Institute of Oceanology, Chinese Academy of Sciences
Article Information
- HU Peng, LIU Bin, MA Qian, LIU Shufang, LIU Xinfu, ZHUANG Zhimeng
- Expression profiles of sex-related genes in gonads of genetic male Takifugu rubripes after 17β-estradiol immersion
- Journal of Oceanology and Limnology, 37(3): 1113-1124
- http://dx.doi.org/10.1007/s00343-019-8060-x
Article History
- Received Mar. 19, 2018
- accepted in principle Apr. 24, 2018
- accepted for publication Jul. 6, 2018
2 Function Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao National Laboratory for Marine Science and Technology & Function Laboratory for Marine Biology and Biotechnology, Qingdao 266000, China
Sex differentiation of teleost fish species may be determined independently by genetic or environmental factors or a combination of both. For species with genetic sex determination (GSD) mechanism, sex differentiation is also labile to effects of extreme environmental factors (i.e., exogenous hormones and temperature), and the turnout phenotypic sex may be contrary to the genetic one (Strüssmann and Nakamura, 2002). Estrogens, for example, are necessary for triggering ovarian differentiation and maintaining ovarian development in fish (Guiguen et al., 2010). Estrogen treatments during early development stages are able to induce changes in gonadal phenotypes of a genetic male from testis to the ovary in fish (Piferrer and Blázquez, 2005). So far, feminization has been conducted in a large number of fish species, including rainbow trout Oncorhynchus mykiss, Nile tilapia Oreochromis niloticus L and yellow catfish Pelteobagrus fulvidraco (Richardson) (Mair et al., 1997; Guiguen et al., 1999; Liu et al., 2013). In these species, treatment of exogenous estrogens within sex differentiation could not only induce primitive gonads in genetic males to differentiate into ovaries but also maintain development and maturation of ovaries. However, for species such as tiger puffer Takifugu rubripes and zebrafish Danio rerio, similar treatment resulted in reversion of ovaries to testes after treatment withdrawal (Baumann et al., 2014; Hu et al., 2017). Still, molecular regulatory mechanisms underlying gonadal sex reversal after estrogen treatments remain unclear.
In natural condition, the fate of gonadal differentiation in vertebrates with GSD is usually defined by the sex-determining gene. Current studies in mammals have indicated that the male-determining gene sry is just expressed within a critical window to initiate its downstream sex-related genes, and then it is lowly or barely expressed in the testes (She and Yang, 2017). Similar results are observed in rainbow trout and Nile tilapia. The male-determining genes in the two species are highly expressed during testicular differentiation but are lowly expressed in the differentiated testes (Yano et al., 2012; Li et al., 2015). Those studies imply that the sex-determining gene just acts as a switch to trigger gonadal differentiation and does not involve in gonadal development after differentiation. This is supported by the study in medaka Oryzias latipes that the male-determining gene dmy did not trigger testicular development once gonadal differentiation was completed, according to the results that the adult pseudo-females maintained ovarian morphology though dmy was expressed in the ovary (Suzuki et al., 2005). Based on the aforementioned conclusion, the sex-determining gene does not participate in morphological changes in gonads of sex-reversed fish after the treatments.
To date, a large number of sex-related genes in the sex determination pathway have been proved to play important roles in gonadal differentiation and maintenance. For instance, foxl2 and cyp19a are central transcription factors for triggering ovarian differentiation and maintaining ovarian development. Foxl2 can up-regulate expression of cyp19a by directly binding to its promoter in humans or by interacting with sf-1 in Nile tilapia (Wang et al., 2007; Fleming et al., 2010). Cyp19a codes aromatase, which is responsible for the conversion of androgens to estrogens in nearly all vertebrates (Uno et al., 2012). Deficiency in foxl2 or cyp19a in female Nile tilapia results in oocyte degeneration and complete sex reversal (Li et al., 2013). Dmrt1, amh, and sox9 act as important transcription factors implicated in testicular differentiation and development. Dmrt1 has been proven to be involved in spermatogonia proliferation in medaka and tiger puffer (Kobayashi et al., 2004; Yamaguchi et al., 2006). Amh can decrease germ cell number, which subsequently triggers testicular development in medaka and zebrafish (Shiraishi et al., 2008; Skaar et al., 2011; Pfennig et al., 2015). In medaka, lack of dmrt1 or amh results in male-tofemale sex reversal (Masuyama et al., 2012; Nakamura et al., 2012). Sox9 is the key target gene of sry, and loss of its function lead to sex reversal of male gonads in mammals (Sekido and Lovell-Badge, 2009). In tiger puffer, two subtypes of sox9, sox9a, and sox9b, have been identified, and both are considered to involve in gonadal differentiation and development (Shen et al., 2007).
Tiger puffer is a differentiated gonochoristic fish with an XX/XY sex determination. It has the most compact genome among vertebrates and is a model species to research gene function related to sex determination and differentiation (Aparicio et al., 2002). The gonadal differentiation of tiger puffer occurred within 42 days post-hatching (dph, Yamaguchi et al., 2006). However, juveniles fed with 100 μg/g 17β-estradiol (E2) developed into intersexual gonads from 21 to 80 dph (Lee et al., 2009). Similar results were observed in the juveniles fed with 100, 150 and 200 μg/g E2 from 30 to 80 dph in our previous study. Then we immersed tiger puffer with 1, 10 and 100 μg/L E2 from 15 to 100 dph (Hu et al., 2017). Results showed that E2 immersion caused conversion of genetic males (XY males) into phenotypic females (XY females). However, recovery from the feminizing effect was observed in part of juveniles once treatment was stopped. In this study, we further analyzed the expression profiles of six important sex-related genes (cyp19a, foxl2, dmrt1, amh, sox9a, and sox9b) in the gonads of XY tiger puffer after E2 treatment. The aim of this study is to gain insights into molecular regulatory mechanisms underlying sex reversal in fish.
2 MATERIAL AND METHOD 2.1 Larvae and juveniles rearingNewly hatched tiger puffer larvae were obtained from a commercial hatchery in Weihai, China, and then reared in a seawater aquaria at the Tianyuan Fisheries Company, Yantai City, China. At 15 dph, about 3 000 larvae were randomly assigned to three 500-L aquaria containing ~1 000 larvae each. The larvae were fed with live rotifers from 16 to 25 dph, and Artemia nauplius from 26 to 40 dph. After 40 dph, metamorphosed juveniles were weaned onto commercial pellets (Marine Yu Bao, Hayashikane Sangyo Co. Ltd., Japan). Water temperature was maintained at 18–21℃ during the rearing period.
