Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHANG Yue, SONG Xiuxian, YU Zhiming, ZHANG Peipei, CAO Xihua, YUAN Yongquan
- Impact assessment of modified clay on embryo-larval stages of turbot Scophthalmus maximus L.
- Journal of Oceanology and Limnology, 37(3): 1051-1061
- http://dx.doi.org/10.1007/s00343-019-8043-y
Article History
- Received Mar. 5, 2018
- accepted in principle Jul. 5, 2018
- accepted for publication Aug. 12, 2018
2 Laboratory of Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China;
3 University of Chinese Academy of Sciences, Beijing 100049, China;
4 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
In recent years, the frequency, intensity, and area of coverage of harmful algal blooms (HABs) have been increasing with the eutrophication and environmental degradation of seawater (Anderson et al., 2002). Outbreaks of HAB have seriously affected the development of fisheries and aquaculture in coastal areas, resulting in huge economic losses (Larkin and Adams, 2007; Jin et al., 2008). A recent report by the National Oceanic and Atmospheric Administration (NOAA) summarized most of the recent literature on HABs and concluded that they are responsible for annual economic losses of $75 million in the United States alone (Larkin and Adams, 2007). To mitigate and manage this problem, new technologies and control methods have been developed that include various physical, chemical and biological approaches. However, most of these technologies have only been tested in the laboratory, and few can currently be used in the field.
Clay is a non-toxic, naturally occurring material that scavenges algal cells from water and carries them to bottom sediments (Anderson, 1997). However, unmodified clay has been found to remove algal cells inefficiently, meaning that a large amount of clay is needed (Liu et al., 2016). Notably, the cell-removal capacity of clay can be greatly increased through modification. Modified clay technology has been proven to be effective in the field and is considered the most promising emergency treatment method because of its high removal efficiency, cost-effectiveness and potentially low environmental impact (Anderson, 1997). To date, modified clay has been employed for emergency control in both freshwater and seawater systems, especially in China, Japan and Korea (Sengco and Anderson, 2004; Lee et al., 2008; Pan et al., 2011).
However, the potential ecological and environmental effects of modified clay when used for HAB mitigation have attracted considerable attention, especially regarding the impacts on non-target organisms. Field application of yellow clay to control blooms of Cochlodinium polykrikoides Margalef was found to not adversely affect fish aquaculture if the clay was dispersed at a concentration below 0.23% (Lee et al., 2013a). Furthermore, aluminized modified zeolite clay was found to neither affect the survival of crayfish nor produce any sub-lethal effects on crayfish mobility or physiology at an application concentration of 350 g/m2 in Lake Okaro (Parkyn et al., 2011). Additionally, previous studies conducted in the laboratory have investigated the effects of clay on adult Penaeus chinensis Osbeck (Sun et al., 2000), Pagrus major (Temminck et Schlegel), Paralichthys olivaceus (Temminck et Schlegel) (Lee et al., 2013b), Perna viridis Linnaeus, milkfish (Chanos chanos Forsskål), sea bass (Lates calcarifer Bloch), rabbitfish (Siganus guttatus Bloch) (Orizar et al., 2013), Daphnia magna, Cyprinus carpio and Limnodrilus hoffmeisteri (Wang et al., 2016). Apparently, the adult stages of aquatic organisms and fishes with better swimming ability strongly resist or actively avoid adverse environmental conditions, whereas the sensitive stages of organisms are usually more vulnerable. Therefore, the sensitive life stages of organisms, such as juvenile Penaeus japonicas Bate (Song et al., 2003), Crassostrea gigas Thunberg (Gao et al., 2007), Crassostrea virginica Gmelin (Urban Jr. and Kirchman, 1992), Mercenaria mercenaria L. (Archambault et al., 2004), Patinopecten yessoensis Jay (Wang et al., 2014a) and Apostichopus japonicus Selenka (Wang et al., 2014b), have been further studied. These studies show that modified clay causes insignificant harm to the survival and growth of these organisms even at concentrations higher than those effective in treating HAB. Previous studies on the ecological effects of modified clay have mainly focused on the adult and larval stages of organisms, but the effects on embryos have rarely been investigated. However, fish embryos have been reported to be the most sensitive life stage to environmental pollutants (Marty et al., 1990; Hamm and Hinton, 2000). Thus, it is necessary to study the effect of modified clay on this particularly sensitive and vulnerable developmental period in organisms.
Turbot (Scophthalmus maximus L.) is considered one of the most promising species for marine fish farming because of its resistance to low temperatures, rapid growth rate, disease resistance and delicious taste. In recent years, there has been an increase in the production of this species, especially in Spain, France, Norway and China (Kim et al., 2005; Paulsen et al., 1998). Embryonic development in turbot is essentially the same as that in other teleost fish with telolecithal eggs that hatch after the blastocyst and gastrula stages, followed by development into the larval and juvenile stages. Thus, turbot, which is a typical teleost fish and an important commercial species, is a good model organism. We hypothesized that moderate concentration of modified clay would cause negligible harm to the embryo-larval stages of turbot, whereas excessive modified clay might lead to higher mortality rate or abnormal development of the embryos and larvae. We also hypothesized the application of modified clay in the field with a limited concentration would not adversely affect the survival and development of embryos and newly hatched larvae. In the present study, the effects of modified clay on the survival and growth of the embryo-larval stages of S. maximus were investigated to provide a reference for the treatment of HAB using modified clay, especially in the spawning and nursery grounds of fishes.
2 MATERIAL AND METHOD 2.1 Embryo and modified clay preparationThe healthy embryos were purchased from a fish farm (Weihai, Shandong, China) and cultured to the blastocyst stage of the experiment. The embryos were reared under natural light conditions at the Institute of Oceanology, Chinese Academy of Sciences, at 18℃, water pH 7.9, the dissolved oxygen content > 5.0 mg/L, and salinity of 30–31.
