Institute of Oceanology, Chinese Academy of Sciences
Article Information
- HUANG Wen, XU Fei, LI Li, QUE Huayong, ZHANG Guofan
- The transcription of iodothyronine deiodinase genes is regulated by thyroid hormone receptor in the Pacific oyster Crassostrea gigas
- Journal of Oceanology and Limnology, 37(4): 1317-1323
- http://dx.doi.org/10.1007/s00343-019-8207-9
Article History
- Received Aug. 1, 2018
- accepted in principle Sep. 25, 2018
- accepted for publication Nov. 1, 2018
2 School of Marine Sciences, Guangxi University, Nanning 530004, China;
3 University of Chinese Academy of Sciences, Beijing 100049, China;
4 Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China;
5 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
In vertebrates, the thyroid hormones (THs), L-thyroxine (T4) and 3, 3′, 5-triiodothyronine (T3), are indispensable for the regulation of growth, development, and metabolism (Yen, 2001; Morvan-Dubois et al., 2008). Abnormal of TH levels induce severe diseases such as endemic cretinism and adult hypothyroidism (Yen, 2001). TH function and its mechanism had been extensively studied in vertebrates; the THs were regarded as hormones exclusive to vertebrates in the past century (Morvan-Dubois et al., 2008). However, in recent years, studies related to THs in invertebrate species have extended our understanding of THs in the evolutionary context. For example, the TH-related functions and signaling pathways in the invertebrate chordates (Cephalochordata and Urochordata) were gradually accepted (Carosa et al., 1998; Paris et al., 2008). However, TH-related studies in non-chordate invertebrates were scarce and scattered in several species; studies had been carried in two abalone species (Haliotis discus and H. gigantea) (Fukazawa et al., 2001), the sea urchin, Lytechinus variegatus (Heyland et al., 2006), two Platyhelminthes species (Schistosoma mansoni and S. japonicum) (Wu et al., 2007; Qiu et al., 2012), the scallop, Chlamys farreri (Wu et al., 2012), the Manila clam, Ruditapes philippinarum (Song et al., 2016), and the snail, Achatina fulica (Lustrino et al., 2017). These fragmentary evidence indicated that TH system might also be present in a wide range of non-chordate invertebrates. Studies on the TH system in non-chordate invertebrates will shed light on the origin of TH system and may have application value in control of biofouling and aquaculture.
Iodothyronine deiodinases are critical enzymes in the regulation of thyroid hormone levels in vertebrates. Vertebrate deiodinases are classified into three types, based on their action on THs. Type Ⅱ deiodinase (D2) initiates TH effects by catalyzing the outer ring deiodination (ORD) to transform the prohormone T4 to active T3; type Ⅲ deiodinase (D3) mainly terminates TH effects by catalyzing inner ring deiodination (IRD) to inactivate T4 and T3; the versatile type Ⅰ deiodinase (D1) is capable of both initiation and termination of TH effects (Bianco et al., 2002). The transcription of deiodinases is regulated by TH status in return via thyroid hormone receptor (TR). This feedback regulation mechanism is critical in maintaining TH homeostasis (Zavacki et al., 2005; Barca-Mayo et al., 2011). Vertebrate D1, D2, and D3 are TH responsive genes and the ligand-dependent transfactor TR regulates their expression through binding TH response element (TRE) in the promoter of target genes (Zhang and Lazar, 2000).
As a well-studied species among the mollusks, the Pacific oyster, Crassostrea gigas (Thunberg) has a clear genetic background with a completed genome project (Zhang et al., 2012). It is also a worldwide aquaculture species. A TH-related study in C. gigas will not only provide evolutionary knowledge of the TH system but also may contribute to applications in oyster culture. We previously reported that T4 and T3 had been measured throughout the developmental stages of C. gigas, the functional characteristics of the thyroid hormone receptor CgTR had been studied (Huang et al., 2015b), and two deiodinases had been identified (Huang et al., 2015a). Here, we further studied the physiological effects of exogenous T4 and the transcription regulation pathway of the two deiodinases (CgDx and CgDy). These results provide important clues regarding the evolution of TH systems.
