Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHU Guoping, LIU Zijun, YANG Yang, WANG Zhen, YANG Wenjie, XU Liuxiong
- Thermal and saline tolerance of Antarctic krill Euphausia superba under controlled in-situ aquarium conditions
- Journal of Oceanology and Limnology, 37(3): 1080-1089
- http://dx.doi.org/10.1007/s00343-019-8002-7
Article History
- Received Jan. 17, 2018
- accepted in principle May. 3, 2018
- accepted for publication Jul. 17, 2018
2 Center for Polar Research, Shanghai Ocean University, Shanghai 201306, China;
3 National Engineering Research Center for Oceanic Fisheries, Shanghai 201306, China;
4 Polar Marine Ecosystem Group, Key Laboratory of Sustainable Exploitation of Oceanic Fisheries Resources, Ministry of Education, Shanghai Ocean University, Shanghai 201306, China
A series of experiments were conducted to establish a mechanistic understanding on how climate change might transform the global biota, including marine organisms (Wernberg et al., 2012). As a keystone species of the Southern Ocean ecosystem, Antarctic krill (Euphausia superba Dana 1850, hereafter krill) is always one of the cores of Antarctic research. Among these, some studies have examined environmental pressures experienced by krill, such as ocean acidification, etc., (McWhinnie and Marciniak, 1964; Hirche, 1984; Jarman et al., 1999; Newman et al., 1999, 2003; Dahms et al., 2011; Kawaguchi et al., 2011, 2013; Tremblay and Abele, 2016), using field observations and/or incubation in the laboratory; more details can be found in the review by Flores et al. (2012). Aarset and Torres (1989) examined the cold resistance and metabolic response of krill to variations in salinity. However, few studies can be found that examined the response of krill to changing thermal or saline environments over the past 30 years, though they have been performed on other crustacean species, such as Homarus americanus (McLeese and Wilder, 1958; Reynolds and Casterlin, 1979a), Ocypode ceratophthalma (Burrows and Hoyle, 1973; Florey and Hoyle, 1976), Astacus astacus (Kivivuori, 1983; Lehti-Koivunen and Kivivuori, 1994), Daphnia magna (McKenzie et al., 1992), and Asellus aquaticus (Lagerspetz, 2003). Potential changes in temperature due to a warming climate may have an effect on salinity (Durack et al., 2012; Rye et al., 2014), particularly in the western Antarctic Peninsula; these areas may overlap with the regions of krill distribution. Changes in the thermal and saline environments may affect several life history stages of krill, particularly larval krill (Aarset and Torres, 1989; Quetin et al., 1996; Loeb et al., 2009). It is potentially very difficult to examine this with field observations, but controlled aquarium experiments can be an effective and feasible alternative. Compared to land-based experiments, insitu experiments based on aquariums at sea can provide more accurate information on the response of krill to changes in the environmental conditions. Therefore, the purpose of the present study was to examine the thermal and saline tolerance of krill and to estimate the thresholds of those tolerances under controlled in-situ aquarium conditions. The results of the present study can provide potential scenarios for predicting the fate of this keystone species in the Southern Ocean.
2 METHOD AND MATERIAL 2.1 Experimental settingKrill have a complex life history (Nicol, 2006; Jia et al., 2014), which means that they experience different thermal and saline environments in the lifetime. To examine the adaptability of krill to different rates of changing temperature and salinity, it is necessary to observe their response under different conditions. Hence, a set of four different experiments were designed in this study: specific thermal and saline scenarios and changing thermal and salinity scenarios. To exclude interference from other factors, krill were not fed during the experiment.
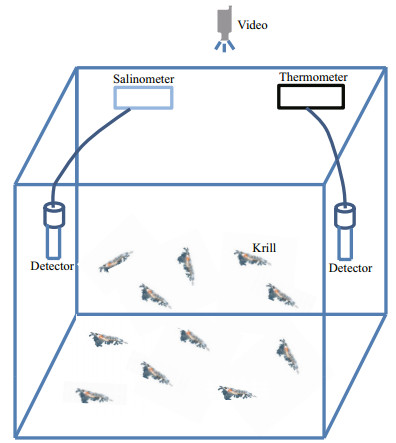
The live krill were incubated in a 300 L aquarium for 24 h under starvation conditions (Fig. 1). Seawater in the maintained aquarium was changed by two thirds every 8 h. Dead individuals, molted carapaces, and feces were discharged from the hole in the bottom of the aquarium. Alive and strong individuals were selected to conduct the controlled experiments. The controlled experiments were conducted in 50 cm×40 cm×30 cm and 50-L incubation aquariums, which included a recycled water system, with water recycling, filtering, and oxygenation (Fig. 2). The ambient light intensity in the aquarium was monitored with an electronic illuminometer (TES-1322A, TES Electrical Electronic Corp., Taipei, China). The temperature and salinity values of the seawater in the incubation aquariums were monitored by thermometer and salinometer, respectively (YM- 2007, Yimeng Electronic Co. Ltd., Handan, Heibei Province, China). The processes of the experiment were monitored by video (GoPro Hero 2 v.312, GoPro, Inc., USA).

