Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHOU He, ZHUANG Zixin, ZHANG Rui, XU Qizheng, LIANG Yuting, JIANG Zhiqiang, LI Xia, MA Tianyu, LI Yajuan
- Temperature-control-induced masculinization in tiger puffer Takifugu rubripes
- Journal of Oceanology and Limnology, 37(3): 1125-1135
- http://dx.doi.org/10.1007/s00343-019-7382-z
Article History
- Received Jan. 2, 2018
- accepted in principle Apr. 10, 2018
- accepted for publication Jul. 9, 2018
The tiger puffer Takifugu rubripes is a valued species (Gui, 2007), abundantly distributed in the Yellow Sea, the Bohai Sea and East Sea of China, the Korean Peninsula and Japan (Jiang et al., 2004; Lei, 2005; Ma et al., 2011). The flesh of this marine fish is white, tender and tasty, earning it a high price category. Although the difference in the growth rate of male and female T. rubripes is insignificant, the market price differs greatly for the two sexes. The meat of mature males is considered superior to that of females, and in Japan, the testes are considered a high-quality food with an average price comparable to Matsusaka beef and tuna, which can reach US$ 220 per kilogram. Moreover, the ovaries of this species are highly toxic, whereas the testes are not poisonous. Thus, the mature male fish command more than doubles of the market price of the same-sized females (Suzuki, 2010). Therefore, devising an all-male production technology for T. rubripes has implications for improving the profits and international competitiveness of farming this species in China and elsewhere.
Sex-determination mechanisms in numerous species of teleost fish have been proved temperature dependent or genotypic. Conover and Kynard (1981) reported that sex determination in Atlantic silverside Menidia menidia is controlled by heredity and environmental factors during larval stages, and different sex ratios could be achieved with different rearing temperatures. T. rubripes has a special sexdetermination mechanism controlled by genotype but regulated by ambient water temperature, thus the species is an ideal model for studying sex determination and differentiation in a fish. Genomewide linkage analyses by Kikuchi et al. (2007) confirmed that the sex-determination mechanism in T. rubripes is the XX-XY type, and that the sexdetermining gene is located in a single area of linkage group 19. Kamiya et al. (2012) reported that a sexdetermining gene anti-Müllerian hormone receptor type 2 (Amhr2) could be achieved by a single nucleotide polymorphism (SNP) marker, namely a cytosine (C) on chromosome X and a guanine (G) on chromosome Y, and the use of the Amhr2 genespecific SNP loci for genetic sex identification could achieve a nearly 100% accuracy rate. In addition, Suzuki et al. (1996) comfirmed that sex differentiation of gonads occurred range 22.02-29.05 mm TL (50 dph) by histology method, and both the ovary and testis are formed directly by the undifferentiated gonad, which showed that the gonad of T. rubripes had gender plasticity within 2-3 months after hatching. In this period, various hormones and environmental induction could cause phenotypic sex reversal. Based on the literature recordation, the nomal growth temperature range of T. rubripes in nature during larval development was 20-24℃ (Li and Fu, 1993). Hattori et al. (2012) reported that 2-3 weeks after hatching, cultivating T. rubripes at low temperatures significantly increased the proportion of males. Studies by Liu et al. (2014) and Zhou et al. (2015) likewise showed that low-temperature conditions (13-15℃) effectively resulted in malebiased sex ratios. Matsuura et al. (1994) and Suzuki et al. (1996) have investigated early sexual differentiation and gonadal development in T. rubripes, but a technology for temperature-induced masculinization has not yet been reported for T. rubripes.
We used orthogonal array testing to explore the best conditions for temperature-induced masculinization of T. rubripes, and we examined the effects of different temperature treatments on growth traits (length and weight) and sex ratios in cultured fish. We aimed to provide basic data for a simple and reliable way to induce all-male T. rubripes production through low-temperature conditions.
2 MATERIAL AND METHOD 2.1 Ethics statementThis study was performed according to the Guide for the Care and Use of Laboratory Animals in Dalian Ocean University, Dalian, China. All animal experiments were approved by the Animal Study Ethical Committee of Dalian Ocean University, and comply with Chinese laws, regulations, and ethics.
2.2 MaterialTwo adult Takifugu rubripes were acquired from Dalian Days Industrial Co. Ltd., Tanghai County Zuidong Farms, China. The male was 44.2 cm TL and 2.4 kg, and the female was 41.2 cm TL and 2.6 kg, and the age of the broodstock was 5 years old.