The clay used for the experiment was a type of kaolin produced in Jiangsu Province, China, and was modified using the method described by Yu et al. (1994). Polyaluminum chloride (PAC) was employed as the modifier at a ratio of 1:5 (w/w), which is the ratio that is typically used in the field. For the subacute experiments, a turbid liquid containing the modified clay was prepared with hyper-pure water at a density of 500 g/L (w/v) and shaken violently.
2.2 Impact assessment testsEighty turbot embryos were assigned randomly to each of 18, acid-rinsed 1-L glass beakers containing 1 L of membrane-filtered (0.45 μm) seawater. The control group beakers did not receive modified clay, while 0.5, 1.0, 1.5, 2 or 10 mL of turbid liquid containing modified clay was slowly and evenly added to the beakers of the experimental groups so that the final modified clay concentrations were 0 (control), 0.25, 0.5, 0.75, 1.0, and 5.0 g/L, respectively. All experiments were conducted in triplicate under the conditions described above. After the modified clay was added, the number of surviving embryos was observed and counted every 8 h to determine the cumulative mortality and the lethal concentration causing 50% mortality (LC50). Embryos were considered dead when they were deposited onto the bottoms of the beakers or turned opaque and white. After all of the larvae were hatched, the following biological indexes of newly hatched larvae were measured:
(1) Hatching rate: percentage of the number of larvae that hatched during the test relative to the number of embryos initially stocked in each beaker (80). The number of larvae was determined by counting with the naked eye after 56 h. No larvae hatched in any treatment after 56 h, the defined deadline of hatching.
(2) Morphological abnormality: percentage of the total number of abnormal larvae out of the cumulative number of larvae that hatched during the test. This index was determined by observing all of the newly hatched larvae under an inverted microscope.
(3) Heart rate: 5 larvae were randomly sampled with the required amount of seawater from each beaker at 56 h, and transferred to 24-well plates, in which 1-min videos were shot using a microscope to record the number of heartbeats. The larvae were then returned to the beakers. At 80 h and 96 h, 5 larvae were randomly sampled again, and their heartbeats were recorded as described above.
(4) Yolk absorption rate: 10 larvae were randomly sampled from each beaker at 56 h and 96 h respectively, and the yolk adsorption rate was defined as follows:R=(V0–Vt)/t, where V0 and Vt are the sizes of the yolk sacs of normally developing individuals at 56 h and 96 h, respectively, and t is the elapsed time in hours. Yolk size (V, mm3) was measured according to the following formula: V=π·a·b2/6, where a and b are the major and minor axes of the yolk sac, respectively.
(5) Specific growth rate (SGR): 10 larvae were randomly selected from each beaker, and SGR was calculated according to the following formula: SGR=100×(eg–1), and g=(lnLt–lnL0)/t, where Lt and L0 are the final (96 h) and initial (56 h) larval lengths of the normally developing individuals, respectively; and t is the test duration in days.
(6) Safe concentration (Wang, 2012): SC=0.3×48 h LC50/(24 h LC50/48 h LC50)2.
2.3 Statistical analysisProbit analysis, as described by Finney (1997), was used to determine the LC50 as well as the median effective concentration (EC50) of modified clay that deformed 50% of the exposed embryos at a 95% confidence level. Prior to analysis of variance (ANOVA), the data were tested for a normal distribution using the Kolmogorov-Smirnov test and for homogeneity of variance using Levene's test. The data of survival rates, hatching rates, morphological abnormality and heart rates met the two assumptions (normality and homogeneity of variance). Difference analysis of these data and the calculated values (mean±SD) of yolk absorption rate and SGR which were derived from the mean and standard deviation of variable V and L at 56 and 96 h according to the formula described by (Harvard University, 2007), were then tested by one-way ANOVA with Tukey post hoc comparisons using SigmaStat 3.5; The results were considered significant when P < 0.05.
3 RESULT 3.1 LC50 of modified clay for embryosThe survival rate of the control group was (92.5±3.8)% due to natural deaths recorded at 24 h and 48 h (Table 1). Compared with the survival rates at 24 h, the survival rates at 48 h were slightly lower, but the trends in their variation were consistent. As the concentration of modified clay increased, the survival rates decreased; 5.0 g/L modified clay resulted in 100% mortality within 24 h, whereas the survival rates in the other experimental groups slightly decreased and did not show a significant difference compared with the control (ANOVA, P > 0.05). The LC50 at 24 h and 48 h were 1.70 g/L (95% confidence interval, 1.42–2.17 g/L) and 1.65 g/L (95% confidence interval, 1.38–2.12 g/L), respectively, and the safe concentration was calculated to be 0.47 g/L according to the formula described above.

|
All fertilized eggs had hatched by 56 h during the experiment. The final hatching rate was 87.9% in the control but slightly lower in the experimental groups (Fig. 1). The groups subjected to modified clay concentrations of 0.25, 0.5, and 0.75 g/L exhibited very similar hatching rates of approximately 80%, and the lowest hatching rate of 74.4% was observed in the group subjected to 1.0 g/L modified clay. No significant differences were observed between the experimental groups and the control (ANOVA, P > 0.05). However, under the 5.0 g/L treatment, all of the embryos had died finally, without the appearance of any larvae.