|
| Fig.1 The maintained aquarium for krill observation |
|
| Fig.2 Schematic figure of the incubation aquarium used for krill under temperature- and salinity-controlled scenarios |
The samples were collected from the large-scale midwater trawler Kaili operating in the proximity of the South Shetland Islands on March 10, 2014. The ambient temperature of the seawater was -0.2℃. The temperature of seawater in the maintained aquariums ranged from -0.2℃ to 0.5℃; the average ambient air temperature was 2℃, and the light intensity was 90 to 150 lx.
2.1.2 Saline tolerance experimentThe samples were collected from the large-scale midwater trawler Longda operating in the proximity of the South Shetland Islands on May 9, 2015. The ambient temperature of the seawater was -0.2℃. The conditions used were identical to those used for the above thermal tolerance experiment.
2.2 Sampling and experimental setupLagerspetz and Vainio (2006) indicated that the 'final thermal preferendum' can be determined based on (1) the 'gravitational method' which means the subjects are left in a gradient for a long period, and (2) the 'acute' method, in which the results of short-term experiments on animals acclimated to different temperatures can be used for graphical or mathematical estimation. Based on these definitions, the present study used two experimental designs, i.e., specific and changing conditions for thermal and saline scenarios.
For the thermal scenario, 120 alive and strong individuals were selected from the maintained aquarium and divided equally into six groups (Table 1). There was no remarkable difference in the standard lengths (SLs) of krill between groups (Kolmogorov-Smirnov test; χ2=4.823, P=0.567 > 0.05). The fluctuation in seawater temperature in the incubation aquarium was controlled to ±0.1℃, and the average light intensity was 25.6 lx. Sufficient oxygen levels were maintained in the water. Alive krill were incubated in the six incubation aquariums with the preset and constant temperatures, and a 3℃-temperature gradient of seawater from 0–15℃ was set up between the incubation aquariums.

|
For the salinity scenario, 200 alive and strong individuals were selected from the maintained aquarium and divided equally into 10 groups (Table 1). In the Southern Ocean, the saline environment could be impacted by seasonal sea ice dynamics. The water could be refreshed by the melting of sea ice in the summer, and vice versa in the winter. Hence, two different saline conditions (under or above average salinity of seawater) were considered in the present study. The standard lengths of the krill under lower saline (Kolmogorov-Smirnov test; P=0.401 > 0.05) or higher saline (Kolmogorov-Smirnov test; P=0.573 > 0.05) scenarios did not differ significantly between groups. The temperature of seawater in the incubation aquarium was controlled at 0.3±0.1℃ and the light intensity was 65±5 lx. Sufficient oxygen levels were maintained in the water.
For changing thermal scenarios, 20 individuals (41.80±5.02 mm SL) were selected and transferred to the 50 L incubation aquarium (0℃ natural seawater). The condition of the krill was recorded every h, before gradually increasing the temperature by 2℃; this process was repeated until an abnormal individual occurred. At this point, the temperature was increased by 1℃ each time. The temperature was considered at the critical point of krill's temperature tolerance if dead individuals occurred. The 1℃ temperature increase was continued until half of the individuals were dead. During the experimental period, the temperatures in the aquariums were real-time monitored using electronic thermometers. To determine the preferred temperature range of krill, the gradient used was wide enough (0–14℃) and was not too close to the avoidance temperatures of that species (Lagerspetz and Vainio, 2006).
Given the unknown effect of changing climate on the salinity of seawater in the Southern Ocean, two salinity scenarios were designed in the present study, i.e., higher and lower salinity scenarios with an average salinity of sea water 34. For the higher salinity scenario, 24 individuals (42.18±4.74 mm SL) were selected and transferred to a 100-L incubation aquarium with natural seawater. The condition of krill was recorded after a 2-h constant period. The salinity of seawater was increased by 2 after krill condition was recorded and again maintained for a 2-h constant period. The 2 salinity increase, constant salinity, and recording processes were repeated until an abnormal individual occurred. The salinity was considered the upper critical point of the krill's saline tolerance if dead individuals occurred. The 2 salinity increase was continued until half of the individuals were dead. For the lower salinity scenario, 24 individuals (41.83±4.45 mm SL) were selected and transferred to the 100 L incubation aquarium with natural seawater. The process was similar to the higher saline scenario, but with a 2-decreasing salinity interval. The salinity was considered as the lower critical point of the krill's saline tolerance if dead individuals occurred.
With the dynamic conditions at sea, it is difficult to conduct in-situ physiological measurements. Thus, the conditions of krill in the aquariums were observed and described arbitrarily in four types of conditions: normal (N), occasionally reclining (I), reclining (L), and dead (D). The observation time lasted for 5 min. The occasionally reclining individual recovered to swimming or moving conditions after a short period reclining at the bottom of the incubation aquarium. When the reclining individual lay on the bottom of the incubation aquarium for a period it was considered abnormal as it showed to be uncomfortable in the thermal or saline conditions.