2.3 Artificial spawning and fertilizationThe mature parental T. rubripes were injected with human chorionic gonadotropin (HCG) (dose: ♀ 20-25 IU/g, ♂ 10-12.5 IU/g). After 24 h, gentle pressure was applied to the abdomen of the female to observe whether eggs could be extruded; once eggs could be discharged, the fish was lifted and squeezed on both sides of the abdomen as the eggs were collected in a clean jar. Next, sperm was similarly collected in the same jar and gently mixed. After 5 min of dry fertilization, seawater was added to the jar for 3-5 min to activate the sperm. Excess sperm was rinsed off the fertilized eggs with seawater, and the eggs were washed 4 or 5 times, for 5 min at a time, to remove stickiness. The fertilized eggs were then placed into an incubator for fish eggs until the fry hatched out after 7 days; the incubation temperature was 21±1℃.
2.4 Orthogonal test designThe effects of orthogonal combinations of the three factors (i.e. treatment starting time, treatment temperature, and treatment duration) on the production rate of male T. rubripes was examined. The controls were reared at the standard cultivation temperature of 21±1℃. The experiments were repeated once. The orthogonal test L9(34) design chosen for the experiments (Pan and He, 1993) is shown in Table 1. The sequence and optimal combination of the three factors and levels in regard to achieving a male-biased sex ratio and the best growth rate (length and weight) were obtained by intuitive analysis.
The offspring in the treatment and control groups were each cultivated in separate tanks, of 1 m diameter and 1 m height, with 250 fish per tank. The fish were fed, in turn, a diet of rotifers (protein content 28%-36%, and lipid content 9%-28% of dry weight), Artemia (protein content 57%-60% of dry weight), fish mud, fish blocks, normal mixed fish, and pelleted feed (Tian et al., 2007). The water was kept running. The normal cultivation temperature was 21±1℃. The culture methods were identical for each experimental group.
2.6 Measuring growth rateEvery 15 days, thirty fish in each treatment group were randomly selected and measured for length (accurate to 0.01 cm) and weight (accurate to 0.01 g).
2.7 Identification of biological sexAfter 230 days, the gonads of thirty randomly sampled fish in each group were dissected, fixed with Bouin solution, and then transferred to 80% alcohol after 24 h. For identification of biological sex, paraffin sections of the gonad samples were prepared and dyed with hematoxylin-eosin (HE) stain for observation under a microscope (Leica).
2.8 Identification of genetic sexSpecific SNP loci in the Amhr2 gene, as established by Kamiya et al. (2012), were used to identify the genetic sex of fish in each treatment group, including the control. Differences in DNA base pairs, namely cytosine (C) on an X chromosome and guanine (G) on a Y chromosome, were used to identify sex. CC indicated XX chromosomes, representing females, and CG indicated XY chromosomes, representing males. The following primers were used for the PCR: SD3exon8F: ACGATGCACACAAACCACCT; SD3exon10R: TCCCAGTGTTGCGGTATGTA.
2.9 Data analysisOne-way analysis of variance (one-way ANOVA), Duncan multiple range alignment and significance tests of the growth rates (with differences considered significant if P≤0.05, and extremely significant if P≤0.01) were conducted using SPSS version 19.0 (Statistical Product and Service Solutions Inc., Chicago, IL, USA). Tukey's method was used to perform pairwise comparisons. A three-level standard deviation of the mean was equal for factors A and B: S=(mean square error/number of horizontal horizontal number of repetitions)1/2=(17.57/3·2)1/2=3.42. The least significant difference (D) value was calculated as: D0.05 (2, 6)=S×F0.05 (2, 6)=17.58, D0.01(2, 6)=S×F0.01 (2, 6)=37.28.
Absolute daily increase in length (ADIL)=(L2-L1)/(T2-T1), where T1 and T2 represent different time sites, and L1 and L2 represent lengths of different fish at respective times.
Absolute daily increase in weight (ADIW)=(W2-W1)/(T2-T1), where T1 and T2 represent different time sites, and W1 and W2 represent weights of different fish at respective times.
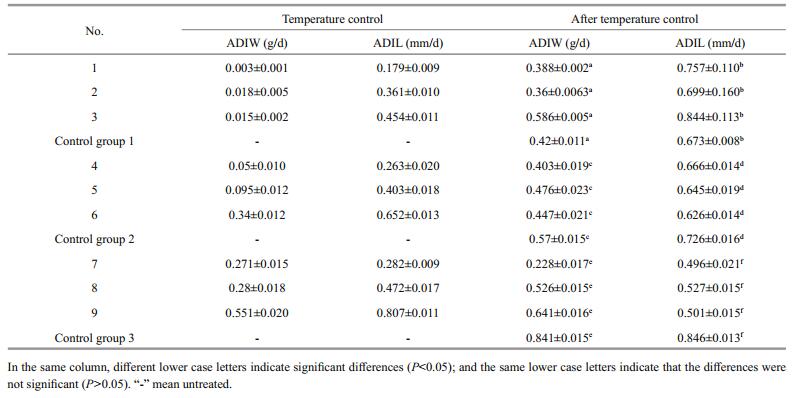
3 RESULT 3.1 Identification of T. rubripes biological sexAt 230 days post-hatch (dph), primary spermatocytes and seminal spermatocytes could be clearly seen in the testis of an average male (Fig. 1a); ovary lobules with oocytes could be seen in an average female (Fig. 1b); and primary spermatocytes and secondary spermatocytes were seen in pseudo males (Fig. 1c).