2 MATERIAL AND METHOD 2.1 T4 treatment in the umbo stageDue to a relatively long time through this developmental stage, umbo larvae were selected to estimate TH effects on the growth of oyster larvae. Umbo larvae (shell height 143.4±16.4 μm) were exposed to four different concentrations of T4. After the eighth day of T4 treatment, larvae were still in the umbo stage. The indexes of growth, shell heights of more than 30 individuals were measured in each bucket. Larvae were cultured in filtered seawater in 60-L plastic buckets in triplicate and the culture water was changed every two days. T4 stocks were prepared at the concentrations of 1.29×10-2, 1.29×10-3, 1.29×10-4, and 1.29×10-5 mol/L and diluted in a ratio of 1:104 in the culture water.
2.2 Oyster collection and THs measurementArtificially bred oyster embryos were divided into four groups and treated with different concentrations of T4 for 8 h: no exogenous T4 (control), 1.29×10-10 mol/L T4, 1.29×10-9 mol/L T4, 1.29×10-8 mol/L T4. Then, the embryos were sampled and stored in -80 ℃ for not more than two months. T3 and T4 were extracted from the embryos with 0.01 mol/L NaOH and quantified with Elecsys 2010 automatic electrochemical immunoanalyzer (Roche, Penzberg, Germany) as per the manufacturer's instructions.
2.3 Dual luciferase reporter assayDual luciferase reporter assay was conducted using the expression vector pcDNA3.1 (Invitrogen) and reporter vector pGL3-Basic (Promega, Madison, WI, USA) in HEK293T cells (ATCC, Manassas, USA). Plasmid pcDNA3.1-CgTR was used to express the full-length CgTR protein. pGL3-pCgDx and pGL3-pCgDy reporter vectors contain the cloned promoter region (~3 Kb) of CgDx and CgDy. Cells were seeded, cultured, and transfected as previously described (Qu et al., 2014). The experiment was carried out in 96- well plates, in which cells from each well were transfected with 5 ng pRL-CMV (Promega), 20 ng reporter vector, and 0, 20, 40, and 80 ng pcDNA3.1-CgTR. pcDNA3.1 was used to keep the total amount of DNA consistent in each well. At 24 h post transfection, the Dual-Luciferase Reporter Assay System (Promega) was used to measure the luciferase activity of each well.
2.4 Electrophoretic mobility shift assay (EMSA)Recombinant full-length CgTR protein with His-tag (rCgTR) was produced using a prokaryotic expression system with expression vector pET30a (Novagen, George Town, KY, USA). The prokaryotic expressed protein rCgTR was identified by SDS-PAGE on 12% gels stained with Coomassie brilliant blue R250 and confirmed using western blot with a monoclonal anti-His antibody (TIANGEN biotech, Beijing, China). Two probes were synthesized and labeled with biotin in Sangon Biotech (Shanghai, China). The probe TRE-Dx (5′-CTTTTGACCTTTGGATTGACCCCGCA-3′) corresponded to the putative TRE in the CgDx promoter region from -2 249 to -2 223 and the probe TRE-Dy (5′-GAATACGAGGTCATAGGTAAAAGCAA-3′), to one of the two putative TREs in the CgDy promoter region from -1914 to -1888 (Huang et al., 2015a). The LightShift Chemiluminescent EMSA kit (Pierce, Rockford, USA) was used according to the manufacturer's instructions in EMSA experiments.
3 RESULT AND DISCUSSION 3.1 High concentrations of T4 retarded umbo larval growthAs the longest developmental stage and as the larvae in this stage has the fastest growth rate, umbo larvae were chosen to evaluate the effects of T4 on oyster larval growth by using the verified immersion method. The results show (Fig. 1) that larvae in the high T4 concentration groups (1.29×10-7 mol/L and 1.29×10-6 mol/L) grew significantly slower than that in the control group, and that the larval growth rate of the low T4 concentration groups (1.29×10-9 mol/L and 1.29×10-8 mol/L) did not differ significantly from that of the control group. The results suggest that high concentrations of T4 are detrimental to umbo larval growth.