|
| Fig.1 Hatching rate of S. maximus embryos under different concentrations of modified clay Mean±SD; ANOVA, Tukey; * significant difference compared with the controls at P < 0.05. |
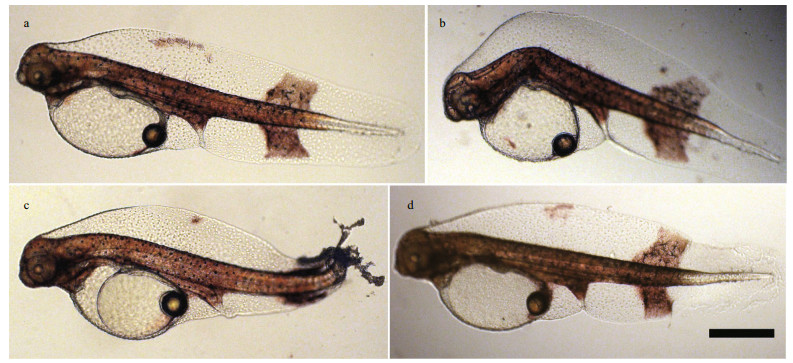
Based on the numbers of deformed larvae, the rate of morphological abnormalities in the control was 16% (Fig. 2). Approximately 12.7% abnormally developed larvae were observed under the 0.25 g/L modified clay treatment, and abnormality increased with the concentration of modified clay to 24.2% at 0.50 g/L. When the modified clay concentrations increased to 0.75 and 1.0 g/L, the abnormality rates were 34.1% and 37.7%, respectively, which were significantly higher than that of the control (ANOVA, P < 0.05). The EC50 based on deformities was 1.34 g/L. In addition, the malformations observed after hatching included tail degeneration, spinal curvature and fin lesions (Fig. 3).

|
| Fig.2 Morphological abnormality of larval S. maximus under different concentrations of modified clay Mean±SD; ANOVA, Tukey; * significant difference compared with controls at P < 0.05. |
|
| Fig.3 Morphological abnormalities in larval S. maximus a. normally developed embryo (56 h, control); b. kyphosis deformity (56 h, 0.5 g/L); c. larva with degenerated tail (56 h, 0.75 g/L); d. larva with abnormal fins (56 h, 0.75 g/L); scale bar represents 200 μm. |
Larval heart rates were measured at 56 h, 80 h, and 96 h after the modified clay was added (Fig. 4), and as the newly hatched larvae developed, their heart rate increased with time, from 102 beats/min at 56 h to 144 beats/min at 96 h in the control. There was a consistent tendency at all three time points; as the modified clay concentrations increased from 0–1.0 g/L, heart rates first increased and then decreased. The groups showing differences in the number of heartbeats compared with the control varied with time: a significant increase was observed in the group of 0.75 g/L at 56 h, and in the 0.5–1.0 g/L and 0.25–1.0 g/L at 82 h and 96 h, respectively (ANOVA, P < 0.05). The maximal heart rates observed at 56 h and 80 h appeared under the 0.75 g/L modified clay treatment, which was 111 and 150 beats/min, respectively. At 96 h after the addition of modified clay, the heartbeat reached a maximum of 161 beats/ min under the 0.5 g/L modified clay treatment, which was 17 beats/min faster than in the control.

|
| Fig.4 Heart rate of larval S. maximus at 56 h, 80 h, and 96 h under different concentrations of modified clay Mean±SD; ANOVA, Tukey; * significant difference compared with controls at P < 0.05. |
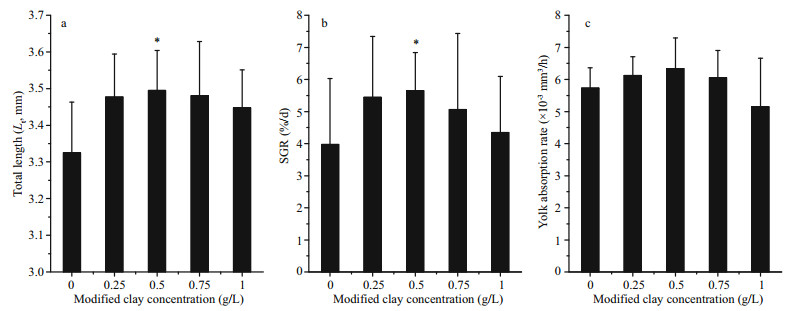
The Lt of the larvae in all experimental groups (0.25–1.0 g/L) first increased to 3.5 mm under the 0.5 g/L treatment with significant difference from the control group (ANOVA, P < 0.05), and then decreased to 3.4 mm under the 1.0 g/L treatment, while it was 3.3 mm in the control (Fig. 5a). Similarly, the SGR from 56–96 h increased to its maximum under the modified clay concentrations of 0.5 g/L and then gradually decreased (Fig. 5b), and the SGR was significantly accelerated under a modified clay concentration of 0.5 g/L (5.7%/d) compared with the control (3.9%/d) (ANOVA, P < 0.05).
|
| Fig.5 Final total length (a), SGR (b), and yolk absorption (c) of S. maximus under varying concentrations of modified clay Mean±SD; ANOVA, Tukey; * significant difference compared with controls at P < 0.05. |
The yolk is a nutrition reservoir for the growth and metabolism of newly hatched larvae, and the yolk adsorption rate is, therefore, another important index of their development. The changes in the yolk adsorption rate followed the same pattern as Lt and SGR (Fig. 5c), but there were no significant differences among groups: 5.7×10-3 mm3/h in the control compared with 6.1×10-3 mm3/h, 6.3×10-3 mm3/h, 6.1×10-3 mm3/h and 5.1×10-3 mm3/h, respectively, under the range of modified clay concentrations from 0.25–1.0 g/L (ANOVA, P < 0.05).