|
| Fig.1 Histological sections of gonads in T. rubriges (230 dph) Testis (a), ovary (b), testis (c), bars=100 μm. PSC: primary spermatocytes; SSC: secondary spermatocytes; OC: oocytes cell; OL: ovary lobule. |
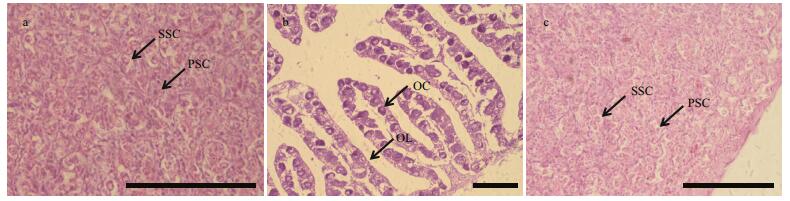
The specific SNP in the sex-determination Amhr2 genes was reported by Kamiya et al. (2012), hence we used it to identify the genetic sex of thirty progeny from each treatment group. As shown in Fig. 2, the genotype of females was C/C (XX) and that of males was C/G (XY). We also found pseudo males whose biological sex was male, but with a C/C genotype revealed by the SNP analysis.
|
| Fig.2 SNP sharp peak of T. rubripes' embryo a. SNP genotype of diploid (female, pseudo male) (C/C); b. SNP genotype of diploid (male) (C/G). |
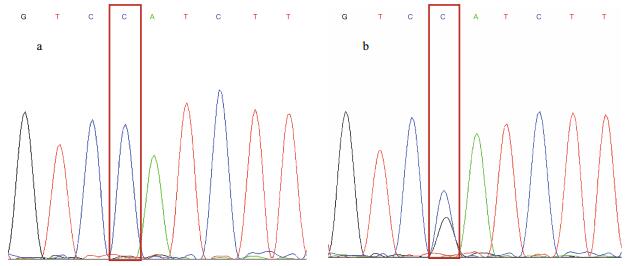
As shown in Table 2, in the controls, the female-tomale sex ratio was 16:14 and the male rate was 46.67% (±4.72), which is consistent with the theoretical male-to-female ratio (1:1). The male rate in all treatment groups was higher than in the controls, and treatment group 4 had the highest proportion of males, at 75%. Genetic sex identification was carried out for the group 7, 14 females (C/C) and 16 males (C/G) had been found. Combing with histological observations, 3 pseudo males (C/C) were screened out from the females (Table 2). According to the range R of the intuitive analysis (Table 2), the order of importance of the three factors in regard to achieving a male-biased sex ratio was: B→A→C. Figure 3 depicts the rate that the sex ratio changed in favor of males as an effect of the three factors at three levels. Factors A and B show greater steepness in the line chart, indicating a larger impact (as the main factors) on the male-production rate, and factor C was the secondary. The optimal combination was A2B1C2 (Fig. 3).
|
| Fig.3 Effects of three factors and their levels on male rate |
Upon comparsions, the difference of each level for factor A and factor B was not significant (P > 0.05) (Table 3), which was consistent with the results of the intuitive analysis.
The results show that the growth rate (as a factor of length and weight) increased commensurate with an increasing rearing temperature (13, 15 and 17℃), but the differences between treatment groups were not significant (P > 0.05). However, the average length of fish in the treatment groups significantly differed to that of the controls (P≤0.05), and among the treatment groups the difference was significant only at 13℃ (P≤0.05) (Table 4); this result indicates that rearing temperature had a large effect on fish length. Second, although the starting time of the temperature treatment (20, 50 and 80 dph) had no significant effect on differences in length and weight (P > 0.05), the difference in growth was a significant when compared with the controls (155.37 mm, 116.98 g) (P≤0.05).
A comparison of the growth traits at 11 dph showed that the average length and weight of each treatment group and the controls increased with age, but the overall growth rates of the treatment groups were significantly lower than that of the controls.
Figure 4a-b shows the changes in length and weight for treatment groups 1 to 3 from 20 dph. Fish in group 1 were maintained at 13℃ for 30 days, group 2 at 15℃ for 45 days, and group 3 at 17℃ for 60 days. The average body lengths in these three treatment groups were less than the average lengths of the controls. One month after the temperature treatment, fish significantly increased in length, especially those of group 1. Length increased significantly from 80 to 200 dph, except for group 3 (Fig. 4a). The differences in average weight among the three temperature-treatment groups and the controls were not significant up to 50 dph. The difference at 65 to 80 dph was also not obvious, but at 95 to 200 dph the average weight of fish in the treatment groups was significantly less than that of the controls. From 110 dph, some difference occurred among the average weights of fish in the three treatment groups: the difference between group 1 and group 3 was not obvious, yet significantly greater than the average weight of group 2 (Fig. 4b).