2.2 17β-estradiol immersionFrom 15 to 100 dph, larvae were exposed to E2 at different concentrations of 0 (control), 10 and 100 μg/L for 2 h once every two days. Stock solutions were prepared by dissolving 0.5 and 5 mg E2 in 1 000-mL absolute ethanol to give a concentration of 0.5 and 5 mg/mL E2, respectively. Prior to the 2-h immersion, the flowing water was halted, and then 10 mL of the respective E2 stock solutions were added to each aquarium to give final concentrations of 10 and 100 μg/L E2. After the final exposure to E2 at 100 dph, juveniles from each group were respectively transferred to three 5-m3 aquaria and reared in a flowthrough seawater system.
2.3 Sample collectionA total of 40 juveniles were collected from each group at 100 dph. The gonads of 30 juveniles were fixed in Davidson's for at least 24 h, washed in 50% ethanol, and then stored in 70% ethanol at 4℃ until histological processing. The gonads from the remaining 10 juveniles were stored at -80℃ for DNA and RNA extraction. Subsequently, 30 juveniles were sampled from each group at 160, 270 and 400 dph. For each juvenile, one of the two gonads was fixed in Davidson's (as above) and the other gonad was stored at -80℃.
2.4 Genetic sex identificationTotal DNA was extracted from gonads using a Marine Animals DNA kit (TLANamp, Beijing, China) following the manufacturer's instructions. DNA quality and quantity were assessed by 1% agarose gel electrophoresis and by UV spectrophotometry (1.8 < OD260/280 < 2.0), respectively.
A single-nucleotide polymorphism (G/C) in amhr2 is associated with sex determination in tiger puffer (Kamiya et al., 2012), and can be used to identify the genetic sex of juveniles. The sense (F) 5′-TAGACACGATGCACACAAACCAC-3′ and antisense (R) 5′-CGCAAAATGAGGCTCTC TATGGAG-3′ primers for the SNP marker were designed with Primer Premier 5.0 software (Premier Biosoft, Palo Alto, CA, USA). The reaction conditions of PCR were 5 min at 95 C, followed by 35 cycles of 1 min at 94℃, 40 s at 58℃ and 50 s at 72℃, with a final extension at 72℃ for 5 min. The products (625 bp) of PCR were sequenced by Sangon Biotech Co., Ltd. (Shanghai, China). In the resulted sequence traces, the genetic male (XY) juveniles and genetic female (XX) juveniles were heterozygous (G/C) and homozygous (C/C) in the SNP position (334 bp), respectively.
2.5 Gonadal phenotype identificationThe gonadal phenotypes of juveniles were identified using histological analysis. Gonads were dehydrated in a series of alcohol, clarified in dimethylbenzene, and then embedded in paraffin. Cross-sections were cut at 5–7 μm with a microtome (Leica RM2235, Nussloch, Germany), stained with hematoxylin and eosin, and observed and photographed using a light microscope (Olympus DP72, Tokyo, Japan).
2.6 Gene expression analysisTotal RNA from gonads was extracted using MiniBEST Universal RNA Extraction kit (Takara, Dalian, China). RNA quality and quantity were assessed by 1% agarose gel electrophoresis (28S:18S > 1) and UV spectrophotometry (1.8 < OD260/280 < 2.0), respectively. To avoid contamination of genomic DNA, total RNA was treated with DNase I (Qiagen) for 30 min at 37℃. The first-stranded cDNA was synthesized using PrimeScriptTM RT reagent kit (TaKaRa, Dalian, China) with 1 μg total RNA according to the manufacturer's instruction. The cDNA was stored at -20℃.
Relative expression of cyp19a, foxl2, dmrt1, sox9a, sox9b and amh genes were determined by quantitative real-time PCR (qRT-PCR) in the gonads of XY fish and XX fish in the control group and the gonads of XY fish in the treatment groups. The qRT-PCR was performed on an ABI StepOnePluse Sequence Detection System (Applied Biosystems, USA) in accordance with the manufacturer's instructions. SYBR Premix Ex TaqTM kit (TaKaRa, China) was used for amplification, and the reaction mixture contained 10 μL of SYBR® Premix Ex TaqTM, 0.8 μL of each primer (10 μmol/L), 0.4 μL of ROX Dye (50×), 2 μL of cDNA sample (25 ng/μL), and 6 μL of sterile distilled water. Initial denaturation was conducted at 95℃ for 10 s, followed by 40 cycles at 95℃ for 5 s and at 60℃ for 30 s. A dissociation protocol was carried out after thermocycling to determine the target specificity. The stability of six commonly used reference genes [18S ribosomal RNA (18s), beta-actin (actb), elongation factor 1-alpha (ef1a), Cathepsin D (ctsd), glyceraldehyde-3-phosphate-dehydrogenase (gapdh), and ribosomal protein 17 (rpl17)] was evaluated using Normfinder (v 0.953). The evaluation revealed that actb is the most stable reference gene in this study (data not shown). Hence, we selected actb as the internal control, and the relative abundance of the target mRNA was normalized to actb by using the 2-∆∆Ct method. The primers for qRT-PCR are listed in Table 1. All samples were amplified in triplicates.
All data were expressed as a mean±standard error of the mean (SEM). Isolated and interactive effects of sex and development time in normal condition were analyzed using Two-way ANOVA. Isolated and interactive effects of E2 concentration and development time in XY individuals were analyzed using Two-way ANOVA. If significant differences were found in factors, Tukey's multiple range tests were used to determine the differences between means. P was taken as statistically significant. Statistical analysis was conducted using SPSS 16.0 software (SPSS Inc., USA).
3 RESULT 3.1 Gonadal phenotypesAt 100 dph, gonadal phenotypes of juveniles were investigated by histological method. The ovary/testis ratio was 16:14 in the control group, whereas all gonads (n=30) were presented as ovaries in 10 and 100 μg/L E2 groups, no testis or intersexual gonad was observed. Ovaries in the two E2 treatment groups indicated that all gonads of XY juveniles were induced into ovaries (XY ovaries) by 10 and 100 μg/L E2.
At 160, 270, and 400 dph, genetic sex of juveniles was identified by detecting sex-associated SNP. Then, the gonadal phenotype of each fish was examined correspondingly. In the control group, the gonads of XY and XX juveniles developed correspondingly into testes (XY testis) and ovaries (XX ovaries) (Fig. 2a–f). In treatment groups, the gonads of XX juveniles were also ovaries, whereas those of XY juveniles exhibited different phenotypes. All gonads of XY juveniles in the 10 μg/L E2 group were presented as intersexual gonads (XY intersexual gonads) at 160 dph (Fig. 2g). But all those intersexual gonads developed into XY testes at 270 and 400 dph (Fig. 2h, I). In the 100 μg/L E2 group, 38% of gonads of XY juveniles remained XY ovaries at 160 dph (Fig. 2j), whereas the rest were observed as XY intersexual gonads (Fig. 2m). Percentages of XY intersexual gonads and XY ovaries in the 100 μg/L E2 group reached 57% and 43% at 270 dph (Fig. 2k, n) and 44% and 56% at 400 dph (Fig. 2l, o), respectively. The intersexual gonads at 160 and 270 dph were identified by few oocytes in the gonad and high expressed levels of cyp19a and dmrt1, which were primarily detected in the ovaries and testes, respectively (Lee et al., 2009). And the intersexual gonads at 400 dph were identified by the simultaneous presence of oocytes and spermatocytes. Figure 1 showed the number of gonadal phenotypes in the control and E2 treatment groups after E2 immersion.