4 DISCUSSIONHABs are responsible for massive fish mortality in coastal aquaculture worldwide (Bruno et al., 1989; Kent et al., 1995; Landsberg, 2002; Thangaraja et al., 2007; Park et al., 2013; Delegrange et al., 2015). They can also have sub-lethal effects such as (1) physical damage to mucous membranes, (2) asphyxiation due to oxygen depletion, (3) gas bubble trauma resulted from photosynthesis driving oxygen hyper-saturation, and (4) ichtyotoxicity (Landsberg, 2002). As one of the few HAB mitigation methods, modified clay technology has been successfully applied in the field. Although the material selection and preparation of modified clays are based on the consideration of methods with minimal adverse environmental impacts, the environmental effects of clay and modified clay addition have still received intensive scrutiny (Yu et al., 2017). The effects of modified clay on non-target organisms are expected to be negligible, or at least less harmful than the HAB itself. Studied have shown that the effects on fishes or embryos vary with factors such as the type, composition, and dosage of clay. Approximately 73% to 100% survival was observed when juvenile and adult milkfish, sea bass and rabbitfish were exposed to 2.0 g/L ball clay for 96 h (Orizar et al., 2013). Aminoclay has also been reported to have a negligible impact on farmed fishes (P. major and P. olivaceus) at a concentration of 0.1% (w/v), which is much higher than the recommended dosage for HAB control (0.001%) (Lee et al., 2013b). Lewis et al. (2003) tested phosphatic clay mixed with a coagulant (PAC), which was very similar to the modified clay used in this study, and found that it did not pose any threat of acute or chronic toxicity to larval sheepshead minnows (Cyprinodon variegatus Lacépède) and grass shrimp embryos (Palaemonetes pugio Holthuis) when concentrations of 0.25 g/L clay and 50 mg/L PAC were applied. The clay subjected to modification by PAC in the present study was a type of kaolin and resulted in negligible adverse effects in the 0.25 g/L treatment (with a PAC concentration of 50 mg/L), which was consistent with the results of the previous study. The survival rate is the most commonly used indicator in toxicity tests. In this study, the experiment was performed immediately after the embryos were transported from the farm to the laboratory, and the embryos developed to the blastocyst stage during transport. Lugowska (2007) observed the highest death rates for common carp embryos in the blastocyst stage, and we suspect that changes in environmental factors, such as temperature and salinity, during transport may have contributed to the natural death of embryos in the sensitive blastocyst stage, leading to the 7.5% death rate observed in the control during cultivation. Notably, the survival rate tended to be stable 8 h after the addition of modified clay (data not shown), and was almost the same at 24 h and 48 h, which indicated that the lethal effect of modified clay on turbot embryos did not accumulate with exposure time after complete flocculation.
Lethal effects mainly occurred during the addition and flocculation of modified clay, when clay particles inevitably met the turbot embryos. Collision with modified clay may influence embryo survival by causing direct physical damage. In addition, the surfaces of aquatic organisms often carry with net negative charge (Chai et al., 2014), and the positively charged modified clay may therefore adhere to their surface. The adhesion of modified clay caused an increase in the weight of the buoyant, floating embryos. Previous studies showed that a high floating rate represented a high quality of the turbot eggs (Jia et al., 2014). It can be inferred that the adhesion of modified clay may influence the embryo quality by decreasing the floating rate. Direct damages mainly occurred immediately after modified clay was added to seawater with a relatively higher concentration. The higher concentrations of modified clay contained more particles, increasing the probability of contact with embryos. As a result, the survival rate decreased with an increasing modified clay concentration, due to collision and adhesion. However, the D90 (particle diameter for which the cumulative undersized volume fraction is equal to 90%) of the clay particles used in this experiment was less than 40 μm (Yu et al., 2017), which is much smaller than that of the embryos (usually greater than 1 mm). Moreover, the sedimentation rate of modified clay was quite high. The majority of the clay particles deposited within 30 min and sedimentation was nearly complete after 2–3 h. As a result, direct lethal effects of the modified clay particles on the turbot embryos were more likely to occur when the modified clay concentration was quite high (for example, 5.0 g/L). In contrast, embryo death was relatively limited, and there were no significant differences between the control and experimental groups at modified clay concentrations of 1.0 g/L or less.
A few studies have tested the acute toxicity of PAC-modified clay to non-target aquatic animals, and the results showed that the 96 h LC50 of PAC-modified clay for infant oyster (C. gigas) (Gao et al., 2007), infant P. yessoensis (Wang et al., 2014a) and A. japonicas (Wang et al., 2014b) were 2.67, 2.3 and 6.01 g/L, respectively. In this study, the 48 h LC50 of PAC-modified clay for turbot embryos was 1.65 g/L, which is lower than the LC50 for previously tested organisms, indicating that turbot embryos are more sensitive to the addition of modified clay. Embryos represent the most vulnerable stage in the life history of fishes due to their lack of motility, which prevents them from avoiding unfavorable environments. On the other hand, turbot produces buoyant embryos that float on the surface of the seawater and may meet the suddenly high concentration of clay immediately after clay is added. In contrast, benthonic and nektonic organisms are more likely to contact with modified clay particles that have already dispersed for some time. In this study, all of the experimental groups had experienced the whole process from the addition to the sedimentation of the modified clay, and the effects of modified clay treatment at different stages were taken into account in the calculation of the LC50. Safe concentrations are calculated to provide a reference for the use of chemicals or toxicants and they are commonly determined based on the LC50. The safe concentration of modified clay for S. maximus embryos was determined to be 0.47 g/L. However, the concentration of modified clay that is often used in the field is approximately 4–10 t/km2, which is equivalent to 4–10 g/m2. Additionally, the spraying of modified clay slurry in the field is carried out more than once at a low dose to reach the final concentration (4–10 g/m2), and the sedimentation rate is faster than in the laboratory due to disturbance, indicating a minimal risk of lethal toxicity from clay application to non-target aquatic animals. Even so, the effects of modified clay on floating eggs and embryos should still be considered with extreme caution, especially in the reproductive season. It is also suggested that the application of modified clay in these sensitive water areas should be carried out at a lower intensity with a longer spraying interval.
