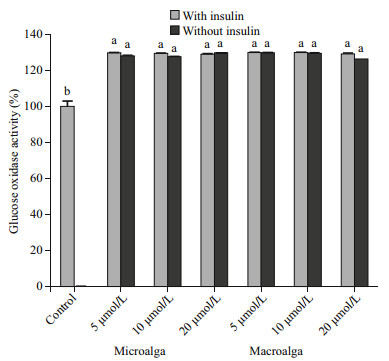
The effect of fucoxanthin on preadipocyte differentiation was determined using lipid accumulation as a marker for adipogenesis. Lipid droplets were quantified by Oil Red-O staining (Fig. 4). The Oil Red-O staining revealed that microand macroalgal fucoxanthin effectively inhibited lipid accumulation in the 3T3-L1 cells in a dose-dependent manner. The Oil Red-O staining level of 3T3-L1 cells administered with 5, 10 and 20 μmol/L microalgal fucoxanthin reduced the lipid accumulation in the 3T3-L1 adipocyte by 90.27%, 88.05%, and 77.43%, respectively, while 86.28%, 83.19%, and 84.07% for macroalgal fucoxanthin. However, fucoxanthin had no activity against glucose-6-phosphate dehydrogenase (G6PD), which was also an effective therapeutic target for obesity and diabetes (Table 1). G6PD is an important factor in the pentose phosphate pathway (PPP) to generate the reduced nicotinamide adenine dinucleotide phosphate (NADPH). NADPH is an essential reducing agent in the lipogenic processes to synthesize fatty acid and cholesterol (Mikami et al., 2013). Obese and diabetic symptoms such as chronic inflammation, oxidative stress, and lipid dysregulation are strongly related to the elevated expression of G6PD (Shin et al., 2008). The macroalgal fucoxanthin suppressed the differentiation of 3T3-L1 predipocytes into adipocytes by downregulating peroxisome proliferator-activated receptor γ (PPARγ) (Maeda et al., 2006; Kang et al., 2011). Macroalgal fucoxanthin significantly suppressed lipid accumulation and decreased lipid peroxidation in hepatocytes, in which it decreased lipogenesis-related transcription factor expression, including sterol regulatory element-binding proteins 1c and peroxisome proliferator-activated receptor γ (Chang et al., 2018). It also reduced fatty acid synthase expression and increased adipose triglyceride lipase and the phosphorylation of hormone-sensitive lipase production for lipolysis. Furthermore, fucoxanthin significantly increased phosphorylation of AMPactivated protein kinase (AMPK), and decreased activity of acetyl-CoA carboxylase for regulating fatty acid synthesis, resulting in increasing lipolysis and inhibiting lipogenesis in oleic acid induced fatty liver cells through the promoted Sirt1/AMPK pathway (Chang et al., 2018). The microalgal fucoxanthin did not affect cell viability or cause cytotoxicity in the 3T3-L1 cells (Fig. 5), which was the same as the macroalgal fucoxanthin of Undaria pinnatifida (Maeda et al., 2006) and Petalonia binghamiae (Kang et al., 2011). Both micro- and macroalgal fucoxanthins increased glucose oxidase activity in the 3T3-L1 cells by more than 29% compared with the control (Fig. 6). The glucose oxidase activities at 5, 10, and 20 μmol/L of microalgal fucoxanthin were 131.30%, 129.57% and 128.63%, respectively, which was similar to 131.00%, 132.33% and 130.80% of macroalgal fucoxnathin. At tested concentrations, the microalgal fucoxanthin (>90% of trans) had the ability to enhance glucose metabolism and to inhibit the differentiation in adipocytes cells, which was the same as did macroalgal fucoxanthin (>95% of trans). Therefore, it could be concluded that the ratio of trans- to cis-isomers did not affect the ability of fucoxanthin on glucose metabolism and the differentiation of adipocytes cells. The antidiabetic activity of fucoxanthin was the same as that of flavonoids and alkaloids by suppressing adipocyte differentiation and modulation of carbohydrate metabolic enzymes (Hii and Howell, 1985; García López et al., 2004). Thus, the microalgal fucoxanthin might be utilized as a functional ingredient for the prevention of obesity or type 2 diabetes.