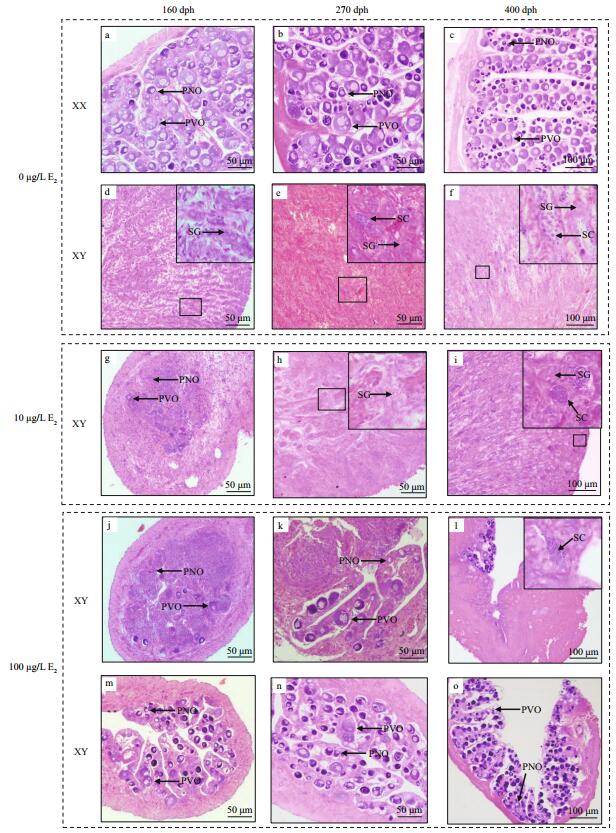
|
| Fig.2 Developmental changes in the gonads of tiger puffer at 160, 270, 400 dph a, b, c. XX ovary in the control (0 μg/L E2) group at 160, 270, 400 dph, respectively; d, e, f. XY testis in the control (0 μg/L E2) group at 160, 270, 400 dph, respectively; g. XY intersexual gonad in the 10 μg/L E2 group at 160 dph; h, i. XY testis in the 10 μg/L E2 group at 270, 400 dph, respectively; j, k, l. XY intersexual goand in the 100 μg/L E2 group at 160, 270, 400 dph, respectively; m, n, o. XY ovary in the 100 μg/L E2 group at 160, 270, 400 dph, respectively. |
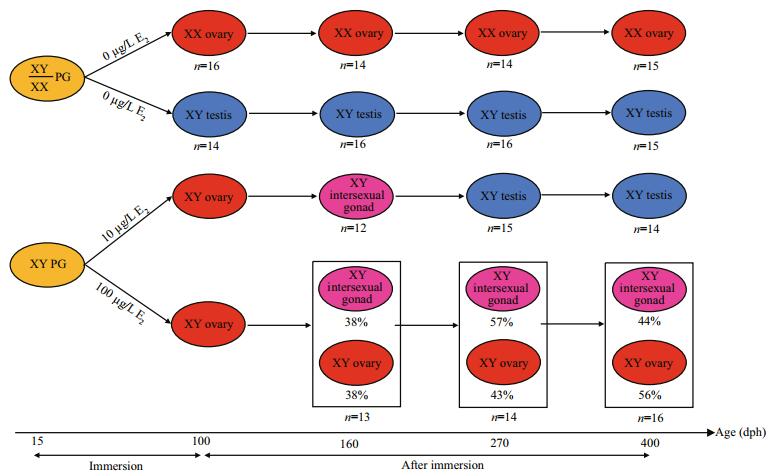
|
| Fig.1 The gonadal phenotypes of tiger puffer in the control and E2 treatment groups after E2 immersion XY ovary, ovary in genetic male; XY intersexual gonad, intersexual gonad in genetic male; XY testis, testis in the genetic male. |
Similar expression profiles were observed between foxl2 and cyp19a in gonads of control and E2treatment groups (Fig. 3). In the control group, foxl2 and cyp19a constantly presented higher mRNA levels in XX ovaries than in XY testes (Fig. 3a, c). After the exposure, the mRNA levels of both the two genes were significantly different in the XY gonads among groups (Fig. 3b, d). In the 10 μg/L E2 group, mRNA levels of the two genes were significantly higher in XY ovaries than in XY testes at 100 dph. Along with XY ovary-to-XY testis transformation in the 10 μg/L E2 group, mRNA levels of foxl2 and cyp19a increased in XY intersexual gonads at 160 dph but subsequently decreased in XY testes at 270 and 400 dph. In the 100 μg/L E2 group, mRNA levels of foxl2 and cyp19a in XY ovaries also increased in comparison with those in control XY testes at 100 dph. Though 38%– 57% of XY ovaries developed into XY intersexual gonads in the 100 μg/L E2 group after 160 dph, mRNA levels of foxl2 and cyp19a gradually increased in the two types of XY gonads and yielded values comparable to those of control XX ovaries.
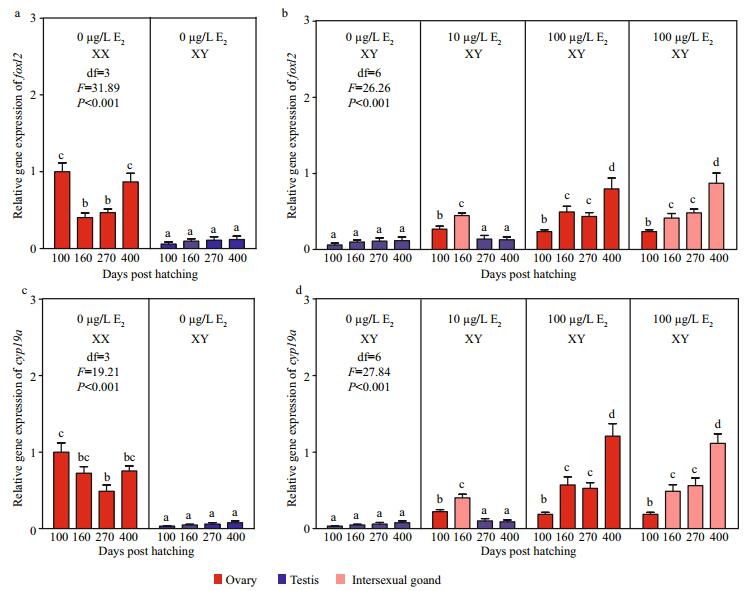
|
| Fig.3 Expression profiles of foxl2 (a, b) and cyp19a (c, d) during the gonadal development of tiger puffer after E2 immersion Values represent mean ±SEM (n=3). The different superscripts represent a significant difference of bars within each plot in a graph (P < 0.05). Red bars represent ovaries; blue bars, testes; and pink bars, intersexual gonads. |
Figure 4 displayed expression profiles of dmrt1 and amh in gonads of control and E2treatment groups. In the control group (0 μg/L E2), dmrt1 and amh were highly expressed in XY testes, and their mRNA levels gradually increased from 100 dph to 400 dph but were low or barely expressed in XX ovaries (Fig. 4a, c). After the exposure, the mRNA levels of both dmrt1 and amh were significantly different in the XY gonads of different groups (Fig. 4b, d). In the 10 and 100 μg/L E2 group, mRNA levels of dmrt1 and amh significantly decreased in XY ovaries compared with those in control XY testes at 100 dph. The mRNA levels of these same genes remained unchanged during development of XY ovaries in the 100 μg/L E2 group until 400 dph. In XY gonads that reverted to testes in the 10 μg/L E2 group or developed into intersexual gonads in the 100 μg/L E2 group, mRNA levels of the two genes gradually increased and yielded comparable values to those in control XY testes after 160 dph.