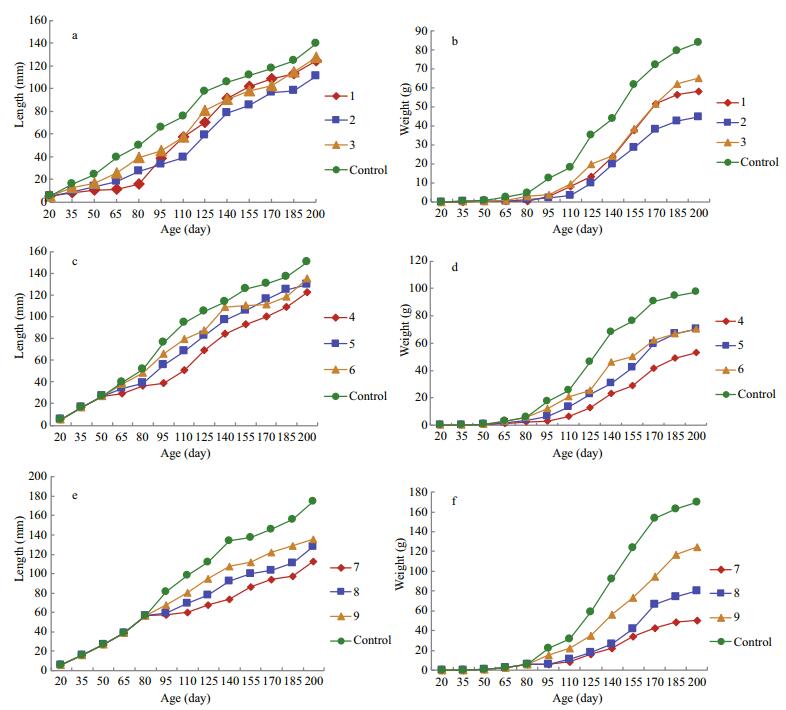
|
| Fig.4 Changes in length for treatment groups 1 to 3 from 20 dph (a), changes in weight for treatment groups 1 to 3 from 20 dph (b), length changes in treatment groups 4 to 6 from 50 dph (c), weight changes in treatment groups 4 to 6 from 50 dph (d), length changes in treatment groups 7 to 9 from 80 dph (e), weight changes in treatment groups 7 to 9 from 80 dph (f) |
Figure 4c-d shows the length and weight changes in treatment groups 4 to 6 from 50 dph. Group 4 was reared at 13℃ for 45 days, group 5 at 15℃ for 60 days, and group 6 at 17℃ for 30 days. The average lengths in these three treatment groups were all less than that of the controls; there was no significant difference between the lengths of group 5 and group 6, but their lengths were greater than group 4 (Fig. 4c). The average weight of fish in each of these three treatment groups was significantly less than that of the controls. After 80 dph, the average weights among the three treatment groups showed some differences: group 5 and group 6 were not obviously different in weight, yet their weights were greater than that of group 4 (Fig. 4d).
Figure 4e-f shows the length and weight changes in treatment groups 7 to 9 from 80 dph. Group 7 was reared at 13℃ for 60 days, group 8 at 15℃ for 30 days, and group 9 at 17℃ for 45 days. The average lengths in these three treatment groups were less than that of the controls, and there were significant differences among the treatment groups. At 200 dph, there was no significant difference in length between groups 8 and 9, but both groups had a longer average body length than that recorded for group 7 (Fig. 4e). The average weight of fish in each of these three treatment groups was significantly lower than that of the controls. The difference in average weight between groups 7 and 8 was not significant before 125 dph, but became significant at 140 to 200 dph, although their average weights were still less than that of group 9 (Fig. 4f).
3.6 Comparisons of absolute increases in body length and weight among the treatment groups and controlsThe absolute daily increase in body length and weight was significantly less during a given temperature treatment than after the treatment (Table 5). In addition, the ADIW and ADIL differed among the treatment groups. The highest growth rates occurred in group 9 (weight: 0.551±0.020 g/day; length: 0.807±0.011 mm/day). The lowest growth rates occurred in group 1 (0.003±0.001 g/day; 0.179±0.009 mm/day). After rearing in the temperature-controlled conditions, the highest ADIW occurred in group 9 (0.641±0.016 g/day) and the highest ADIL occurred in group 3 (0.844± 0.113 mm/day). The lowest ADIW and ADIL both occurred in group 7 (0.228±0.017 g/day; 0.496± 0.021 mm/day). Among all nine groups, only group 3 displayed absolute daily increases in both weight and length that were greater than those of the controls. These rates in the other treatment groups were all significantly lower than those of the controls. Thus, as regards ADIW and ADIL whether during or after lowtemperature treatment, the highest rates occurred in fish treated at 17℃ and the lowest in fish treated at 13℃.