Fish embryos are particularly sensitive to contaminants in the aquatic environment during the hatching period, and researchers often use hatchability as a key factor in investigating the toxic effects of substances on the early life stages of fish (Fraysse et al., 2006). Studies have shown that the hatching success of typical teleost fish under optimum conditions is usually above 70% (Jezierska et al., 2009). None of the embryos in the 5.0 g/L modified clay treatment hatched successfully due to the 100% mortality. The hatchability rates of the other groups were all above 70% and showed no significant differences from each other, which was consistent with the results of previous studies. Fish hatching results from interacting biochemical (enzymatic), biophysical (mechanical) and osmotic mechanisms (Cao et al., 2009). The results regarding hatchability in this study indicated that a moderate concentration of modified clay might not influence key hatching factors, such as hatching related enzymes and the mobility of pro-larvae.
In this study, the deformity rates of newly hatched larvae were quantified in all groups. The most common deformities in fish larvae have been reported to be spinal curvature, body shortening, and heart and yolk sac swelling (Sfakianakis et al., 2015). There was a significantly higher deformity rate than the control under treatment with a modified clay concentration above 0.5 g/L, and the deformity rate in the experimental groups increased with an increasing modified clay concentration. The abnormal larvae identified in these groups mostly exhibited spinal and tail deformities. We speculate that the adhesion and collision of clay particles were the main causes of these deformities. Greig et al. (2005) found that the addition of clay sediment reduced oxygen consumption by Atlantic salmon eggs by restricting oxygen availability and blocking micropore canals in the membrane. Although the clay particles did not kill these embryos, their attachment likely restricted the availability of oxygen and blocked micropore canals in the membrane, thus affecting normal embryo development. As a result, more abnormally developed larvae appeared in the groups with higher modified clay concentrations. To further assess the risk of modified clay regarding the deformity of newly hatched larvae, the teratogenic index (TI) was calculated. TI is defined as the LC50/EC50 ratio, with a TI > 1.5 indicating a high risk of embryo malformation in the absence of significant embryonic mortality (American Society for Testing and Materials, 1992). The TI calculated in this study was 1.2, indicating that a moderate concentration of modified clay did not result in a high risk of malformation in the turbot larvae.
Changes in heart rate are often considered a reflection of the toxic effects of substances on the early life stages of fish. Disorders of the heart rate can adversely affect various physiological processes in fish, including their metabolism, growth, and survival; thus, the heart rate can provide information about environmental impacts on fish physiological states, thereby broadening our understanding of the general relationship between fish and their environment (Priede and Tytler, 1977). In this study, the heart rate of the larvae in all groups gradually accelerated with time, similar to the changes in heartbeat observed in other typical teleost fishes. Studies have shown that when the concentrations of toxic substances exceed species tolerance, the heart rates of larval fishes significantly decelerate, which accompanies a delay in metamorphosis (Cao et al., 2009; Dambal et al., 2017). In the present study, deceleration of the heart rate was not observed in the experimental groups. In contrast, the number of heartbeats was relatively higher in all of the experimental groups than in the control, and the number of heartbeats in the experimental groups at 80 h was very similar to that in the control at 96 h, indicating potentially increased growth.
The total length, SGR and yolk absorption rate of the newly hatched larvae were also measured to assess the effect of modified clay on larval growth and development. Because the yolk sac is the only source of nutrition for growth and development before larvae enter the feeding stage, the changes in the yolk absorption rate were similar to the changes in the SGR and the total length of the larvae; this tendency was consistent with previous studies (Cao et al., 2009). After sedimentation was completed, the effects of modified clay on water quality began to play the dominant role in affecting the growth and development of newly hatched larvae. Previous studies have shown that heavy metals and other environmental stressors in the early life stages of fish activate energyconsuming detoxification processes that increase the metabolic oxygen demand of the body. Thus, extra energy is required for detoxification, which corresponds to less energy being available for growth and development and leads to a smaller body size (Jezierska et al., 2009). Both laboratory studies and field monitoring data have shown that an appropriate dose of modified clay can effectively improve water quality by, for example, decreasing the content of heavy metals in water, such as Cu, Cr, Cd, Pb, Zn and Ni (Rybicka et al., 1995; Malakul et al., 1998), and removing bacteria or inhibiting their adhesion in seawater (Lind et al., 1997; Larraza et al., 2011; Stuart et al., 2016). Therefore, a moderate amount of modified clay may alleviate survival pressure on newly hatched larvae to a certain extent, which is beneficial to their growth and development. It has previously been reported that fish tanks receiving clay exhibit a lower abundance of bacteria than tanks containing algae paste or live algae; cod larvae in tanks with clay initiated exponential growth earlier and present a significantly higher dry weight (Attramadal et al., 2012). In this study, the 0.5 g/L treatment group showed significant increases in total length and SGR, however, the yolk absorption rate showed an insignificant difference compared with the control group, which indicated that the energy contained in yolk might be mainly used for the growth of larvae. When the concentration exceeded 0.5 g/L, modified clay may have put pressure on the growth of larvae that counteracted the effects of the improvement of water quality.