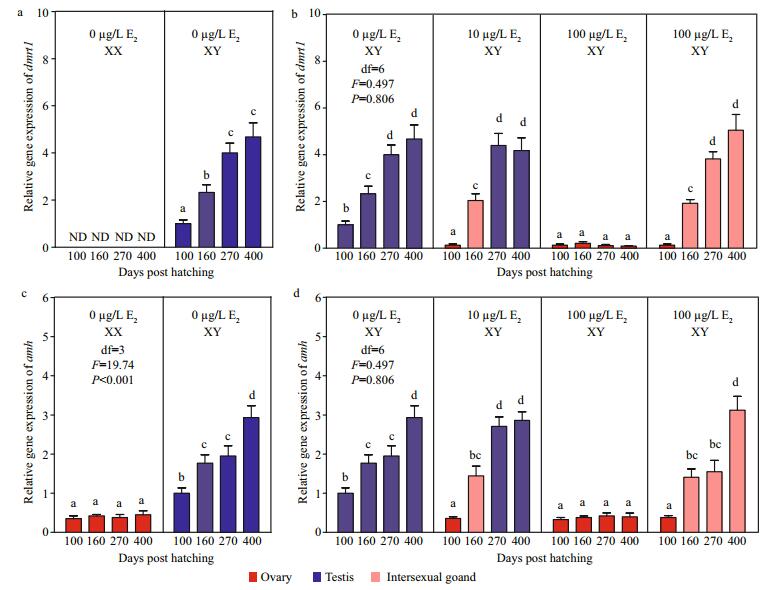
|
| Fig.4 Expression profiles of dmrt1 (a, b) and amh (c, d) during the gonadal development of tiger puffer after E2 immersion Values represent means ± SEM (n=3). The different superscripts represent a significant difference of bars within each plot in a graph (P < 0.05). Red bars represent ovaries; blue bars, testes; and pink bars, intersexual gonads. ND: not detected. |
Sox9a and sox9b showed different expression profiles in gonads of control and E2 treatment groups (Fig. 5). In the control group, sox9a was abundant in XX ovaries and XY testes, with higher mRNA levels in XY testes (Fig. 5a). By contrast, sox9b was highly expressed in XX ovaries but barely expressed in XY testes (Fig. 5c). After the exposure, the mRNA levels of both the two genes were significantly different during the development of XY gonads (Fig. 5b, d).Treatment with 10 and 100 μg/L E2 did not affect mRNA levels of sox9a but increased those of sox9b in XY ovaries compared with control XY testes at 100 dph. In the 10 μg/L E2 group, mRNA levels of sox9a were unchanged in XY intersexual gonads at 160 dph but increased in XY testes at 270 and 400 dph, whereas those of sox9b gradually decreased during XY ovary-to-XY testis transformation. In the 100 μg/L E2 group, mRNA levels of sox9a remained unchanged, but those of sox9b increased during the development of XY ovaries from 100 dph to 400 dph. In the XY intersexual gonads of the 100 μg/L E2 group, mRNA levels of sox9a and sox9b exhibited no changes at 160 dph but significantly increased to 270 and 400 dph.
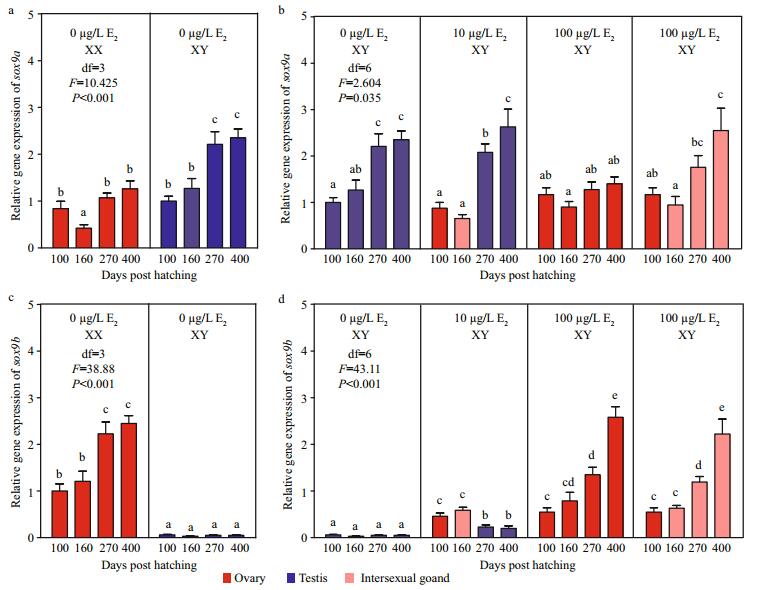
|
| Fig.5 Expression profiles of sox9a (a, b) and sox9b (c, d) during the gonadal development of tiger puffer after E2 immersion Values represent the mean ±SEM (n=3). The different superscripts represent a significant difference of bars within each plot in a graph (P < 0.05). Red bars represent ovaries; blue bars, testes; and pink bars, intersexual gonads. |
For most gonochoristic fish species, gonadal differentiation is usually the results of antagonism between genetic sex determination (GSD) and environmental sex determination (ESD). Gonadal differentiation is normally defined by GSD, but the process is easily affected by environmental factors (Baroiller and D'Cotta, 2001). The best example for this phenomenon is that administration of exogenous estrogens at the time of gonadal differentiation is able to cover male sex determination and induce lifetime ovarian development in male fish (Strüssmann and Nakamura, 2002; Vizziano-Cantonnet et al., 2008). In this study, E2 immersion during gonadal differentiation caused ovarian development in XY tiger puffer as reported in other fish species. However, most ovaries in the XY males recovered into testes or intersexual gonads after the immersion. The possible reason for this phenomenon is that the method of E2 immersion used in our present study is insufficient to induce complete feminization of genetic male tiger puffer. Our results suggested that male sex determination still had the ability to redefine the fate of gonads even through the gonads differentiated into ovaries in XY tiger puffer.