Sex determination and differentiation in fish can be divided into two general types: genetic sex determination (GSD) and environmental sex determination (ESD). GSD refers to when the sex of offspring is primarily determined genetically, as the embryo will develop into a male or female depending on a sex-determining gene on a chromosome or the sex chromosome composition. ESD refers to the gender of offspring being mainly determined by environmental factors—often the environment in which an egg is fertilized (temperature, humidity, pH) or some other uncertain factors that are likely to affect the sex of the offspring. Since fish are ectothermic, temperature plays a vital role in sex determination (Yang et al., 2007). Temperature-dependent sex determination (TSD) is similar in fish and reptiles. Ewert and Nelson (1991) divided TSD into three types: 1) The MF mode, where higher temperatures generate more males and lower temperatures generate more females. Several fishes belong to this type, such as loach Misgurnus anguillicaudatus and Paramisgurnus dabryanus. Embryos of those species developed at 20-30℃; when the water temperature was increased, the proportion of males increased significantly, reaching 82% and 90%, respectively (Nan et al., 2005); 2) the FM mode, which is opposite to the first type of TSD, where higher temperatures produce females and lower temperatures produce males, as in darkbarbel catfish Pseudobagrus vachelli. At approximately 34℃, the gonads of juveniles of that species tended to develop as female, with the production of males only 26.4% (Cheng et al., 2007); 3) the MFM mode, where both relatively high or low temperatures can induce all males, and intermediate temperatures tend to balance the sex rate. Species of Pleuronectiformes, including the southern flounder Paralichthys lethostigma, belong to this type (Luckenbach et al., 2009). However, some researchers assert that TSD in fish is not as prevalent as currently thought (Ospina-Álvarez and Piferrer, 2008). Conover (2004) pointed out that evidence supporting the presence of TSD in fish has often been obtained under laboratory temperatures, and the experimental species have rarely a contact with nature. He also formulated a standard for a species to demonstrate TSD: the sex ratio must occur within its range of natural temperature (RNT). However, the thermosensitive period in most species examined by previous studies usually happened during early development, especially in the larval stages (Devlin and Nagahama, 2002; Conover, 2004). Hence, Ospina-Álvarez and Piferrer (2008) considered that the temperature range of TSD should include the thermosensitive period, namely the RTD. Therefore, verification of TSD should meet two conditions: 1) the species has no sex chromosomes; and 2) the sex ratio responded at the RTD. Based on this standard, experimental results for 59 fish species that had been widely confirmed as TSD were examined by statistical analysis. The results showed that, excluding species of the genus Apistogramma, the species had been overwhelmingly proved as TSD, while sex-ratio shifts of 75% under some extreme temperatures were most likely the consequence of thermal effects on GSD, namely GSD+TE, not TSD. In the FM mode, analysis of data on channel catfish Ictalurus punctatus showed no difference with respect to the 1:1 sex ratio (Chi-square test=1.42, P=0.233), and sockeye salmon Oncorhynchus nerka has been previously suggested as mode MF (Craig et al., 1996). However, both these species had sex chromosomes, and the test temperatures were out of the natural range. In mode MFM, both Japanese flounder Paralichthys olivaceus (Yamamoto, 1999) and southern flounder P. letho (Luckenbach et al., 2003) failed one criterion, thus they were deemed not real TSD species, though this outcome was different from the majority. Furthermore, when compared with cultivated strains, sex-ratio shifts in wild populations are more easily affected by genetic and environmental factors (Baroiller and D'Cotta, 2016). Therefore, the mechanisms of TSD in fish require additional research.
This study chose an L9(34) orthogonal array to determine the optimal combination for achieving a high proportion of male tiger puffer T. rubripes while maintaining the most favorable growth rate despite low-temperature-controlled conditions: the ideal time to start treatment was 50 days post-hatch, the best treatment temperature was 13℃, and the most favorable treatment duration was 45 days. The maleproduction rate with this method was up to 75%. The three factors determining a male-biased sex rate and their ideal sequence was treatment-temperature > days after hatching > processing time. Thus, before or during the course of gonadal differentiation in the fry of this species, low-temperature conditions could effectively control the direction of gonadal differentiation, and effectively increased the proportion of males. Our results are consistent with a study by Hattori et al. (2012), Liu et al. (2014) and Lee et al. (2009), which used a water temperature of approximately 20-32℃ to culture T. rubripes larvae from the time of hatching to 32-43 days after hatching. After nine months, tissue sections and RT-PCR were used to examine the gonadal morphology and determine the expression of vasa mRNA as seen in germ cells. The results showed that culturing the T. rubripes at 32℃ caused germ-cell apoptosis and gonadal ovary-cell masculinization. This phenomenon of sex reversal can also be found in Nile Tilapia Oreochromis niloticus, which is classified as Temperature-induced sex reversal (TISR) species (Baroiller and D'Cotta, 2016).