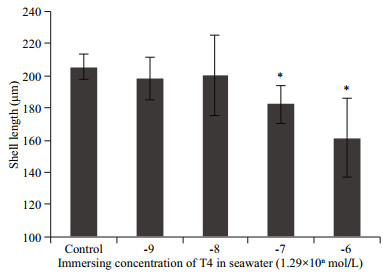
|
| Fig.1 High concentrations of T4 retard the growth of umbo larvae The larvae were cultured in 60-L plastic buckets in triplicate and in the umbo stage, subjected to different concentrations of T4 (1.29×10-9 mol/L, 1.29×10-8 mol/L, 1.29×10-7 mol/L, and 1.29×10-6 mol/L). The shell height was measured on the eighth day of T4 treatment. The data are presented as mean±SD (n=3). Statistically significant differences, determined by Student's t-test, are indicated by asterisks (*P < 0.05). |
Many exogenous TH immersion experiments have been conducted in fish species to assess the effects of THs on growth. The observed effects were inconsistent, which seemed to be dependent on the dose and the developmental stage. Appropriate doses of exogenous THs accelerate growth, whereas higher doses retard growth (Power et al., 2001; Brown et al., 2014). Our T4 treatment experiments demonstrated that high concentrations of T4 retard umbo larval growth (Fig. 1). It is worth noting that natural TH levels are low in the umbo stage of oyster larvae (Huang et al., 2015b). It is possible that the THs available from food or synthesized endogenously are sufficient in the umbo stage, and that no exogenous T4 is needed. When the larvae immersed in 1.29× 10-8 mol/L T4, the concentration of T4 and T3 were about 6 times as a control group, which represented the normal TH level (Fig. 2). THs have wide effects on growth, development, and metabolism. The THs levels were precisely controlled by feedback mechanism (Yen, 2001). If THs levels were out of control, the disordered endocrine system may induce abnormal development or growth (Morvan-Dubois et al., 2008). It is predictable that the concentration of THs was above 6 times of normal level when larvae were immersed in 1.29×10-7 mol/L or 1.29×10-6 mol/L T4. Thus, the high dose of T4 may induce endocrine system disorders and retards the growth of oyster larvae. There are some consistent results from studies in fish that exogenous THs can be detrimental to larval growth, as observed in striped bass, Morone saxatilis and piracanjuba, Brycon orbignyanus (Huang et al., 1996; Landines et al., 2010). It has proved that the effective THs in oysters is not T4 or T3 (Huang et al., 2015b), which may lead to TH-response of oysters required a high dose of T4 compared to that of vertebrates. This is the first and primary study on the physiological effect of THs in bivalve. Although, no application value was detected in oyster umbo stage, it still worthy to investigate the effects of THs in early development and metamorphosis in future. In gastropods, metamorphosis is induced by T4 and T3 in two abalone species (Haliotis discus and H. gigantean) (Fukazawa et al., 2001).
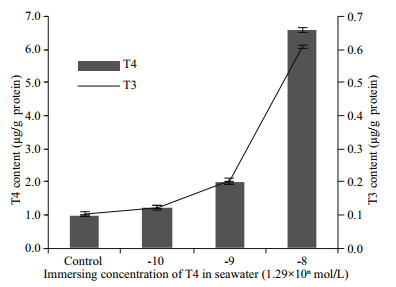
|
| Fig.2 T4 and T3 contents in oyster embryos after immersion in different concentrations of T4 added in sea water The embryos were subjected to different concentrations of T4 for 8 h from 2 hpf (hours post fertilization) to 10 hpf (trochophore). The data are represented as mean±SD of triplicate independent experiments. |
As shown in Fig. 2, when oyster embryos were cultured in seawater containing different concentrations of T4 for 8 h, the T4 content in oyster embryos was shown to be increased depending on the concentration of T4 they were immersed in. This result suggested that T4 permeated into oyster larvae in a dose-dependent manner using the immersion method; hence, the procedure can be used as a feasible method for further studies on the effects of THs in mollusks. Furthermore, T3 content was also increased along with T4, and the T3 and T4 contents in 1.29×10-9 mol/L and 1.29×10-8 mol/L T4 groups were two and six times those in the control group, respectively. These results suggested that T4 was transformed to T3 in vivo, and represented the outer ring deiodinase activity as possessed by vertebrate D1 and D2. The oyster homologs of vertebrates D1, D2, and D3 are CgDx and CgDy. They have the common sequence characteristics of in-frame TGA codon coding for selenocysteine and selenocysteine insertion sequence elements in the 3′ untranslated region (Huang et al., 2015a). Therefore, we speculated that these genes were responsible for the deiodinase activity detected. However, it did not directly prove the deiodinase activity of CgDx or CgDy. It was hard to infer which deiodinase exerted this activity, as the phylogenetic tree showed that the gene duplicated and diverged independently between oyster deiodinases and vertebrate deiodinases (Huang et al., 2015a). Actually, an RNA interference experiment supported that the scallop deiodinase (CfDx) also had the preserved activity of transforming T4 into T3 (Wu et al., 2012). These results suggest that THs metabolism system is also present in mollusks.