|
| Fig.4 Levels of stained intracellular lipid according to elute Oil Red-O of micro- and macroalgal fucoxanthins on the 3T3-L1 cells after adipogenic differentiation (A) and lipid accumulation of 3T3-L1 cells (B) a, b: mean values with different superscripts are significantly different (P < 0.05). |
|
| Fig.5 Effects of micro- and macroalgal fucoxanthins on the 3T3-L1 cell viability a: significantly not different (P < 0.05) compared to untreated control. |
|
| Fig.6 Effect of fucoxanthin on glucose oxidase activity in the 3T3-L1 cells a, b: mean values with different superscripts are significantly different (P < 0.05). |
One therapeutic approach for preventing obesity and diabetes mellitus is to retard or decrease the absorption of glucose as well as lipid accumulation. The microalgal fucoxanthin exerted strong inhibitory activity against α-amylase, whereas weak inhibitory activity against α-glucosidase. The microalgal fucoxanthin also improved glucose metabolism through the induction of glucose oxidase activity and reduced lipid accumulation, with no cytotoxicity in the 3T3-L1 cells. Moreover, the microalgal fucoxanthin might exhibit antidiabetic activity through the inhibition of carbohydrate-digesting enzymes by delaying the absorption of dietary carbohydrates from the digestive organs, suppressing the increased blood glucose level after a meal, and decreased lipid accumulation. In addition, the ratio of trans- to cis-isomers did not affect the ability of fucoxanthin on glucose metabolism and the differentiation of adipocytes cells. Therefore, the microalgal fucoxanthin has the potential application to prevent obesity and type 2 diabetes and could be utilized as an ingredient for the manufacture of functional foods or dietary supplement.
5 DATA AVAILABILITY STATEMENTThe datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
6 ACKNOWLEDGMENTThis research was a part of the project titled 'Future Marine Technology Development' funded by the Ministry of Oceans and Fisheries, Republic of Korea.
Baron A D. 1998. Postprandial hyperglycaemia and α-glucosidase inhibitors. Diabetes Res. Clin. Pract., 40(S1): S51-S55.
|
Bernfeld P.1955. Amylases, alpha and beta. In: Colowick S P, Kaplan N O eds. Methods in Enzymology. Academic Press, New York. p.149-158.
|
Chang Y H, Chen Y L, Huang W C, Liou C J. 2018. Fucoxanthin attenuates fatty acid-induced lipid accumulation in FL83B hepatocytes through regulated Sirt1/AMPK signaling pathway. Biochem. Biophys. Res. Commun., 495(1): 197-203.
DOI:10.1016/j.bbrc.2017.11.022 |
Chethan S, Sreerama Y N, Malleshi N G. 2008. Mode of inhibition of finger millet malt amylases by the millet phenolics. Food Chem., 111(1): 187-191.
DOI:10.1016/j.foodchem.2008.03.063 |
Dixon M. 1953. The determination of enzyme inhibitor constants. Biochem. J., 55(1): 170-171.
DOI:10.1042/bj0550170 |
García López P M, de la Mora P G, Wysocka W, Maiztegui B, Alzugaray M E, Del Zotto H, Borelli M I. 2004. Quinolizidine alkaloids isolated from Lupinus species enhance insulin secretion. Eur. J. Pharmacol., 504(1-2): 139-142.
DOI:10.1016/j.ejphar.2004.09.008 |
Giugliano D, Ceriello A, Paolisso G. 1996. Oxidative stress and diabetic vascular complications. Diabetes Care, 19(3): 257-267.
DOI:10.2337/diacare.19.3.257 |
Gumucio D L, Wiebauer K, Caldwell R M, Samuelson L C, Meisler M H. 1988. Concerted evolution of human amylase genes. Mol. Cell. Biol., 8(3): 1197-1205.
DOI:10.1128/MCB.8.3.1197 |
Guyton A C, Hall J E. 2015. Insulin, glucagon, and diabetes mellitus. In: Hall J E. Textbook of Medical Physiology. 13th edn. Saunders, Philadelphia. p.961-978.
|
Haugan J A, Aakermann R, Liaaen-Jensen S. 1992. Isolation of fucoxanthin and peridinin. Meth. Enzymol, 213: 231-245.
DOI:10.1016/0076-6879(92)13124-G |
Heo S J, Hwang J Y, Choi J I, Han J S, Kim H J, Jeon Y J. 2009. Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown algae, a potent α-glucosidase and α-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur. J. Pharmacol, 615(1-3): 252-256.
DOI:10.1016/j.ejphar.2009.05.017 |
Hii C S T, Howell S L. 1985. Effects of flavonoids on insulin secretion and 45Ca2+ handling in rat islets of Langerhans. J. Endocrinol., 107(1): 1-8.
DOI:10.1677/joe.0.1070001 |
Horii S, Fukase H, Matsuo T, Kameda Y, Asano N, Matsui K. 1986. Synthesis and α-D-glucosidase inhibitory activity of N-substituted valiolamine derivatives as potential oral antidiabetic agents. J. Med. Chem., 29(6): 1038-1046.
DOI:10.1021/jm00156a023 |
Jung H A, Islam N, Lee C M, Jeong H O, Chung H Y, Woo H C, Choi J S. 2012. Promising antidiabetic potential of fucoxanthin isolated from the edible brown algae Eisenia bicyclis and Undaria pinnatifida. Fish Sci., 78(6): 1321-1329.