2.3 Statistical analysisA logistic model (Lysack, 1980) was used to describe the relationship between the proportion of dead krill individuals (PX) for each time period in the specific thermal or saline scenarios, or for each temperature or salinity in the changing thermal or saline scenarios. The 50% lethal temperature (salinity) in the changing thermal and saline scenarios, or 50% lethal time in the specific temperature and salinity scenarios were then estimated by substituting PX=0.5 into the below equation:
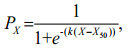
where X represents temperature, salinity, or time, X50 represents 50% lethal temperature/salinity in the changing thermal/saline scenarios, or 50% lethal time in the specific temperature/salinity scenarios, and k represents the rate at which death occurred. The logistic parameters were estimated by the non-linear minimization of a negative binomial log-likelihood of the form (Zhu et al., 2010).
3 RESULT 3.1 Specific thermal or saline scenariosExcept for group 6 (15℃), the temperaturecontrolled experiments were continued for 24 h. Under the group 1 (0℃) and group 2 (3℃) scenarios, most individuals showed a normal condition after 24 h, and none of the krill had died. Under the group 3 (6℃) scenario, the first krill showing an abnormality occurred after 4 h, and the number of individuals showing an abnormal condition increased gradually over time. Six individuals showed no abnormal condition at all after 24 h, and no individuals died. Under the group 4 (9℃) scenario, three individuals were normal and one individual lay on the bottom but not dead after 2 h, and one died when the experiment was continued to 24 h. Under the group 5 (12℃) scenario, the individuals responded rapidly to the temperature; eight abnormal individuals and one dead individual were found after 2 h. After 24 h, 14 individuals were dead. Under the group 6 (15℃) scenario, the experiment was only continued to 7 h, and all individuals were dead after this time. The observed critical lethal time was 24 h, 2 h, and 0.5 h under the 9℃, 12℃, and 15℃ scenarios, respectively, and the estimated 50% lethal time were approximately 17.1 h and 1.7 h under the 12℃ and 15℃ scenarios, respectively (Fig. 3).

|
| Fig.3 Mortality of krill under different temperatures The 50% lethal time under different thermal scenarios (12℃ and 15℃) can be estimated using a logistic model. |
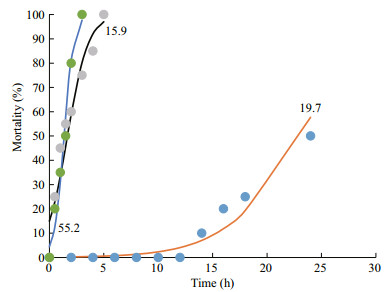
For the lower saline condition, five group (15.9, 19.7, 25.8, 30.2, and 34.4, respectively) scenarios were conducted. Except for group 5 (15.9), four groups were continued to 24 h. Under the group 1 (34.4) and group 2 (30.2) scenarios, only one individual was abnormal after 24 h. Under the group 3 (25.8) scenario, four individuals were laying on the bottom after 12 h, although they were still alive after 24 h. Under the group 4 (19.7) scenario, 11 individuals were abnormal after 2 h, and two individuals were dead after 14 h. After 24 h, all individual krill showed abnormal conditions and 10 individuals had died. Under the group 5 (15.9) scenario, no individuals were normal and five of them were dead after 0.5 h. All krill were dead after 5 h. The observed critical lethal time was approximately 14 h and 0.5 h under the 19.7 and 15.9 scenarios, respectively. The estimated 50% lethal time was approximately 22.9 h and 1.7 h under the 19.7 and 15.9 scenarios, respectively (Fig. 4).
|
| Fig.4 Mortality of krill under different salinities The 50% lethal time under different saline scenarios (19.7, 15.9, and 55.2) can be estimated using a logistic model. |
For the higher saline condition, 5 group (34.4, 39.2, 43.2, 50.5 and 55.2, respectively) scenarios were conducted. Except for group 5 (55.2), all groups were continued to 24 h. Under groups 1 (34.4) and 2 (39.2) scenarios, only one individual was abnormal after 24 h. Under the group 3 (43.2) scenario, three individuals were abnormal after 2 h, and three individuals were lying on the bottom but not dead after 24 h. Under the group 4 (50.5) scenario, nine individuals were abnormal and three of them were lying on the bottom but not dead after 2 h; one dead individual occurred after 10 h, and seven were dead after 24 h. Under the group 5 (55.2) scenario, no individuals were normal and four of them were dead after 0.5 h. All krill were dead after 3 h. The observed critical and 50% lethal times were 0.5 h and approximately 1.4 h, respectively, under the 55.2 scenario (Fig. 4).
3.2 Changing thermal or saline scenariosFor changing thermal scenarios, the experiment was continued for 16 h (Fig. 5). All individuals were swimming normally after 3 h. One individual was abnormal and one was lying on the bottom when the temperature was increased to 7℃ and 9℃, respectively. One individual died when the temperature was increased to 13℃ after 11 h. After that, the number of dead individuals increased rapidly, and all individuals were dead after 16 h (13℃). The observed critical and 50% lethal temperatures were 13℃ and about 14.2℃, respectively (Fig. 6). The upper thermal preference of krill can be considered to be 6℃ (Fig. 5).