The morphological recovery in the gonads of XY tiger puffer after the E2 immersion is similar to the gonadal changes in some natural sex-reversing fish species. In those fish species, all gonads initially differentiate into ovaries, then approximately half ovaries in the undifferentiated gonochoristic fish species (i.e., zebrafish and Labeo victorianus) and all of them in the protogynous hermaphroditic fish species (i.e., the genera Epinephelus and red porgy Pagrus pagrus) transform into testes (Bhandari et al., 2003; Maack and Segner, 2003; Kokokiris et al., 2006; Rutaisire et al., 2008). Numerous attempts have tried to reveal the sex determination mechanism in zebrafish, but neither sex chromosomes nor a sexdetermining gene has been identified (Tong et al., 2010). So it is generally believed that the gonadal differentiation in zebrafish is mediated by genetic signals from sex-related genes on the autosomes. Correspondingly the sex-related genes in the sex determination pathways of mammals, birds and differentiated gonochoristic fish species also involve in the gonad changes in protogynous hermaphroditic fish species (Xia et al., 2007; Hu et al., 2015; Nozu et al., 2015; Wu et al., 2017; Horiguchi et al., 2018). Until now the sex-determining gene has been identified in 11 vertebrate species, including tiger puffer (Pan et al., 2016). But studies in mammals, rainbow trout, and Nile tilapia have indicated that the sex-determining gene just acts as a switch to initiate gonadal differentiation and does not play roles in the development of differentiated gonads (Yano et al., 2012; Li et al., 2015; She and Yang, 2017). Thereby, the ovary-to-testis recovery in XY tiger puffer after E2 immersion is most possibly controlled by the sexrelated genes in the sex determination pathways, as studied in undifferentiated gonochoristic fish species and protogynous hermaphroditic fish species.
Dmrt1, amh, and sox9 are known as the essential transcription factors in male sex determination pathway in mammals, birds and differentiated gonochoristic fish species. During normal gonadal differentiation, they are usually highly expressed in the male gonads and are lowly or barely in the female gonads (Vizziano et al., 2007; Ijiri et al., 2008). However, Studies in mice, chicken, and Nile tilapia have indicated that overexpression of dmrt1, amh or sox9 in the female gonads can induce varying degrees of ovarian degeneration, and even induce complete sex reversal (Wang et al., 2010; Kim et al., 2011; Lambeth et al., 2014, 2016; Zhao et al., 2015). High expression of dmrt1, amh, and sox9 were also proved to be important for ovary-to-testis sex reversal in zebrafish and protogynous hermaphroditic fish species. In zebrafish, dmrt1 mutant causes abnormal testicular development and eventually lost germ cell in males, suggesting that dmrt1 is necessary for testicular development and male germ cells maintenance in zebrafish (Webster et al., 2017). Similarly high dmrt1 expression correlated with the proliferation of spermatogonia in orange-spotted grouper Epinephelus coioides and the three-spot wrasse Halichoeres trimaculatus, as studied in tiger puffer (Yamaguchi et al., 2006; Xia et al., 2007; Nozu et al., 2015). Different from dmrt1, amh is proved to inhibit spermatogonia proliferation during the ovary-to-testis sex change in zebrafish, orange-spotted grouper and the black porgy Acanthopagrus schlegelii (Skaar et al., 2011; Wu et al., 2015, 2017). The amh-arrested spermatogonia may suppress ovarian development by hindering intercellular communication in the gonad (Wu et al., 2015). As reported in tiger puffer, two subtypes of sox9 (namely sox9a and sox9b) are isolated in zebrafish (Chiang et al., 2001). Sox9a is restricted to the testis of zebrafish, and it controls juvenile ovary-to-testis transformation by enhancing the production of extracellular matrix required for testis cord formation and inducing follicle disassembly (Sun et al., 2013). Sox9b is restricted to the ovary, but its function is still not clear in zebrafish. In the present, the mRNA levels of dmrt1, amh, and sox9a were gradually increased during the ovary-to-testis change as well as in the development of intersexual gonads after the E2 immersion but were unchanged in the XY ovaries. The results indicated the potential roles of dmrt1, amh, and sox9a in the regulation of gonad sex reversal in XY tiger puffer after E2 immersion. Additionally, the mRNA levels of dmrt1 and amh were quickly increased during the morphological changes in the gonads of XY tiger puffer, suggesting that the up-regulation of dmrt1 and amh is required to initiate sex change.
Then a new question emerged: why the XY intersexual gonads did not develop into XY testes from the 100 μg/L E2 group as observed in the XY gonads from the 10 μg/L E2 group? By comparing the gene profiles between the two types of gonads, we further found that the mRNA levels of foxl2, cyp19a, and sox9b were increased during the development of XY intersexual gonads but were decreased along with the morphological change from XY ovary-to-XY testis. The results imply that up-regulation of foxl2, cyp19a, and sox9b are associated with the development of ovarian tissues in the intersexual gonads. Studies in mammals indicated that foxl2 is one of the earliest markers of ovarian determination and its primary function is the lifetime protection of granulosa cell from the repression of male-related genes in vertebrates (Georges et al., 2013). Cyp19a is the most important gene for the synthesis of estrogen, the critical hormone for ovarian differentiation and maintenance in all most vertebrates (Guiguen et al., 2010). Foxl2 and cyp19a are always high expressed in the female gonads of both gonochoristic fish species and protogynous hermaphroditic fish species (Blázquez et al., 2008). However, knockout of foxl2 or cyp19a are able to cause complete sex reversal in Nile tilapia and zebrafish (Lau et al., 2016; Yang et al., 2017; Zhang et al., 2017). Similarly, decreased expression levels of foxl2 and cyp19a were observed during sex reversal in the rice field eel and the three-spot wrasse (Liu et al., 2009; Wu et al., 2010; Hu et al., 2014). All the researches demonstrated that suppression of foxl2 and cyp19a expression is necessary for complete ovary-totestis recovery. Though the function of sox9b is not yet clear in tiger puffer, its predominant expression in the ovaries suggested that sox9b mainly involves in ovarian development as foxl2 and cyp19a. Consequently, suppression of sox9b expression is associated with the ovarian degeneration during ovaryto-testis sex reversal in tiger puffer.