The addition of aluminum in the modifier during the flocculation and sedimentation of modified clay and its effects on the embryonic-larval stages of fish cannot be neglected. Previous studies have found complex impacts of aluminum on fish. Although aluminum is not an essential trace metal for fish, it is often introduced through the addition of zeolite, which is employed to promote fish growth, improve metabolism, enhance disease resistance, remove ammonia nitrogen and improve water quality (James and Sampath, 1999; Yıldırım et al., 2009). An analysis of the aluminum content in Salmonidae showed great differences among different life stages; the aluminum content reached 49–77 mg/kg during early embryonic development and the hatching period but fell below 4 mg/kg after continuous growth, suggesting that aluminum played an important role in early salmonid development (Hugh, 1991). The apparent decrease in aluminum during growth may be related to tissue and bone formation (Hugh, 1991). However, many studies on aluminum toxicity have shown adverse effects on fish growth and development. Excess aluminum inhibits enzyme activities and calcium absorption, which cause metabolic disorders and damage the liver and gills of fish (Hugh, 1991; Barcarolli and Martinez 2004; Monette et al., 2008). It is worth noting that damage from aluminum has only been found in acidic water environments (Gibbs and Özkundakci, 2011). The amount of added to seawater in this study resulted in an aluminum concentration of approximately 1.14×10-3 mol/L under the 1.0 g/L modified clay treatment, and the pH of the seawater decreased from 7.68 to 7.47 as the concentration of modified clay rose to 1.0 g/L; i.e., the pH decreased slightly but was still alkaline. PAC shows a strong tendency to hydrolyze to aluminum hydroxide precipitates in the neutral to alkaline pH range (Wang et al., 2004). As a result, the concentration of Al3+ is limited. Moreover, heavy metals often delay the hatching process or cause premature hatching or deformation or death of newly hatched larvae. These disturbances result in reduced numbers and poor-quality larvae with a small body size and reduced viability (Jezierska et al., 2009); however, these phenomena were not observed in the present study.
5 CONCLUSIONIn conclusion, the safe modified clay concentration calculated for turbot (S. maximus) was 0.47 g/L, which is higher than the concentration used in the field for HAB control. Modified clay had negligible effects on turbot hatching, and the addition of modified clay at a level below its safe concentration may have the potential to promote the development and growth of newly hatched larvae without increasing the number of abnormal individuals, whereas more than 0.5 g/L modified clay showed a risk of causing more deformity. Turbot embryos are vulnerable organisms and could be regarded as a model organism to provide references to a certain extent for the use of modified clay. However, the environments are more complex in fields, caution should be exercised in the application of modified clay, especially in the spawning and nursery grounds of fishes in the reproductive seasons. Experiments with additional types of organisms at larger scales are necessary to further assess methods of modified clay application.
6 DATA AVAILABILITY STATEMENTThe raw datasets generated during and/or analyzed during the current study are not publicly available because they will be used in writing Ph.D. thesis of the first author and so requires secure protection prior to thesis submission and graduation but are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENTWe would like to thank Dr. YOU Feng for her valuable advice during the experiment.
American Society for Testing and Materials. 1992. Standard guide for conducting the frog embryo teratogenesis assayXenopus (FETAX). In: American Society for Testing and Materials ed. Annual Book of ASTM Standards. Philadelphia: American Society for Testing and Materials. p.1 199-1 209. https://www.astm.org/Standards/E1439.htm
|
Anderson D M, Glibert P M, Burkholder J M. 2002. Harmful algal blooms and eutrophication: nutrient sources, composition, and consequences. Estuaries, 25(4): 704-726.
DOI:10.1007/BF02804901 |
Anderson D M. 1997. Turning back the harmful red tide. Nature, 388(6642): 513-514.
DOI:10.1038/41415 |
Archambault M C, Bricelj V M, Grant J, Anderson D M. 2004. Effects of suspended and sedimented clays on juvenile hard clams, Mercenaria mercenaria, within the context of harmful algal bloom mitigation. Marine Biology, 144(3): 553-565.
DOI:10.1007/s00227-003-1222-5 |
Attramadal K J K, T ndel B, Salvesen I, ie G, Vadstein O, Olsen Y. 2012. Ceramic clay reduces the load of organic matter and bacteria in marine fish larval culture tanks. Aquacultural Engineering, 49: 23-34.
DOI:10.1016/j.aquaeng.2012.02.003 |
Barcarolli I F, Martinez C B R. 2004. Effects of aluminum in acidic water on hematological and physiological parameters of the neotropical fish Leporinus macrocephalus (Anostomidae). Bulletin of Environmental Contamination and Toxicology, 72(3): 639-646.
DOI:10.1007/s00128-004-0291-6 |
Bruno D W, Dear G, Seaton D D. 1989. Mortality associated with phytoplankton blooms among farmed Atlantic salmon, Salmo salar L., in Scotland.. Aquaculture, 78(3-4): 217-222.
DOI:10.1016/0044-8486(89)90099-9 |
Cao L, Huang W, Shan X X, Xiao Z Z, Wang Q Y, Dou S Z. 2009. Cadmium toxicity to embryonic-larval development and survival in red sea bream Pagrus major. Ecotoxicology and Environmental Safety, 72(7): 1 966-1 974.
DOI:10.1016/j.ecoenv.2009.06.002 |
Chai S L, Robinson J, Chong Mei F C. 2014. A review on application of flocculants in wastewater treatment. Process Safety and Environmental Protection, 92(6): 489-508.
DOI:10.1016/j.psep.2014.04.010 |
Dambal V Y, Selvan K P, Lite C, Barathi S, Santosh W. 2017. Developmental toxicity and induction of vitellogenin in embryo-larval stages of zebrafish (Danio rerio) exposed to methyl Paraben. Ecotoxicology and Environmental Safety, 141: 113-118.
DOI:10.1016/j.ecoenv.2017.02.048 |
Delegrange A, Vincent D, Duret M, Amara R. 2015. The use of mussels for mitigating the noxious effect of phytoplankton spring blooms on farmed fish. Aquacultural Engineering, 66: 52-61.
DOI:10.1016/j.aquaeng.2015.03.001 |
Finney D J. 1971. Probit Analysis. 3rd edn. Cambridge University Press, Cambridge. p.197-199.
|
Fraysse B, Mons R, Garric J. 2006. Development of a zebrafish 4-day toxicity of embryo-larval bioassay to assess chemicals. Ecotoxicology and Environmental Safety, 63(2): 253-267.
DOI:10.1016/j.ecoenv.2004.10.015 |
Gao Y H, Yu Z M, Song X X, Cao X X. 2007. Impact of modified clays on the infant oyster (Crassostrea gigas). Marine Science Bulletin, 26: 53-60.