|
| Fig.5 Individual conditions of krill under the changing thermal scenario N: normal; I: occasionally reclining; L: reclining; D: dead. |

|
| Fig.6 Mortality of krill under different temperatures The 50% lethal temperature can be estimated using a logistic model. |
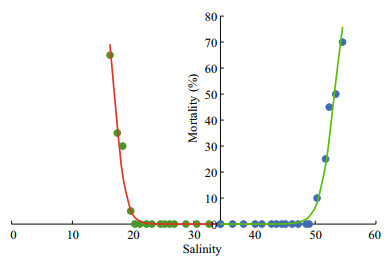
For the lower salinity scenario, in which the salinity was decreased starting from 34.4, the experiment was continued for 30 h (Fig. 7). All individuals were moving normally when the salinity was decreased to 26.8 after 8 h. An individual lay on the bottom after 14 h (24.5). One dead individual occurred when the salinity was decreased to 19.6 after 24 h. Thirteen individuals were dead after 30 h (16.2). The observed critical and 50% lethal salinity values were 19.6 and 17.5, respectively (Fig. 8).

|
| Fig.7 Individual conditions of krill under changing lower (upper panel) and higher (lower panel) salinity scenarios N: normal; I: occasionally reclining; L: reclining; D: dead. |
|
| Fig.8 The proportion of dead krill under different salinities The 50% lethal salinity under different saline scenarios can be estimated using a logistic model. |
For the higher salinity scenario, in which the salinity was increased from 34.4, the experiment was continued for 34 h (Fig. 7). The individuals were moving normally before the salinity was increased to 41.2. Two individuals lay on the bottom after 16 h and two individuals died when the salinity was increased to 50.2 after 26 h. Fourteen were dead after the experiment finished and the corresponding salinity was 54.5. The observed critical and 50% lethal salinity was 50.3 and 53.2, respectively (Fig. 8). The saline preference of krill can be considered to be 26.8–41.2 (Fig. 7).
4 DISCUSSION 4.1 Preferred thermal rangeThe experimental design was similar to the definitions of Lagerspetz and Vainio (2006); i.e., (1) the gravitational method corresponds to the specific thermal scenario and method, and (2) the acute method corresponds to the changing thermal scenario in the present study. During the present study, the specific thermal scenario was not conducted using a stepwise increase of 1℃, but of 2℃, limiting the comparability of both thermal experiments. However, in both experiments, no individuals died when the temperature was below 6℃. Using the bluegill sunfish (Lepomis macrochirus), Reynolds and Casterlin (1979b) indicated that equal results can be concluded based on the above two approaches. Similar findings were also available for the prawn Macrobrachium rosenbergii (Díaz Herrera and Bückle Ramirez, 1993). Moreover, for the specific thermal scenarios below 6℃, although no dead individuals occurred, different conditions of krill, such as normal, occasionally reclining and reclining, could be found, and they varied when changing the thermal scenario, in which all individuals were normal when the temperature was below 6℃. The effects of thermal acclimation on krill and the suitability of krill to the changing thermal environment should be considered, particularly under the changing thermal scenario, although the preferred upper thermal range was identified as 6℃ in the present study. This value is higher than the expected one stated by Atkinson et al. (2006). They indicated that krill did not tolerate temperatures above 3.5℃ for a long period of time (Atkinson et al., 2006; Tarling et al., 2006). Thus, future studies shall focus on the interval at which the temperature is changed and temporal ranges for observation when conducting the changing thermal scenario; these studies could be used to identify the precise preferred thermal range of krill, because a gradual increase in the mean temperature may reflect the real thermal stress on krill populations (Whitehouse et al., 2008). Additionally, with the limitation of insitu experiments, the present study did not examine the lower thermal range of krill, as shown by Aarest and Torres (1989), who indicated that the critical lower temperature that krill can tolerate was -9℃. Additional to the behavioral observations, the future detailed investigation should be conducted using physiological, biochemical, and molecular approaches (Kawaguchi et al., 2011), because temperature changes can affect molting, growth, and maturation of krill at different life stages (Poleck and Denys, 1982; Brown et al., 2010). Moreover, in the present study, the experiments were accomplished under starvation conditions. In fact, krill could survive for more than 200 days without food in the laboratory (Ikeda and Dixon, 1982) and the short-term starvation had no significant effect on the metabolism of the krill (Van Ngan et al., 1997). Thus, compared to the thermal effect, the potential effect of short-term starvation (less than 36 h) on the adaptability of krill to thermal change should not be substantial.