5 CONCLUSIONIn this study, the gonads in XY tiger puffer were induced to differentiate into ovaries by E2 immersion, and ovary-to-testis recovery was initiated when the E2 immersion was halted. Our study provides a potential way to precisely trace the molecular processes underlying gonadal transformation from start to finish, which is hardly carried out in natural sexreversing fish species (Tong et al., 2010). We first detected the expression profiles of six important sexrelated genes during the gonadal transformation in XY tiger puffer in the present study. And the results indicated their potential roles in regulating ovary-totestis sex reversal. But in order to further discover a clue to the puzzle of sex reversal, future researches should focus on the molecular mechanisms underlying the initiation of gonadal transformation using transcriptome sequencing or microarray analysis.
Aparicio S, Chapman J, Stupka E, Putnam N, Chia J M, Dehal P, Christoffels A, Rash S, Hoon S, Smit A, Gelpke M D, Roach J, Oh T, Ho I Y, Wong M, Detter C, Verhoef F, Predki P, Tay A, Lucas S, Richardson P, Smith S F, Clark M S, Edwards Y J, Doggett N, Zharkikh A, Tavtigian S V, Pruss D, Barnstead M, Evans C, Baden H, Powell J, Glusman G, Rowen L, Hood L, Tan Y H, Elgar G, Hawkins T, Venkatesh B, Rokhsar D, Brenner S. 2002. Whole-genome shotgun assembly and analysis of the genome of Fugu rubripes. Science, 297(5585): 1301-1310.
DOI:10.1126/science.1072104 |
Baroiller J F, D'Cotta H. 2001. Environment and sex determination in farmed fish. Comparative Biochemistry and Physiology C: Toxicology & Pharmacology, 130(4): 399-409.
|
Baumann L, Knörr S, Keiter S, Rehberger K, Volz S, Schiller V, Fenske M, Holbech H, Segner H, Braunbeck T. 2014. Reversibility of endocrine disruption in zebrafish (Danio rerio) after discontinued exposure to the estrogen 17α-ethinylestradiol. Toxicology and Applied Pharmacology, 278(3): 230-237.
DOI:10.1016/j.taap.2014.04.025 |
Bhandari R K, Komuro H, Nakamura S, Higa M, Nakamura M. 2003. Gonadal restructuring and correlative steroid hormone profiles during natural sex change in protogynous honeycomb grouper (Epinephelus merra). Zoological Science, 20(11): 1399-1404.
DOI:10.2108/zsj.20.1399 |
Blázquez M, González A, Papadaki M, Mylonas C, Piferrer F. 2008. Sex-related changes in estrogen receptors and aromatase gene expression and enzymatic activity during early development and sex differentiation in the European sea bass (Dicentrarchus labrax). General and Comparative Endocrinology, 158(1): 95-101.
DOI:10.1016/j.ygcen.2008.06.001 |
Chiang E F L, Pai C I, Wyatt M, Yan Y L, Postlethwait J, Chung B C. 2001. Two Sox9 genes on duplicated zebrafish chromosomes: expression of similar transcription activators in distinct sites. Developmental Biology, 231(1): 149-163.
DOI:10.1006/dbio.2000.0129 |
Fleming N I, Knower K C, Lazarus K A, Fuller P J, Simpson E R, Clyne C D. 2010. Aromatase is a direct target of FOXL2: C134W in granulosa cell tumors via a single highly conserved binding site in the ovarian specific promoter. PLoS One, 5(12): e14389.
DOI:10.1371/journal.pone.0014389 |
Georges A, Auguste A, Bessière L, Vanet A, Todeschini A L, Veitia R A. 2013. FOXL2: a central transcription factor of the ovary. Journal of Molecular Endocrinology, 52(1): R17-R33.
DOI:10.1530/JME-13-0159 |
Guiguen Y, Baroiller J F, Ricordell M, Iseki K, Mcmeel O M, Martin S A M, Fostier A. 1999. Involvement of estrogens in the process of sex differentiation in two fish species: the rainbow trout (Oncorhynchus mykiss) and a tilapia (Oreochromis niloticus). Molecular Reproduction and Development, 54(2): 154-162.
DOI:10.1002/(ISSN)1098-2795 |
Guiguen Y, Fostier A, Piferrer F, Chang C F. 2010. Ovarian aromatase and estrogens: a pivotal role for gonadal sex differentiation and sex change in fish. General and Comparative Endocrinology, 165(3): 352-366.
DOI:10.1016/j.ygcen.2009.03.002 |
Horiguchi R, Nozu R, Hirai T, Kobayashi Y, Nakamura M. 2018. Expression patterns of sex differentiation-related genes during gonadal sex change in the protogynous wrasse, Halichoeres trimaculatus. General and Comparative Endocrinology, 257: 67-73.
DOI:10.1016/j.ygcen.2017.06.017 |
Hu P, Liu B, Meng Z, Liu X F, Jia Y D, Yang Z, Lei J L. 2017. Recovery of gonadal development in tiger puffer Takifugu rubripes after exposure to 17β-estradiol during early life stages. Chinese Journal of Oceanology and Limnology, 35(5): 613-623.
|
Hu Q, Guo W, Gao Y, Tang R, Li D P. 2015. Molecular cloning and characterization of amh and dax1 genes and their expression during sex inversion in rice-field eel Monopterus albus. Scientific Reports, 5: 16667.
DOI:10.1038/srep16667 |
Hu Q, Guo W, Gao Y, Tang R, Li D P. 2014. Molecular cloning and analysis of gonadal expression of Foxl2 in the ricefield eel Monopterus albus. Scientific Reports, 4: 6884.
|
Ijiri S, Kaneko H, Kobayashi T, Wang D S, Sakai F, PaulPrasanth B, Nakamura M, Nagahama Y. 2008. Sexual dimorphic expression of genes in gonads during early differentiation of a teleost fish, the Nile tilapia Oreochromis niloticus. Biology of Reproduction, 78(2): 333-341.
DOI:10.1095/biolreprod.107.064246 |
Kamiya T, Kai W, Tasumi S, Oka A, Matsunaga T, Mizuno N, Fujita M, Suetake H, Suzuki S, Hosoya S, Tohari S, Brenner S, Miyadai T, Venkatesh B, Suzuki Y, Kikuchi K. 2012. A trans-species missense SNP in Amhr2 is associated with sex determination in the tiger pufferfish, Takifugu rubripes (Fugu). PLoS Genetics, 8(7): e1002798.
DOI:10.1371/journal.pgen.1002798 |
Kim Y, Muroki H, Yamamoto K, Deng J M, Behringer R R, Nakamura T, Akiyama H. 2011. Generation of transgenic mice for conditional overexpression of Sox9. Journal of Bone and Mineral Metabolism, 29(1): 123-129.