(in Chinese with English abstract) |
Gibbs M, Özkundakci D. 2011. Effects of a modified zeolite on P and N processes and fluxes across the lake sedimentwater interface using core incubations. Hydrobiologia, 661(1): 21-35.
DOI:10.1007/s10750-009-0071-8 |
Greig S M, Sear D A, Smallman D, Carling P A. 2005. Impact of clay particles on the cutaneous exchange of oxygen across the chorion of Atlantic salmon eggs. Journal of Fish Biology, 66(6): 1 681-1 691.
DOI:10.1111/jfb.2005.66.issue-6 |
Hamm J T, Hinton D E. 2000. The role of development and duration of exposure to the embryotoxicity of diazinon. Aquatic Toxicology, 48(4): 403-418.
DOI:10.1016/S0166-445X(99)00065-X |
Harvard University. 2007. A summary of error propagation. Physical Sciences 2. http://ipl.physics.harvard.edu/wpuploads/2013/03/PS3_Error_Propagation_sp13.pdf.
|
Hugh A. Poston. 1991. Effects of dietary aluminum on growth and composition of young Atlantic salmon. North American Journal of Aquaculture, 53(1): 7-10.
|
James R, Sampath K. 1999. Effect of zeolite on the reduction of cadmium toxicity in water and a freshwater fish, Oreochromis mossambicus. Bulletin of Environmental Contamination and Toxicology, 62(2): 222-229.
DOI:10.1007/s001289900863 |
Jezierska B, Ługowska K, Witeska M. 2009. The effects of heavy metals on embryonic development of fish (a review). Fish Physiology and Biochemistry, 35(4): 625-640.
DOI:10.1007/s10695-008-9284-4 |
Jia Y D, Meng Z, Liu X F, Lei J L. 2014. Biochemical composition and quality of turbot (Scophthalmus maximus) eggs throughout the reproductive season. Fish Physiology and Biochemistry, 40(4): 1 093-1 104.
|
Jin D, Thunberg E, Hoagland P. 2008. Economic impact of the 2005 red tide event on commercial shellfish fisheries in New England. Ocean & Coastal Management, 51(5): 420-429.
|
Kent M L, Whyte J N C, Latrace C. 1995. Gill lesions and mortality in seawater pen-reared Atlantic salmon Salmo salar associated with a dense bloom of Skeletonema costatum and Thalassiosira species. Diseases of Aquatic Organisms, 22(1): 77-81.
|
Kim W S, Oh M J, Jung S J, Kim Y J, Kitamura S I. 2005. Characterization of an iridovirus detected from cultured turbot Scophthalmus maximus in Korea. Diseases of Aquatic Organisms, 64(2): 175-180.
|
Landsberg J H. 2002. The effects of harmful algal blooms on aquatic organisms. Reviews in Fisheries Science, 10(2): 113-390.
DOI:10.1080/20026491051695 |
Larkin S L, Adams C M. 2007. Harmful algal blooms and coastal business:economic consequences in Florida. Society & Natural Resource, 20(9): 849-859.
|
Larraza I, Peinado C, Abrusci C, Catalina F, Corrales T. 2011. Hyperbranched polymers as clay surface modifiers for UV-cured nanocomposites with antimicrobial activity. Journal of Photochemistry and Photobiology A: Chemistry, 224(1): 46-54.
DOI:10.1016/j.jphotochem.2011.09.005 |
Lee C K, Kim W S, Park Y T, Jo Q T. 2013a. Effect of yellow clay on the oxygen consumption rate of Korean rockfish, Sebastes schlegelii. Journal of the Korean Society of Marine Environment & Safety, 19(3): 241-247.
|
Lee Y C, Jin E S, Jung S W, Kim Y M, Chang K S, Yang J W, Kim S W, Kim Y O, Shin H J. 2013b. Utilizing the algicidal activity of aminoclay as a practical treatment for toxic red tides. Science Reports, 3: 1 292.
DOI:10.1038/srep01292 |
Lee Y J, Choi J K, Kim E K, Youn S H, Yang E J. 2008. Field experiments on mitigation of harmful algal blooms using a Sophorolipid-Yellow clay mixture and effects on marine plankton. Harmful Algae, 7(2): 154-162.
DOI:10.1016/j.hal.2007.06.004 |
Lewis M A, Dantin D D, Walker C C, Kurtz J C, Greene R M. 2003. Toxicity of clay flocculation of the toxic dinoflagellate, Karenia brevis, to estuarine invertebrates and fish. Harmful Algae, 2(4): 235-246.
DOI:10.1016/S1568-9883(03)00041-6 |
Lind U T, Chrzanowski T H, Dávalos-Lind L. 1997. Clay turbidity and the relative production of bacterioplankton and phytoplankton. Hydrobiologia, 353(1-3): 1-18.
|
Liu Y, Cao X H, Yu Z M, Song X X, Qiu L X. 2016. Flocculation of harmful algal cells using modified clay:effects of the properties of the clay suspension. Journal of Applied Phycology, 28(3): 1 623-1 633.
DOI:10.1007/s10811-015-0735-x |
Lugowska K. 2007. The effect of cadmium and cadmium/ copper mixture during the embryonic development on deformation common carp larvae. Electronic Journal of Ichthyology, 2: 46-60.
|
Malakul P, Srinivasan K R, Wang H Y. 1998. Metal adsorption and desorption characteristics of surfactant-modified clay complexes. Industrial & Engineering Chemistry Research, 37(11): 4 296-4 301.
|
Marty G D, Núñez J, Lauren D J, Hinton D E. 1990. Agedependent changes in toxicity of N-nitroso compounds to Japanese medaka (Oryzias latipes) embryos. Aquatic Toxicology, 17(1): 45-62.