4.2 Tolerance to temperature and salinityChanges in the environmental temperature were shown to have immediate and transient effects on crustacean motor activity, and the effect of temperature on motor activity depended on the rate of the temperature change (Lagerspetz and Vainio, 2006). Except for larger semi-terrestrial crabs exposed to air, all crustaceans, including krill, are poikilotherms (Lagerspetz and Vainio, 2006). In many studies, animals were maintained for 0.5–1.0 h at a stable temperature before their motor activity was measured. In the present study, in the groups 1 and 2 (specific thermal scenario), the individuals were almost all under normal conditions after 2 h, but for group 3 onward, the activity of krill was affected in different conditions; thus, 6℃ was critical to krill survival. When krill were transferred from their natural water temperature (-0.2℃ to 0.5℃) to controlledtemperature incubation aquariums at 9℃, 12℃, and 15℃, the number of dead (or heat shocked) individuals increased from 1 to 20 after 24 h. Similar results were observed in Asellus aquaticus, when they were transferred from 13℃ to 18℃ water for a few minutes, the frequency and angle of the movement increased. However, when transferred from 13℃ to 25℃ and 28℃, the number of stops was increased and the posture of the animals was also affected. Lagerspetz (2003) considered that this represented the first behavioral and neural effects of heat shock because of its rapid and transient effects. Although Asellus aquaticus can tolerate 28℃ for 1 h (Korhonen and Lagerspetz, 1996), krill do not have this heat tolerance. Hence, the results derived from the present study, to some extent, reflect the vulnerability of krill in the Southern Ocean and their sensitivity to temperature changes.
As one of the important attributes of sea water, salinity has a significant effect on the physiological behavior of aquatic organisms; aquatic organisms can present different tolerances under different saline environments (Li, 2008). Krill are associated with sea ice and depend on ice algae as their food source, especially during the winter season, and they will be exposed to the seasonal changes in physical parameters as the ice melts or freezes (Aarset and Torres, 1989; Meyer et al., 2009; Schaafsma et al., 2017). The tolerance of aquatic organisms to salinity has been examined in copepods (Lance, 1963; Gradinger and Schnack-Schiel, 1998), gastropods (Sander and Moore, 1979), and crustaceans (Rokneddine and Chentoufi, 2004; Dissanayake and Ishimatsu, 2011, Torres et al., 2011). However, the salinity tolerance of krill has hitherto has not been well examined. Aarset and Torres (1989) indicated that variation in salinity can impact the metabolism of krill, and found that krill are osmoconformers in the salinity range of 25–45. This range was narrower than that observed in the present study (19.6–50.3 for the critical salinity range). However, basically, both studies demonstrated that krill have a significant tolerance to changes in salinity. Therefore, this result may provide a possible explanation for the under-ice feeding of krill along the dynamically changing marginal ice zone and under-ice habitat. Moreover, during the molt period, krill must conduct a series of matter circulations and energy transfers from the ambient environment; this process needs suitable salinity to maintain the permeation pressure (Brown et al., 2010). Thus, when krill are exposed to high or low salinity media at a constant temperature, they will conform osmotically to within the tolerated salinity range (Aarset and Torres, 1989). Similar results can be found for the amphipod Orchomene plebs in other Antarctic communities (Rakusa-Suszczewski and McWhinnie, 1976), the sea ice amphipod Gammarus wilkitzkii (Aarset and Aunaas, 1987) and Northern krill Meganyctiphanes norvegica (Forward and Fyhn, 1983) in the Arctic.
5 CONCLUSIONThe Southern Ocean, particularly the western Antarctic Peninsula, has undergone significant environmental changes in the past few decades (Cook et al., 2005; Meredith and King, 2005). These changes could potentially affect the living resources in this region. Understanding the response of krill, a keystone species in the Southern Ocean, to those changes can provide insights for the entire marine ecosystem in the Southern Ocean. Maintaining krill in the onboard aquarium was a difficult work, and opportunities to collect krill in the wild are generally limited. In addition, the control of environmental conditions on the vessel also requires some compromises. This is a reason why such studies have been progressing very slowly in the past decades. The present study examined the thermal and saline tolerances of krill under in-situ aquarium conditions with different controlled scenarios. The results provide a potential scenario for predicting the possible fate of this key species in the Southern Ocean. However, samples from different seasons, developmental stages, and geographical regions, and controlled experiments under more environmental conditions could provide a more comprehensive understanding of the environmental tolerance of this species. It would be very helpful to further explore the response mechanism of krill to changing environmental variables when the specific object-oriented aquarium was developed and being used in the field.
6 DATA AVAILABILITY STATEMENTThe datasets generated and/or analyzed during the current study ae not publicly available due to the data management regulations of Shanghai Ocean University but are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENTThe authors are especially grateful to the captain and scientific observers on board trawlers Kaili and Longda for their help in collecting the samples used in this study and providing experimental space that made our research possible. We would also like to extend our thanks to Ms. Fokje Schaafsma at the Wageningen University & Research (WUR) and another anonymous reviewer for their valuable comments and suggestions, which improved significantly the present study. We also thank the Key Laboratory of Sustainable Exploitation of Oceanic Fisheries Resources, Shanghai Ocean University for their support and the utilization of their laboratory facilitates.