DOI:10.1007/s12562-012-0552-y |
Kang S I, Ko H C, Shin H S, Kim H M, Hong Y S, Lee N H, Kim S J. 2011. Fucoxanthin exerts differing effects on 3T3-L1 cells according to differentiation stage and inhibits glucose uptake in mature adipocytes. Biochem.Biophys. Res. Commun., 409(4): 769-774.
DOI:10.1016/j.bbrc.2011.05.086 |
Kawamura-Konishi Y, Watanabe N, Saito M, Nakajima N, Sakaki T, Katayama T, Enomoto T. 2012. Isolation of a new phlorotannin, a potent inhibitor of carbohydratehydrolyzing enzymes, from the brown alga Sargassum patens. J. Agric. Food Chem., 60(22): 5565-5570.
DOI:10.1021/jf300165j |
Kawee-Ai A, Kim S M. 2014. Application of microalgal fucoxanthin for the reduction of colon cancer risk:inhibitory activity of fucoxanthin against β-glucuronidase and DLD-1 cancer cells. Nat. Prod. Commun., 9(7): 921-924.
|
Kawee-Ai A, Kuntiya A, Kim S M. 2013. Anticholinesterase and antioxidant activities of fucoxanthin purified from the microalga Phaeodactylum tricornutum. Nat. Prod.Commun., 8(10): 1381-1386.
|
Kim K Y, Choi K S, Kurihara H, Kim S M. 2008. ?-Glucuronidase inhibitory activity of bromophenols purified from Grateloupia elliptica. Food Sci. Biotechnol., 17(5): 1110-1114.
|
Kim S M, Kang S W, Kwon O N, Chung D, Pan C H. 2012. Fucoxanthin as a major carotenoid in Isochrysis aff Galbana:characterization of extraction for commercial application. J. Korean Soc. Appl. Biol. Chem., 55(4): 477-483.
|
King G L, Kunisaki M, Nishio Y, Inoguchi T, Shiba T, Xia P. 1996. Biochemical and molecular mechanisms in the development of diabetic vascular complications. Diabetes, 45(S3): S105-S108.
|
Kurihara H, Mitani T, Kawabata J, Takahashi K. 1999. Inhibitory potencies of bromophenols from Rhodomelaceae algae against α-glucosidase activity. Fish Sci., 65(2): 300-303.
DOI:10.2331/fishsci.65.300 |
Lam S H, Chen J M, Kang C J, Chen C H, Lee S H. 2008. α-glucosidase inhibitors from the seeds of Syagrus romanzoffiana. Phytochemistry, 69(5): 1173-1178.
DOI:10.1016/j.phytochem.2007.12.004 |
Li B, Huang Y, Paskewitz S M. 2006. Hen egg white lysozyme as an inhibitor of mushroom tyrosinase. FEBS Lett., 580(7): 1877-1882.
DOI:10.1016/j.febslet.2006.02.051 |
Li Y Y, Wu H S, Tang L, Feng C R, Yu J H, Li Y, Yang Y S, Yang B, He Q J. 2007. The potential insulin sensitizing and glucose lowering effects of a novel indole derivative in vitro and in vivo. Pharmacol. Res., 56(4): 335-343.
DOI:10.1016/j.phrs.2007.08.002 |
Lineweaver H, Burk D. 1934. The determination of enzyme dissociation constants. J. Am. Chem. Soc., 56(3): 658-666.
DOI:10.1021/ja01318a036 |
Lo Piparo E, Scheib H, Frei N, Williamson G, Grigorov M, Chou C J. 2008. Flavonoids for controlling starch digestion:structural requirements for inhibiting human α-amylase. J. Med. Chem., 51(12): 3555-3561.
DOI:10.1021/jm800115x |
Maeda H, Hosokawa M, Sashima T, Funayama K, Miyashita K. 2005. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys.Res. Commun., 332(2): 392-397.
DOI:10.1016/j.bbrc.2005.05.002 |
Maeda H, Hosokawa M, Sashima T, Miyashita K. 2007. Dietary combination of fucoxanthin and fish oil attenuates the weight gain of white adipose tissue and decreases blood glucose in obese/diabetic KK-Ay mice. J. Agric.Food Chem., 55(19): 7701-7706.
DOI:10.1021/jf071569n |
Maeda H, Hosokawa M, Sashima T, Murakami-Funayama K, Miyashita K. 2009. Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model. Mol. Med. Rep., 2(6): 897-902.
|
Maeda H, Hosokawa M, Sashima T, Takahashi N, Kawada T, Miyashita K. 2006. Fucoxanthin and its metabolite, fucoxanthinol, suppress adipocyte differentiation in 3T3-L1 cells. Int. J. Mol. Med., 18: 147-152.
|
Maeda H, Kanno S, Kodate M, Hosokawa M, Miyashita K. 2015. Fucoxanthinol, metabolite of fucoxanthin, improves obesity-induced inflammation in adipocyte cells. Mar.Drugs, 13(8): 4799-4813.