DOI:10.1007/s00774-010-0206-z |
Kobayashi T, Matsuda M, Kajiura-Kobayshi H, Suzuki A, Saito N, Nakamoto M, Shibata N, Nagahama Y. 2004. Two DM domain genes, DMY and DMRT1, involved in testicular differentiation and development in the medaka, Oryzias latipes. Developmental Dynamics, 231(3): 518-526.
DOI:10.1002/(ISSN)1097-0177 |
Kokokiris L, Fostire A, Athanassopoulou F, Petridis D, Kentouri M. 2006. Gonadal changes and blood sex steroids levels during natural sex inversion in the protogynous Mediterranean red porgy, Pagrus pagrus (Teleostei: Sparidae). General and Comparative Endocrinology, 149(1): 42-48.
DOI:10.1016/j.ygcen.2006.05.002 |
Lambeth L S, Morris K, Ayers K L, Wise T G, O'Neil T, Wilson S, Cao Y, Sinclair A H, Cutting A D, Doran T J, Smith C A. 2016. Overexpression of anti-Müllerian hormone disrupts gonadal sex differentiation, blocks sex hormone synthesis, and supports cell autonomous sex development in the chicken. Endocrinology, 157(3): 1258-1275.
DOI:10.1210/en.2015-1571 |
Lambeth L S, Raymond C S, Roeszler K N, Kuroiwa A, Nakata T, Zarkower D, Smith C A. 2014. Over-expression of DMRT1 induces the male pathway in embryonic chicken gonads. Developmental Biology, 389(2): 160-172.
DOI:10.1016/j.ydbio.2014.02.012 |
Lau E S, Zhang Z W, Qin M M, Ge W. 2016. Knockout of zebrafish ovarian aromatase gene (cyp19a1a) by TALEN and CRISPR/Cas9 leads to all-male offspring due to failed ovarian differentiation. Scientific Reports, 6: 37357.
DOI:10.1038/srep37357 |
Lee K H, Yamaguchi A, Rashid H, Kadomura K, Yasumoto S, Matsuyama M. 2009. Estradiol-17β treatment induces intersexual gonadal development in the pufferfish, Takifugu rubripes. Zoological Science, 26(9): 639-645.
DOI:10.2108/zsj.26.639 |
Li M H, Sun Y L, Zhao J, Shi H J, Zeng S, Ye K, Jiang D N, Zhou L Y, Sun L N, Tao W J, Nagahama Y, Kocher T D, Wang D S. 2015. A tandem duplicate of anti-Müllerian hormone with a missense SNP on the Y chromosome is essential for male sex determination in Nile tilapia, Oreochromis niloticus. PLoS Genetics, 11(11): e1005678.
DOI:10.1371/journal.pgen.1005678 |
Li M H, Yang H H, Li M R, Sun Y L, Jiang X L, Xie Q P, Wang T R, Shi H J, Sun L N, Zhou L Y, Wang D S. 2013. Antagonistic roles of Dmrt1 and Foxl2 in sex differentiation via estrogen production in tilapia as demonstrated by TALENs. Endocrinology, 154(12): 4814-4825.
DOI:10.1210/en.2013-1451 |
Liu H Q, Guan B, Xu J, Hou C C, Tian H, Chen H X. 2013. Genetic manipulation of sex ratio for the large-scale breeding of YY super-male and XY all-male yellow catfish (Pelteobagrus fulvidraco (Richardson)). Marine Biotechnology, 15(3): 321-328.
DOI:10.1007/s10126-012-9487-7 |
Liu J F, Guiguen Y, Liu S J. 2009. Aromatase (P450arom) and 11β-hydroxylase (P45011β) genes are differentially expressed during the sex change process of the protogynous rice field eel, monopterus albus. Fish Physiology and Biochemistry, 35(3): 511-518.
DOI:10.1007/s10695-008-9255-9 |
Maack G, Segner H. 2003. Morphological development of the gonads in zebrafish. Journal of Fish Biology, 62(4): 895-906.
DOI:10.1046/j.1095-8649.2003.00074.x |
Mair G C, Abucay J S, Abella T A, Beardmore J A, Skibinski D O F. 1997. Genetic manipulation of sex ratio for the large scale production of all-male tilapia Oreochromis niloticus. Canadian Journal of Fisheries and Aquatic Sciences, 54(2): 396-404.
DOI:10.1139/f96-282 |
Masuyama H, Yamada M, Kamei Y, Fujiwara-Ishikawa T, Todo T, Nagahama Y, Matsuda M. 2012. Dmrt1 mutation causes a male-to-female sex reversal after the sex determination by Dmy in the medaka. Chromosome Research, 20(1): 163-176.
DOI:10.1007/s10577-011-9264-x |
Nakamura S, Watakabe I, Nishimura T, Picard J Y, Toyoda A, Taniguchi Y, di Clemente N, Tanaka M. 2012. Hyperproliferation of mitotically active germ cells due to defective anti-Müllerian hormone signaling mediates sex reversal in medaka. Development, 139(13): 2283-2287.
DOI:10.1242/dev.076307 |
Nozu R, Horiguchi R, Kobayashi Y, Nakamura M. 2015. Expression profile of doublesex/male abnormal-3-related transcription factor-1 during gonadal sex change in the protogynous wrasse, Halichoeres trimaculatus. Molecular Reproduction and Development, 82(11): 859-866.
DOI:10.1002/mrd.22527 |
Pan Q W, Anderson J, Bertho S, Herpin A, Wilson C, Postlethwait J H, Schartl M, Guiguen Y. 2016. Vertebrate sex-determining genes play musical chairs. Comptes Rendus Biologies, 339: 258-262.
DOI:10.1016/j.crvi.2016.05.010 |
Pfennig F, Standke A, Gutzeit H O. 2015. The role of Amh signaling in teleost fish-Multiple functions not restricted to the gonads. General and Comparative Endocrinology, 223: 87-107.
DOI:10.1016/j.ygcen.2015.09.025 |
Piferrer F, Blázquez M. 2005. Aromatase distribution and regulation in fish. Fish Physiology and Biochemistry, 31(2-3): 215-226.
DOI:10.1007/s10695-006-0027-0 |
Rutaisire J, Levavi-Sivan B, Nyatia N, Booth A. 2008. Juvenile intersexuality in the cyprinid fish Labeo victorianus. Cybium: International Journal of Ichthyology, 32(2): 232.
|
Sekido R, Lovell-Badge R. 2009. Sex determination and SRY: down to a wink and a nudge?. Trends in Genetics, 25(1): 19-29.
DOI:10.1016/j.tig.2008.10.008 |
She Z Y, Yang W X. 2017. Sry and SoxE genes: How they participate in mammalian sex determination and gonadal development?. Seminars in Cell and Developmental Biology, 63: 13-22.