Gonad differentiation in many ectotherm species is associated with the environmental temperature during early development. However, it remains unknown how these species respond to artificial rearing temperatures and the definite transfer mechanisms between the ambient water temperature and gonad differentiation. In fish, especially some temperaturesensitive species, high temperature has been widely reported that can affect the expression of genes and cortisol levels, which can skew the sex ratio towards males. Studies had found that high levels of cortisol could be found in pejerrey Odontesthes bonariensis larvae during the sex determination period at high temperature. This glucocorticoid produced by the renal cells could promote the production of 11-ketotestoterone (11-KT) and drive the morphogenesis of testes (Hattori et al., 2009; Fernandino et al., 2012, 2013). In Japanese flounder, high levels of cortisol were also found to inhibit the expression of ovary-type aromatase (cyp19a1) mRNA, which caused female-to-male sex reversal (Yamaguchi et al., 2010). This phenomenon was also confirmed in Medaka Oryzias latipes (Kitano et al., 2012). Additionally, high temperature could also unregulate the mRNA expression of the retinoiddegrading enzyme (cyp26b1), which played an important role in the onset of cell meiosis in fish ovaries. (Yamaguchi and Kitano, 2012). Furthermore, the researchers discovered that a sex-determination gene amhy both existed in silverside Odontesthes hatcheri and pejerrey, which agained underlined the genetic environment could determine the phenotype in sensitive fish species. (Yamamoto et al., 2014). For T. rubripes, it is still unclear whether the increase of the male ratio at the high and low temperatures is related to the reasons given above or is the result of other mechanisms. We intend to continue our research on this topic in future studies.
The growth of cultured aquatic animals is affected not only by genetic factors but also by temperature, illumination, dissolved oxygen, pH, and density. Temperature is importantly used to influence growth rates in fish culture. Within a certain temperature range, the growth rate of fish increases as the ambient water temperature increases. A number of investigations have reported on different temperature effects in relation to fish growth traits. For instance, in the range of 13-25℃, the growth rate of European bass Dicentrarchus labrax gradually increased with increasing temperatures but decreased at 29℃ (Person et al., 2004). Cultured at 15.2-24℃, the growth rate of turbot Scophthalmusmaximus decreased with increasing temperatures. The suitable temperature should not exceed 18℃ (Sahí, 2001). When culturing tongue sole Cynoglossus semilaevis at 17.5-27.5℃, the growth rate increased with increasing temperature (Tian et al., 2011). In this study, we monitored changes in body length and weight of different low-temperature treatment groups (13, 15 and 17℃) and a control group (21±1℃) up to 200 days old, and found that the growth rate (length and weight) increased with increasing temperatures. The average lengths and weights of fish in the three low-temperature-treatment groups did not significantly differ (P > 0.05). Furthermore, examination of ADIL and ADIW among nine orthogonal groups during or after rearing in lowtemperature-controlled conditions showed that these growth rates within a given treatment group were significantly lower during the treatment than after the treatment. The growth rates (length and weight) of the treatment groups did not significantly differ from those of the controls (P > 0.05). This result shows that although the growth rate was impacted during the temperature-controlled period, the growth rate returned to normal after the treatment. This research provides a baseline technique for temperaturecontrol-induced masculinization of T. rubripes, which can improve the value of production of this species.
5 CONCLUSIONThis study establishes a low-temperature-induced masculinization technology for T. rubripes, we can get the highest proportion of males with the processing condition combination: treatment starting times of 50 dph, treatment temperatures of 13℃, and treatment durations 45 days. The result shows that although the growth rate was impacted during the temperaturecontrolled period, the growth rate returned to normal after the treatment. This research provides a baseline technique for temperature-control-induced masculinization of T. rubripes, which can improve the value of production of this species.
6 DATA AVAILABILITY STATEMENTThe data that support the findings of this study are available from the corresponding author on request.
Baroiller J-F, D'Cotta H. 2016. The reversible sex of gonochoristic fish: insights and consequences. Sex Development, 10(5-6): 242-266.
DOI:10.1159/000452362 |
Cheng X C, Lin D J, You Y L. 2007. Influence of temperature on sex differentiation of teleost, Pseudobagrus vachelli. Zoological Research, 28(1): 73-80.