3.3 Transcription regulation of CgTR on deiodinasesAs reported previously, the expression of CgDx and CgDy could be stimulated by continuous immersion in T4. At the same time, one atypical putative TRE was found in the promoter of CgDx and two TREs in that of CgDy (Huang et al., 2015a). To further elucidate the TH feedback regulatory mechanism, the dual luciferase reporter assay was performed in HEK293T cells in the absence of THs. Co-transfection of increasing amounts of pcDNA3.1-CgTR (0, 20, 40, and 80 ng) with the pGL3-pCgDx and pGL3-pCgDy reporter genes resulted in transcriptional activation of the promoters in a dose-dependent manner (Fig. 3). The CgTR regulation activity of each of the two promoters was different. The highest activation of the CgDx promoter was 15.7 folds compared to the control, and that of the CgDy promoter was 4.7 folds.
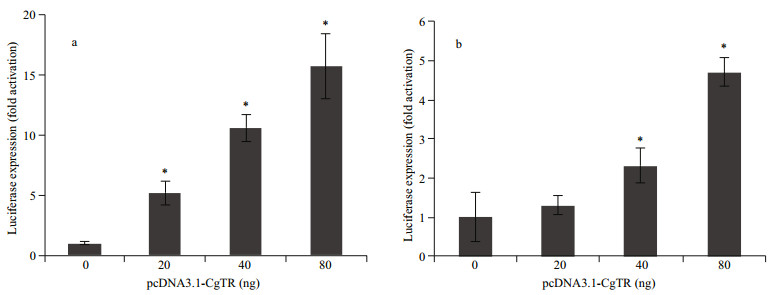
|
| Fig.3 Regulation of transcription of the CgDx (a) and CgDy (b) promoters by increasing amounts of pcDNA3.1-CgTR (0, 20, 40, and 80 ng) in the absence of THs The data are represented as the mean±SD of triplicate independent experiments. Statistically significant differences determined by Student's t-test are indicated by asterisks (*P < 0.05). |
Furthermore, EMSA experiments were conducted to test whether CgTR could bind to the atypical putative TREs in the promoters of CgDx and CgDy. The full-length CgTR protein was expressed in the prokaryotic expression system and confirmed using SDS-PAGE and western blot with anti-His antibody (Fig. 4a). The putative TRE in the promoter of CgDx is DR6 (direct repeats of half-site separated by six nucleotides), which was used to design the probe TRE-Dx. There are two putative TREs in the CgDy promoter, and one of them (named TRE-Dy) was chosen as a representative to test the ability of CgTR binding (Fig. 4b). The EMSA showed clear gel shift bands with similar binding intensity for biotin-labeled probes TRE-Dx and TRE-Dy. These shift bands faded when 100-fold cold probes were added. At the same time, supershift bands were also detected when the antibody was included (Fig. 4c). These results indicated that recombinant CgTR (rCgTR) could bind to the tested probes with comparable binding affinities. In other words, CgTR could regulate the transcription of CgDx and CgDy by binding to the TREs in their promoters. Although vertebrate TRs specifically bind to DR0 or DR4 (Umesono et al., 1991), invertebrate TR from C. gigas (CgTR) and Schistosoma mansoni (SmTRα) could bind to DR0–DR5 (Wu et al., 2007; Huang et al., 2015b). Interestingly, this study further showed that CgTR can also bind to the atypical DR1 (TRE-Dy) and DR6 (TRE-Dx), suggesting that the invertebrate TH receptors (TRs) may have a wider TRE repertoire than expected.