Aarset A V, Aunaas T. 1987. Physiological adaptations to low temperature and brine exposure in the circumpolar amphipod Gammarus wilkitzkii. Polar Biology, 8(2): 129-133.
DOI:10.1007/BF00297067 |
Aarset A V, Torres J J. 1989. Cold resistance and metabolic responses to salinity variations in the amphipod Eusirus antarcticus and the krill Euphausia superba. Polar Biology, 9(8): 491-497.
DOI:10.1007/BF00261032 |
Atkinson A, Shreeve R S, Hirst A G, Rothery P, Tarling G A, Pond D W, Korb R E, Murphy E J, Watkins J L. 2006. Natural growth rates in Antarctic krill (Euphausia superba):Ⅱ. Predictive models based on food, temperature, body length, sex, and maturity stage. Limnology and Oceanography, 51(2): 973-987.
DOI:10.4319/lo.2006.51.2.0973 |
Brown M, Kawaguchi S, Candy S, Virtue P. 2010. Temperature effects on the growth and maturation of Antarctic krill (Euphausia superba). Deep Sea Research Part Ⅱ:Topical Studies in Oceanography, 57(7-8): 672-682.
DOI:10.1016/j.dsr2.2009.10.016 |
Burrows M, Hoyle G. 1973. The mechanism of rapid running in the ghost crab, Ocypode ceratophthalma. Journal of Experimental Biology, 58: 327-349.
|
Cook A J, Fox A J, Vaughan D G, Ferrigno J G. 2005. Retreating glacier fronts on the Antarctic Peninsula over the past half-century. Science, 308(5721): 541-544.
DOI:10.1126/science.1104235 |
Dahms H U, Dobretsov S, Lee J S. 2011. Effects of UV radiation on marine ectotherms in polar regions. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 153(4): 363-371.
|
Díaz Herrera F, Bückle Ramirez L F. 1993. Thermoregulatory behaviour of Macrobrachium rosenbergii (Crustacea, Palaemonidae). Tropical Ecology, 43: 199-203.
|
Dissanayake A, Ishimatsu A. 2011. Osmoregulatory ability and salinity tolerance in several decapod crustaceans (Palaemonidae & Penaeidae) of the East China Sea. Plankton and Benthos Research, 6(3): 135-140.
DOI:10.3800/pbr.6.135 |
Durack P J, Wijffels S E, Matear R J. 2012. Ocean salinities reveal strong global water cycle intensification during 1950 to 2000. Science, 336(6080): 455-458.
DOI:10.1126/science.1212222 |
Flores H, Atkinson A, Kawaguchi S, Krafft B A, Milinevsky G, Nicol S, Reiss C, Tarling G A, Werner R, Bravo Rebolledo E, Cirelli V, Cuzin-Roudy J, Fielding S, Groeneveld J J, Haraldsson M, Lombana A, Marschoff E, Meyer B, Pakhomov E A, Rombolá E, Schmidt K, Siegel V, Teschke M, Tonkes H, Toullec J Y, Trathan P N, Tremblay N, Van De Putte A P, Van Franeker J A, Werner T. 2012. Impact of climate change on Antarctic krill. Marine Ecology Progress Series, 458: 1-19.
DOI:10.3354/meps09831 |
Florey E, Hoyle G. 1976. The effects of temperature on a nerve-muscle system of the Hawaiian ghost crab, Ocypode ceratophthalma (Pallas). Journal of Comparative Physiology, 110(1): 51-64.
|
Forward R B Jr, Fyhn H J. 1983. Osmotic regulation of the krill Meganyctiphanes norvegica. Comparative Biochemistry and Physiology Part A:Physiology, 74(2): 301-305.
DOI:10.1016/0300-9629(83)90604-7 |
Gradinger R, Schnack-Schiel S B. 1998. Potential effect of ice formation on Antarctic pelagic copepods:salinity induced mortality of Calanus propinquus and Metridia gerlachei in comparison to sympagic acoel turbellarians. Polar Biology, 20(2): 139-142.
DOI:10.1007/s003000050288 |
Hirche H J. 1984. Temperature and metabolism of plankton-Ⅰ. Respiration of Antarctic zooplankton at different temperatures with a comparison of antarctic and Nordic krill. Comparative Biochemistry and Physiology Part A:Physiology, 77(2): 361-368.
DOI:10.1016/0300-9629(84)90074-4 |
Ikeda T, Dixon P. 1982. Body shrinkage as a possible overwintering mechanism of the Antarctic krill, Euphausia superba Dana. Journal of Experimental Marine Biology and Ecology, 62(2): 143-151.
DOI:10.1016/0022-0981(82)90088-0 |
Jarman S, Elliott N, Nicol S, McMinn A, Newman S. 1999. The base composition of the krill genome and its potential susceptibility to damage by UV-B. Antarctic Science, 11(1): 23-26.
DOI:10.1017/S0954102099000048 |
Jia Z N, Virtue P, Swadling K M, Kawaguchi S. 2014. A photographic documentation of the development of Antarctic krill (Euphausia superba) from egg to early juvenile. Polar Biology, 37(2): 165-179.