(in Chinese with English abstract) |
Conover D O, Kynard B E. 1981. Environmental sex determination: interaction of temperature and genotype in a fish. Science, 213(4507): 577-579.
DOI:10.1126/science.213.4507.577 |
Conover D O. 2004. Temperature-dependent sex determination in fishes. In: Temperature-Dependent Sex Determination in Vertebrates. Smithsonian Books, Washington. p.11-20.
|
Craig J K, Foote C J, Wood C C. 1996. Evidence for temperature-dependent sex determination in sockeye salmon (Oncorhynchus nerka). Canadian Journal of Fisheries and Aquatic Sciences, 53(1): 141-147.
DOI:10.1139/f95-152 |
Devlin R H, Nagahama Y. 2002. Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture, 208(3-4): 191-364.
DOI:10.1016/S0044-8486(02)00057-1 |
Ewert M A, Nelson C E. 1991. Sex determination in turtles: diverse patterns and some possible adaptive values. Copeia, 1991(1): 50-69.
DOI:10.2307/1446248 |
Fernandino J I, Hattori R S, Kishii A, Strüssmann C A, Somoza G M. 2012. The cortisol and androgen pathways crosstalk in high temperature-induced masculinization: the 11β-hydroxysteroid dehydrogenase as a key enzyme. Endocrinology, 153(12): 6003-6011.
DOI:10.1210/en.2012-1517 |
Fernandino J I, Hattori R S, Moreno Acosta O D, Strüssmann C A, Somoza G M. 2013. Environmental stress-induced testis differentiation: androgen as a by-product of cortisol inactivation. General and Comparative Endocrinology, 192: 36-44.
DOI:10.1016/j.ygcen.2013.05.024 |
Gui J F. 2007. Genetic Basis and Artificial Control of Sexually and Reproduction in Fish. Science Press, China, p.62. (in Chinese)
|
Hattori N, Miyashita S, Sawada Y. 2012. Effective masculinization method of tiger puffer by temperature control under culture condition. Journal of the Japan Fisheries Society, 78(1): 87.
|
Hattori R S, Fernandino J I, Kishii A, Kimura H, Kinno T, Oura M, Somoza G M, Yokota M, Strüssmann C A, Watanabe S. 2009. Cortisol-induced masculinization: does thermal stress affect gonadal fate in pejerrey, a teleost fish with temperature-dependent sex determination?. PLoS One, 4(8): e6548.
DOI:10.1371/journal.pone.0006548 |
Jiang Z Q, Wu L X, Hao L D, Bai L J, Feng Z X. 2005. Mariculture Fish Biology and Aquaculture. Ocean Press, Beijing, China. p.61.
(in Chinese)
|
Kamiya T, Kai W, Tasumi S, Oka A, Matsunaga T, Mizuno N, Fujita M, Suetake H, Suzuki S, Hosoya S, Tohari S, Brenner S, Miyadai T, Venkatesh B, Suzuki Y, Kikuchi K. 2012. A trans-species missense SNP in Amhr2 is associated with sex determination in the tiger pufferfish, Takifugu rubripes (Fugu). PLoS Genetics, 8(7): e1002798.
DOI:10.1371/journal.pgen.1002798 |
Kikuchi K, Kai W, Hosokawa A, Mizuno N, Suetake H, Asahina K, Suzuki Y. 2007. The sex-determining locus in the tiger pufferfish, Takifugu rubripes. Genetics, 175(4): 2039-2042.
DOI:10.1534/genetics.106.069278 |
Kitano T, Hayashi Y, Shiraishi E, Kamei Y. 2012. Estrogen rescues masculinization of genetically female medaka by exposure to cortisol or high temperature. Molecular Reproduction and Development, 79(10): 719-726.
DOI:10.1002/mrd.v79.10 |
Lee K H, Yamaguchi A, Rashid H, Kadomura K, Yasumoto S, Matsuyama M. 2009. Germ cell degeneration in hightemperature treated pufferfish, Takifugu rubripes. Sexual Development, 3(4): 225-232.
DOI:10.1159/000228723 |
Lei J L. 2005. Marine Fish Culture Theory and Techniques. China Agriculture Press, Beijing, China. p.683-702.
(in Chinese)
|
Li Y Q, Fu Z. 1993. The effect of different temperature on the growth and survival of larvals and juveniles in Takifugu rubripes. Hebei Yuy, (2): 26-27.
(in Chinese) |
Liu Y X, Zhou Q, Zhang H T, Jiang C B, Zhang F C. 2014. Effect of temperature on growth and sex differentiation at early developmental stage of redfin puffer (Takifugu rubripes). South China Fisheries Science, 10(5): 24-29.