DOI:10.1016/j.semcdb.2016.07.032 |
Shen X Y, Cui J Z, Yang G P, Gong Q L, Gu Q Q. 2007. Expression detection of DMRTs and two sox9 genes in Takifugu rubripes (Tetraodontidae, Vertebrata). Journal of Ocean University of China, 6(2): 182-186.
DOI:10.1007/s11802-007-0182-7 |
Shiraishi E, Yoshinaga N, Miura T, Yokoi H, Wakamatsu Y, Abe S, Kitano T. 2008. Müllerian inhibiting substance is required for germ cell proliferation during early gonadal differentiation in medaka (Oryziaslatipes). Endocrinology, 149(4): 1813-1819.
DOI:10.1210/en.2007-1535 |
Skaar K S, Nóbrega R H, Magaraki A, Olsen L C, Schulz R W, Male R. 2011. Proteolytically activated, recombinant antiMüllerian hormone inhibits androgen secretion, proliferation, and differentiation of Spermatogonia in adult zebrafish testis organ cultures. Endocrinology, 152(9): 3527-3540.
DOI:10.1210/en.2010-1469 |
Strüssmann C A, Nakamura M. 2002. Morphology, endocrinology, and environmental modulation of gonadal sex differentiation in teleost fish. Fish Physiology and Biochemistry, 26(1): 13-29.
|
Sun D, Zhang Y, Wang C, Hua X, Zhang X A, Yan J. 2013. Sox9-related signaling controls zebrafish juvenile ovarytestis transformation. Cell Death and Disease, 4(11): e930.
DOI:10.1038/cddis.2013.456 |
Suzuki A, Nakamoto M, Kato Y, Shibata N. 2005. Effects of estradiol-17β on germ cell proliferation and DMY expression during early sexual differentiation of the medaka Oryzias latipes. Zoological Science, 22(7): 791-796.
DOI:10.2108/zsj.22.791 |
Tong S K, Hsu H J, Chung B C. 2010. Zebrafish monosex population reveals female dominance in sex determination and earliest events of gonad differentiation. Developmental Biology, 344(2): 849-856.
DOI:10.1016/j.ydbio.2010.05.515 |
Uno T, Ishizuka M, Itakura T. 2012. Cytochrome P450 (CYP) in fish. Environmental Toxicology and Pharmacology, 34(1): 1-13.
DOI:10.1016/j.etap.2012.02.004 |
Vizziano-Cantonnet D, Baron D, Mahè S, Cauty C, Fostier A, Guiguen Y. 2008. Estrogen treatment up-regulates female genes but does not suppress all early testicular markers during rainbow trout male-to-female gonadal transdifferentiation. Journal of Molecular Endocrinology, 41(5): 277-288.
DOI:10.1677/JME-08-0039 |
Vizziano D, Randuineau G, Baron D, Gauty C, Guiguen Y. 2007. Characterization of early molecular sex differentiation in rainbow trout, Oncorhynchus mykiss. Developmental Dynamics, 236(8): 2198-2206.
DOI:10.1002/dvdy.v236:8 |
Wang D S, Kobayashi T, Zhou L Y, Paul-Prasanth B, Ijiri S, Sakai F, Okubo K, Morohashi K, Nagahama Y. 2007. Foxl2 up-regulates aromatase gene transcription in a female-specific manner by binding to the promoter as well as interacting with Ad4 binding protein/steroidogenic factor. Molecular Endocrinology, 21(3): 712-725.
DOI:10.1210/me.2006-0248 |
Wang D S, Zhao L Y, Kobayashi T, Matsuda M, Shibata Y, Sakai F, Nagahama Y. 2010. Doublesex- and Mab-3-related transcription factor-1 repression of aromatase transcription, a possible mechanism favoring the male pathway in tilapia. Endocrinology, 151(3): 1331-1340.
DOI:10.1210/en.2009-0999 |
Webster K A, Schach U, Ordaz A, Steinfeld J S, Draper B W, Siegfried K R. 2017. Dmrt1 is necessary for male sexual development in zebrafish. Developmental Biology, 422(1): 33-46.
DOI:10.1016/j.ydbio.2016.12.008 |
Wu G C, Li H W, Luo J W, Chen C, Chang C F. 2015. The potential role of Amh to prevent ectopic female development in testicular tissue of the protandrous black porgy, Acanthopagrus schlegelii. Biology of Reproduction, 92(6): 158.
|
Wu G C, Tomy S, Lee M F, Lee Y H, Yueh W S, Lin C J, Lau E L, Chang C F. 2010. Sex differentiation and sex change in the protandrous black porgy, Acanthopagrus schlegeli. General and Comparative Endocrinology, 167(3): 417-421.
DOI:10.1016/j.ygcen.2009.11.003 |
Wu G C, Li H W, Tey W G, Lin C J, Chang C F. 2017. Expression profile of amh/Amh during bi-directional sex change in the protogynous orange-spotted grouper Epinephelus coioides. PLoS One, 12(10): e0185864.
DOI:10.1371/journal.pone.0185864 |
Xia W, Zhou L, Yao B, Li C J, Gui J F. 2007. Differential and spermatogenic cell-specific expression of DMRT1 during sex reversal in protogynous hermaphroditic groupers. Molecular and Cellular Endocrinology, 263(1-2): 156-172.
DOI:10.1016/j.mce.2006.09.014 |
Yang Y J, Wang Y, Li Z, Zhou L, Gui J F. 2017. Sequential, divergent and cooperative requirements of foxl2a and foxl2b in ovary development and maintenance of zebrafish. Genetics, 205(4): 1551-1572.
DOI:10.1534/genetics.116.199133 |
Yano A, Guyomard R, Nicol B, Jouanno E, Quillet E, Klopp C, Cabau C, Bouchez O, Fostier A, Guiguen Y. 2012. An immune-related gene evolved into the master sexdetermining gene in rainbow trout, Oncorhynchus mykiss. Current Biology, 22(15): 1423-1428.
DOI:10.1016/j.cub.2012.05.045 |
Yamaguchi A, Lee K H, Fujimoto H, Kadomura K, Yasumoto S, Matsuyama M. 2006. Expression of the DMRT gene and its roles in early gonadal development of the Japanese pufferfish Takifugu rubripes. Comparative Biochemistry and Physiology Part D: Genomics and Proteomics, 1(1): 59-68.
DOI:10.1016/j.cbd.2005.08.003 |
Zhang X B, Li M R, Ma H, Liu X Y, Shi H J, Li M H, Wang D S. 2017. Mutation of foxl2 or cyp19a1a results in female to male sex reversal in XX Nile tilapia. Endocrinology, 158(8): 2634-2647.
|
Zhao L, Svingen T, Ng E T, Koopman P. 2015. Female-to-male sex reversal in mice caused by transgenic overexpression of Dmrt1. Development, 142(6): 1083-1088.
DOI:10.1242/dev.122184 |
 2019, Vol. 37
2019, Vol. 37