DOI:10.1007/s00300-013-1420-7 |
Kawaguchi S, Ishida A, King R, Raymond B, Waller N, Constable A, Nicol S, Wakita M, Ishimatsu A. 2013. Risk maps for Antarctic krill under projected Southern Ocean acidification. Nature Climate Change, 3(9): 843-847.
DOI:10.1038/nclimate1937 |
Kawaguchi S, Kurihara H, King R, Hale L, Berli T, Robinson J P, Ishida A, Wakita M, Virtue P, Nicol S, Ishimatsu A. 2011. Will krill fare well under Southern Ocean acidification?. Biology Letters, 7(2): 288-291.
DOI:10.1098/rsbl.2010.0777 |
Kivivuori L. 1983. Temperature acclimation of walking in the crayfish Astacus astacus L. Comparative Biochemistry and Physiology Part A:Physiology, 75(3): 375-378.
DOI:10.1016/0300-9629(83)90096-8 |
Korhonen A I, Lagerspetz K Y H. 1996. Heat shock response and thermal acclimation in Asellus aquaticus. Journal of Thermal Biology, 21(1): 49-56.
DOI:10.1016/0306-4565(95)00020-8 |
Lagerspetz K Y H, Vainio L A. 2006. Thermal behaviour of crustaceans. Biological Reviews, 81(2): 237-258.
DOI:10.1017/S1464793105006998 |
Lagerspetz K Y H. 2003. Thermal acclimation without heat shock, and motor responses to a sudden temperature change in Asellus aquaticus. Journal of Thermal Biology, 28(5): 421-427.
DOI:10.1016/S0306-4565(03)00027-5 |
Lance J. 1963. The salinity tolerance of some estuarine planktonic copepods. Limnology and Oceanography, 8(4): 440-449.
DOI:10.4319/lo.1963.8.4.0440 |
Lehti-Koivunen S M, Kivivuori L A. 1994. Effect of temperature acclimation in the crayfish Astacus astacus L. on the locomotor activity during a cyclic temperature change.. Journal of Thermal Biology, 19(5): 299-304.
|
Li E C. 2008. Physiological effects of ambient salinity on Litopenaeus vannamei and nutrient modulation. East China Normal University, Shanghai. 155 pp. (in Chinese with English abstract)
|
Loeb V J, Hofmann E E, Klinck J M, Holm-Hansen O, White W B. 2009. ENSO and variability of the Antarctic Peninsula pelagic marine ecosystem. Antarctic Science, 21(2): 135-148.
DOI:10.1017/S0954102008001636 |
Lysack W. 1980. 1979 Cedar Lake Winnipeg Fish Stock Assessment Program. MS Report No. 30. Manitoba Department of Natural Resources, Canada.
|
McKenzie J D, Calow P, Clyde J, Miles A, Dickinson R, Lieb W R, Franks N P. 1992. Effects of temperature on the anaesthetic potency of halothane, enflurane and ethanol in Daphnia magna (Cladocera:Crustacea). Comparative Biochemistry and Physiology Part C:Comparative Pharmacology, 101(1): 15-19.
DOI:10.1016/0742-8413(92)90193-B |
McLeese D W, Wilder D G. 1958. The activity and catchability of the lobster (Homarus americanus) in relation to temperature. Journal of the Fisheries Research Board of Canada, 15(6): 1 345-1 354.
DOI:10.1139/f58-073 |
McWhinnie M A, Marciniak P. 1964. Temperature responses and tissue respiration in Antarctic crustacea with particular references to the krill Euphausia superba. In: Lee M O ed. Biology of the antarctic seas. American Geophysical Union, Washington, DC. p.63-72. https://agupubs.onlinelibrary.wiley.com/doi/abs/10.1029/AR001p0063
|
Meredith M, King J C. 2005. Rapid climate change in the ocean west of the Antarctic Peninsula during the second half of the 20th century. Geophysical Research Letters, 32(19): L19604.
|
Meyer B, Fuentes V, Guerra C, Schmidt K, Atkinson A, Spahic S, Cisewski B, Freier U, Olariaga A, Bathmanna U. 2009. Physiology, growth, and development of larval krill Euphausia superba in autumn and winter in the Lazarev Sea, Antarctica. Limnology and Oceanography, 54(5): 1 595-1 614.
DOI:10.4319/lo.2009.54.5.1595 |
Newman S J, Nicol S, Ritz D, Marchant H. 1999. Susceptibility of Antarctic krill (Euphausia superba Dana) to ultraviolet radiation. Polar Biology, 22(1): 50-55.
DOI:10.1007/s003000050389 |
Newman S J, Ritz D, Nicol S. 2003. Behavioural reactions of Antarctic krill (Euphausia superba Dana) to ultraviolet and photosynthetically active radiation. Journal of Experimental Marine Biology and Ecology, 297(2): 203-217.
DOI:10.1016/j.jembe.2003.07.007 |
Nicol S. 2006. Krill, currents, and sea ice:Euphausia superba and its changing environment. BioScience, 56(2): 111-120.