DOI:10.3390/md13084799 |
Marshall J J, Lauda C M. 1975. Purification and properties of phaseolamin, an inhibitor of alpha-amylase, from the kidney bean, Phaseolus vulgaris. J. Biol. Chem., 250(20): 8030-8037.
|
Mikami D, Kurihara H, Kim S M, Takahashi K. 2013. Red algal bromophenols as glucose 6-phosphate dehydrogenase inhibitors. Mar. Drugs, 11(10): 4050-4057.
DOI:10.3390/md11104050 |
Molinski T F, Dalisay D S, Lievens S L, Saludes J P. 2009. Drug development from marine natural products. Nat.Rev. Drug Discov., 8(1): 69-85.
DOI:10.1038/nrd2487 |
Mori K, Ooi T, Hiraoka M, Oka N, Hamada H, Tamura M, Kusumi T. 2004. Fucoxanthin and its metabolites in edible brown algae cultivated in deep seawater. Mar. Drugs, 2(2): 63-72.
DOI:10.3390/md202063 |
Okada Y, Ishimaru A, Suzuki R, Okuyama T. 2004. A new phloroglucinol derivative from the brown alga Eisenia bicyclis:potential for the effective treatment of diabetic complications. J. Nat. Prod., 67(1): 103-105.
DOI:10.1021/np030323j |
Olive C, Geroch M E, Levy H R. 1971. Glucose 6-phosphate dehydrogenase from Leuconostoc mesenteroides. J. Biol.Chem., 246: 2047-2057.
|
Petrushkina M, Gusev E, Sorokin B, Zotko N, Mamaeva A, Filimonova A, Kulikovskiy M, Maltsev Y, Yampolsky I, Guglya E, Vinokurov V, Namsaraev Z, Kuzmin D. 2017. Fucoxanthin production by heterokont microalgae. Algal Res., 24: 387-393.
DOI:10.1016/j.algal.2017.03.016 |
Priya S, Kaur N, Gupta A K. 2010. Purification, characterization and inhibition studies of α-amylase of Rhyzopertha dominica. Pestic. Biochem. Physiol., 98(2): 231-237.
DOI:10.1016/j.pestbp.2010.06.012 |
Shin E S, Park J, Shin J M, Cho D, Cho S Y, Shin D W, Ham M, Kim J B, Lee T R. 2008. Catechin gallates are NADP+-competitive inhibitors of glucose-6-phosphate dehydrogenase and other enzymes that employ NADP+ as a coenzyme. Bioorg. Med. Chem., 16(7): 3580-3586.
DOI:10.1016/j.bmc.2008.02.030 |
Shobana S, Sreerama Y N, Malleshi N G. 2009. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics:mode of inhibition of α-glucosidase and pancreatic amylase. Food Chem., 115(4): 1268-1273.
|
Tadera K, Minami Y, Takamatsu K, Matsuoka T. 2006. Inhibition of α-glucosidase and α-amylase by flavonoids. J. Nutr. Sci. Vitaminol., 52(2): 149-153.
DOI:10.3177/jnsv.52.149 |
Terasaki M, Hirose A, Narayan B, Baba Y, Kawagoe C, Yasui H, Saga N, Hosokawa M, Miyashita K. 2009. Evaluation of recoverable functional lipid components of several brown seaweeds (Phaeophyta) from Japan with special reference to fucoxanthin and fucosterol contents. J.Phycol., 45(4): 974-980.
DOI:10.1111/j.1529-8817.2009.00706.x |
Tewari N, Tiwari V K, Mishra R C, Tripathi R P, Srivastava A K, Ahmad R, Srivastava R, Srivastava B S. 2003. Synthesis and bioevaluation of glycosyl ureas asα-glucosidase inhibitors and their effect on mycobacterium. Bioorg. Med. Chem., 11(13): 2911-2922.
DOI:10.1016/S0968-0896(03)00214-1 |
Xia S, Wang K, Wan L L, Li A F, Hu Q, Zhang C W. 2013. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar. Drugs, 11(7): 2667-2681.
DOI:10.3390/md11072667 |
Yan X J, Chuda Y, Suzuki M, Nagata T. 1999. Fucoxanthin as the major antioxidant in Hijikia fusiformis, a common edible seaweed. Biosci., Biotechnol., Biochem., 63(3): 605-607.
DOI:10.1271/bbb.63.605 |
 2019, Vol. 37
2019, Vol. 37