(in Chinese with English abstract) |
Luckenbach J A, Borski R J, Daniels H V, Godwin J. 2009. Sex determination in flatfishes: Mechanisms and environmental influences. Seminars in Cell & Developmental Biology, 20(3): 256-263.
|
Luckenbach J A, Godwin J, Daniels H V, Borski R J. 2003. Gonadal differentiation and effects of temperature on sex determination in southern flounder (Paralichthys lethostigma). Aquaculture, 216(1-4): 315-327.
DOI:10.1016/S0044-8486(02)00407-6 |
Ma A J, Lu L J, Chen C, Wang X A, Meng X S, Li W Y, Liu S C. 2011. Breeding and genetic research of major economic species of Fugu. Marine Sciences, 35(11): 128-133.
(in Chinese) |
Matsuura S, Naito T, Shincho M, Yoshimura K, Matsuyama M. 1994. Gonadal sex differentiation in tiger puffer, Takifgu rubripes. Aquaculture Science (Japan), 42(4): 619-625.
|
Nan P, Du Q Y, Yan S G, Chang Z J. 2005. Effects of temperature on sex differentiation of gonads and the cloning and expression of CYP19a in two species of loaches. Journal of Fishery Sciences of China, 12(4): 407-413.
(in Chinese with English abstract) |
Ospina-Álvarez N, Piferrer F. 2008. Temperature-dependent sex determination in fish revisited: prevalence, a single sex ratio response pattern, and possible effects of climate change. PLoS One, 3(7): e2837.
DOI:10.1371/journal.pone.0002837 |
Pan C Y, He Y H. 1993. Mathematical Statistics Theory and Method. Tongji University Press, Shanghai, China. p.292-293.
(in Chinese)
|
Person-Le Ruyet J, Mahé K, Le Bayon N, Le Delliou H. 2004. Effects of temperature on growth and metabolism in a Mediterranean population of European sea bass, Dicentrarchus labrax. Aquaculture, 237(1-4): 269-280.
DOI:10.1016/j.aquaculture.2004.04.021 |
Sahí T. 2001. Effect of water temperature on growth of hatchery reared Black Sea Turbot, Scophthalmus maximus (Linnaeus, 1758). Turkish Journal of Zoology, 25(3): 183-186.
|
Suzuki N, Okada K, Kamiya N. 1996. Gonadal development, sexual differentiation and sex ratio of tiger puffer, Takifugu rubripes. Bulletin of the National Fisheries Research Institute (Japan), 29(2): 39-48.
|
Suzuki Y. 2010. According to the genetic breeding of new varieties tiger puffer varieties as the goal. Biological Function Development Research Institute (Japan), 10: 9-23.
|
Tian B J, Li Y W, Ding Q, Geng L N. 2007. A comparison of rotifer and brine shrimp in nutrient value. Hebei Fisheries, (12): 45-47.
(in Chinese) |
Tian Y S, Wang D, Xu Y, Chen S L. 2011. Effects of rearing temperature on growth and sex determination in the halfsmooth tongue sole (Cynoglossus semilaevis). Journal of Fisheries of China, 35(2): 176-182.
(in Chinese with English abstract) |
Yamaguchi T, Kitano T. 2012. High temperature induces cyp26b1 mRNA expression and delays meiotic initiation of germ cells by increasing cortisol levels during gonadal sex differentiation in Japanese flounder. Biochemical and Biophysical Research Communications, 419(2): 287-292.
DOI:10.1016/j.bbrc.2012.02.012 |
Yamaguchi T, Yoshinaga N, Yazawa T, Gen K, Kitano T. 2010. Cortisol is involved in temperature-dependent sex determination in the Japanese flounder. Endocrinology, 151(8): 3900-3908.
DOI:10.1210/en.2010-0228 |
Yamamoto E. 1999. Studies on sex-manipulation and production of cloned populations in hirame, Paralichthys olivaceus (Temminck et Schlegel). Aquaculture, 173(1-4): 235-246.
DOI:10.1016/S0044-8486(98)00448-7 |
Yamamoto Y, Zhang Y, Sarida M, Hattori R S, Strüssmann C A. 2014. Coexistence of genotypic and temperaturedependent sex determination in pejerrey Odontesthes bonariensis. PLoS One, 9(7): e102574.
DOI:10.1371/journal.pone.0102574 |
Yang J, Wu H D, Fan Z T. 2007. Advances on sex determination mechanism of fish. Fisheries Economy Research, (3): 14-19.
(in Chinese with English abstract) |
Zhou H, Li J Q, Ma H Y, Li Y J, Jiang Z Q, Li X, Xu W, Jiang Z H. 2015. Low temperature-induced masculinization and histological observation of gonadal differentiation in redfin puffer Takifugu rubripes. Journal of Dalian Fisheries University, 30(1): 41-47.
(in Chinese with English abstract) |
 2019, Vol. 37
2019, Vol. 37