DOI:10.1641/0006-3568(2006)056[0111:KCASIE]2.0.CO;2 |
Poleck T P, Denys C J. 1982. Effect of temperature on the molting, growth and maturation of the Antarctic krill Euphausia superba (Crustacea:Euphausiacea) under laboratory conditions. Marine Biology, 70(3): 255-265.
DOI:10.1007/BF00396844 |
Quetin, L B, Ross R M, Frazer T K, Haberman K L. 1996. Factors affecting distribution and abundance of zooplankton, with an emphasis on Antarctic krill, Euphausia superba. In: Ross R M, Hofmann E E, Quetin L B eds. Foundations for Ecological Research West of the Antarctic Peninsula. American Geo-physical Union, Washington, DC. p.357-371. https://agupubs.onlinelibrary.wiley.com/doi/10.1029/AR070p0357
|
Rakusa-Suszczewski S, McWhinnie M A. 1976. Resistance to freezing by Antarctic fauna:supercooling and osmoregulation. Comparative Biochemistry and Physiology Part A:Physiology, 54(3): 291-300.
DOI:10.1016/S0300-9629(76)80114-4 |
Reynolds W W, Casterlin M E. 1979a. Behavioral thermoregulation and activity in Homarus americanus. Comparative Biochemistry and Physiology Part A:Physiology, 64(1): 25-28.
DOI:10.1016/0300-9629(79)90424-9 |
Reynolds W W, Casterlin M E. 1979b. Behavioral thermoregulation and the "Final Preferendum" paradigm. Integrative and Comparative Biology, 19(1): 211-224.
|
Rokneddine A, Chentoufi M. 2004. Study of salinity and temperature tolerance limits regarding four crustacean species in a temporary salt water swamp (Lake Zima, Morocco). Animal Biology, 54(3): 237-253.
DOI:10.1163/1570756042484719 |
Rye C D, Naveira Garabato A C, Holland P R, Meredith M P, George Nurser A J, Hughes C W, Coward A C, Webb D J. 2014. Rapid sea-level rise along the Antarctic margins in response to increased glacial discharge. Nature Geoscience, 7(10): 732-735.
DOI:10.1038/ngeo2230 |
Sander F, Moore E. 1979. Temperature and salinity tolerance limits of the marine gastropod Murex pomum. Comparative Biochemistry and Physiology Part A:Physiology, 64(2): 285-289.
DOI:10.1016/0300-9629(79)90662-5 |
Schaafsma F L, Kohlbach D, David C, Lange B A, Graeve M, Flores H, Van Franeker J A. 2017. Spatio-temporal variability in the winter diet of larval and juvenile Antarctic krill, Euphausia superba, in ice-covered waters. Marine Ecology Progress Series, 580: 101-115.
DOI:10.3354/meps12309 |
Tarling G A, Shreeve R S, Hirst A G, Atkinson A, Pond D W, Murphy E J, Watkins J L. 2006. Natural growth rates in Antarctic krill (Euphausia superba):Ⅰ.Improving methodology and predicting intermolt period. Limnology and Oceanography, 51(2): 959-972.
|
Torres G, Giménez L, Anger K. 2011. Growth, tolerance to low salinity, and osmoregulation in decapod crustacean larvae. Aquatic Biology, 12(3): 249-260.
DOI:10.3354/ab00341 |
Tremblay N, Abele D. 2016. Response of three krill species to hypoxia and warming:an experimental approach to oxygen minimum zones expansion in coastal ecosystems. Marine Ecology, 37(1): 179-199.
DOI:10.1111/maec.2016.37.issue-1 |
Van Ngan P, Gomes V, Carvalho P S M, De A C R Passos M J. 1997. Effect of body size, temperature and starvation on oxygen consumption of Antarctic krill Euphausia superba. Revista Brasileira de Oceanografia, 45(1-2): 1-10.
DOI:10.1590/S1413-77391997000100001 |
Wernberg T, Smale D A, Thomsen M S. 2012. A decade of climate change experiments on marine organisms:procedures, patterns and problems. Global Change Biology, 18(5): 1 491-1 498.
DOI:10.1111/j.1365-2486.2012.02656.x |
Whitehouse M J, Meredith M P, Rothery P, Atkinson A, Ward P, Korb R E. 2008. Rapid warming of the ocean around South Georgia, Southern Ocean, during the 20th century:forcings, characteristics and implications for lower trophic levels. Deep Sea Research Part Ⅰ:Oceanographic Research Papers, 55(10): 1 218-1 228.
DOI:10.1016/j.dsr.2008.06.002 |
Zhu G P, Dai X J, Xu L X, Zhou Y Q. 2010. Reproductive biology of bigeye tuna, Thunnus obesus, (Scombridae) in the eastern and central tropical Pacific Ocean. Environmental Biology of Fishes, 88(3): 253-260.
DOI:10.1007/s10641-010-9636-7 |
 2019, Vol. 37
2019, Vol. 37