DOI:10.1016/0166-445X(90)90011-D |
Monette M Y, Bj rnsson B T, McCormick S D. 2008. Effects of short-term acid and aluminum exposure on the parrsmolt transformation in Atlantic salmon (Salmo salar): disruption of seawater tolerance and endocrine status. General and Comparative Endocrinology, 158(1): 122-130.
DOI:10.1016/j.ygcen.2008.05.014 |
Orizar I S, Rivera P P L, Azanza R V. 2013. Harmful algal bloom (HAB) mitigation using ball clay:effect on nontarget organisms. Journal of Environmental Science and Management, 5(2): 36-43.
|
Pan G, Chen J, Anderson D M. 2011. Modified local sands for the mitigation of harmful algal blooms. Harmful Algae, 10(4): 381-387.
DOI:10.1016/j.hal.2011.01.003 |
Park T G, Lim W A, Park Y T, Lee C K, Jeong H J. 2013. Economic impact, management and mitigation of red tides in Korea. Harmful Algae, 30(S1): S131-S143.
|
Parkyn S M, Hickey C W, Clearwater S J. 2011. Measuring sub-lethal effects on freshwater crayfish (Paranephrops planifrons) behaviour and physiology:laboratory and in situ exposure to modified zeolite. Hydrobiologia, 661(1): 37-53.
DOI:10.1007/s10750-010-0241-8 |
Paulsen H, Poulsen N E, Iglesias J, Olmedo M, Korsgaard B, Lavens P, Burkhardt-Holm P. 1998. Indicators of nutritional status of turbot Scophthalmus maximus (L., 1758) larvae. Boletin-Instituto Espanol de Oceanografia, 14(1): 5-18.
|
Priede I G, Tytler P. 1977. Heart rate as a measure of metabolic rate in teleost fishes; Salmo gairdneri, Salmo trutta and Gadus morhua. Journal of Fish Biology, 10(3): 231-242.
DOI:10.1111/jfb.1977.10.issue-3 |
Rybicka E H, Calmano W, Breeger A. 1995. Heavy metals sorption/desorption on competing clay minerals; an experimental study. Applied Clay Science, 9(5): 369-381.
DOI:10.1016/0169-1317(94)00030-T |
Sengco M R, Anderson D M. 2004. Controlling harmful algal blooms through clay flocculation. The Journal of Eukaryotic Microbiology, 51(2): 169-172.
DOI:10.1111/jeu.2004.51.issue-2 |
Sfakianakis D G, Renieri E, Kentouri M, Tsatsakis A M. 2015. Effect of heavy metals on fish larvae deformities:a review. Environmental Research, 137: 246-255.
DOI:10.1016/j.envres.2014.12.014 |
Song X X, Yu Z M, Gao Y H. 2003. Removal of different species of red tide organisms with an effective claycomplex system. Chinese Journal of Applied Ecology, 14(7): 1 165-1 168.
(in Chinese) |
Stuart K, Rotman F, Drawbridge M. 2016. Methods of microbial control in marine fish larval rearing:clay-based turbidity and passive larval transfer. Aquaculture Research, 47(8): 2 470-2 480.
DOI:10.1111/are.2016.47.issue-8 |
Sun X X, Zhang B. 2000. Toxicity study of anti red tide agents to Penaeus chinensis. Marine Environmental Science, 19(4): 5-8.
(in Chinese with English abstract) |
Thangaraja M, Al-Aisry A, Al-Kharusi L. 2007. Harmful algal blooms and their impacts in the middle and outer ROPME sea area. International Journal of Oceans & Oceanography, 2(1): 85-98.
|
Urban E R Jr, Kirchman D L. 1992. Effect of kaolinite clay on the feeding activity of the eastern oyster Crassostrea virginica (Gmelin). Journal of Experimental Marine Biology and Ecology, 160(1): 47-60.
DOI:10.1016/0022-0981(92)90109-N |
Wang D S, Sun W, Xu Y, Tang H X, Gregory J. 2004. Speciation stability of inorganic polymer flocculant-PACl. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 243(1-3): 1-10.
|
Wang X Q. 2012. Effects of dimethylhydantoin and carbohydrate on growth and immunity of Litopenaeus vannamei. Advanced Materials Research, 356-360: 146-151.
|
Wang Z B, Zhang H G, Pan G. 2016. Ecotoxicological assessment of flocculant modified soil for lake restoration using an integrated biotic toxicity index. Water Research, 97: 133-141.
DOI:10.1016/j.watres.2015.08.033 |
Wang Z F, Yu Z M, Song X X, Cao X H, Liu K. 2014b. Impact of modified clay on the growth of the infant Apostichopus japonicas selenka in habs controling. Oceanologia et Limnologia Sinica, 45(2): 233-238.
(in Chinese with English abstract) |
Wang Z F, Yu Z M, Song X X, Cao X H. 2014a. Effects of modified clay on the infant of Patinopecten yessoensis for HABs control. Marine Environmental Science, 33(6): 817-821.
(in Chinese with English abstract) |
Yıldırım Ö, Türker A, Şenel B. 2009. Effects of natural zeolite (clinoptilolite) levels in fish diet on water quality, growth performance and nutrient utilization of tilapia (Tilapia zillii) Fry. Fresenius Environmental Bulletin, 18(9): 1 567-1 571.
|
Yu Z M, Song X X, Cao X H, Liu Y. 2017. Mitigation of harmful algal blooms using modified clays:theory, mechanisms, and applications. Harmful Algae, 69: 48-64.
DOI:10.1016/j.hal.2017.09.004 |
Yu Z M, Zou J Z, Ma X N. 1994. A new method to improve the capability of clays for removing red tide organisms. Oceanologia et Limnologia Sinica, 25(2): 226-232.
(in Chinese with English abstract) |
 2019, Vol. 37
2019, Vol. 37