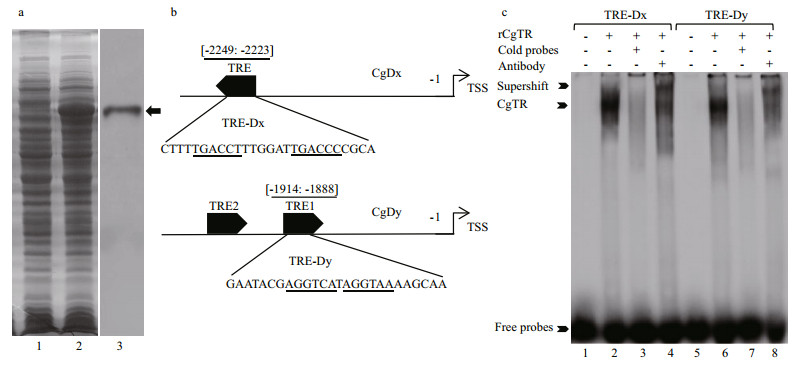
|
| Fig.4 The result of EMSA shows that recombinant CgTR (rCgTR) binds to TREs in the promoters of CgDx and CgDy in vitro a. SDS-PAGE and western blot showing the recombinant CgTR (indicated with black arrow). Lane 1: bacteria with the pET30a-CgTR recombinant plasmid without IPTG induction; lane 2: bacteria with the pET30a-CgTR recombinant plasmid with IPTG induction for 12 h at 30℃; lane 3: western blot confirming the recombinant CgTR with anti-His antibody; b. detailed position and sequences of probes used in EMSA experiments: TRE: thyroid hormone responsive element; TSS: transcriptional start sites; c. EMSA demonstrating that rCgTR could bind to the probes TRE-Dx and TRE-Dy. Lanes 1 and 5 contain the reaction mixture without rCgTR as negative control; lanes 2 and 6 contain rCgTR; lanes 3 and 7 contain rCgTR with a 100-fold concentration of the cold probe; lanes 4 and 8 contain rCgTR with anti-His antibody. |
In vertebrates, the feedback regulatory mechanism is critical in maintaining TH homeostasis. The function of deiodinases is the transformation of prohormone T4 to its active form T3 or the inactivation of T3, which determines the TH status. Interestingly, D1, D2, and D3 are TH-responsive genes and their expression is, in turn, regulated by the TH status via the TR (Zavacki et al., 2005; Barca-Mayo et al., 2011). The TR binds to the TREs in the promoter of deiodinases to activate or repress gene expression depending on the TH status (Bianco et al., 2002). For example, human D1 (hD1) has an ideal TRE (DR4, AGGTCANNNNAGGTCA) in its promoter, on which TR can bind, determined by EMSA experiments. Luciferase reporter experiments revealed that the expression of hD1 is regulated by TR and T4 in vivo (Jakobs et al., 1997; Bianco et al., 2002). In this study, the dual luciferase reporter assays also demonstrated that the overexpression of the unligand CgTR (in the absence of ligand) caused transcriptional activation of the CgDx and CgDy promoters (Fig. 3). Furthermore, atypical putative TREs were found in the promoters, and EMSA experiments confirmed the binding capacity of CgTR to the atypical putative TREs (TRE-Dx and TRE-Dy, Fig. 4). Thus, it is clear that the expression of CgDx and CgDy can be regulated by CgTR via binding to TREs in their promoters. Furthermore, the growth of umbo larva was retarded (in this study) and the expression of CgTR and the two deiodinases was induced by exogenous T4 (Huang et al., 2015a, b ). However, the single CgTR could not bind to T4, T3, or triiodothyroacetic acid (TRIAC), which breaks the key point of the conserved TH feedback loop. Nevertheless, it has been shown in amphioxus that the ligand of TR has changed from T4 or T3 to TRIAC (Paris et al., 2008). Hence, it is reasonable to speculate that the ligand of CgTR may be some other derivative of the THs. The ligand of CgTR requires further study in the follow-up experiments.
4 CONCLUSIONIn this study, the primary physiological effect of T4 on oyster growth was investigated in the umbo stage. High concentrations of T4 retarded umbo larval growth, indicating that THs may have important physiological effects in mollusks. Furthermore, the deiodinase activity of transformation of T4 to T3 was detected in C. gigas in vivo and the previously identified oyster deiodinases (CgDx and CgDy) were speculated to be responsible for this outer ring deiodinase activity. This indicated that the TH metabolic system was also presented in mollusks. Finally, the promoters of CgDx and CgDy were activated by TH receptor (CgTR) and the atypical TREs in the promoters were bound by recombinant CgTR. Thus, the expression of oyster deiodinases was regulated by CgTR through binding to the TREs in their promoters as in vertebrates. These results give important clues in understanding the evolution of TH systems.
5 DATA AVAILABILITY STATEMENTThe datasets generated during the current study are available from the corresponding author on reasonable request.
Barca-Mayo O, Liao X H, Alonso M, Di Cosmo C, Hernandez A, Refetoff S, Weiss R E. 2011. Thyroid hormone receptor α and regulation of type 3 deiodinase. Molecular Endocrinology, 25(4): 575-583.
DOI:10.1210/me.2010-0213 |
Bianco A C, Salvatore D, Gereben B, Berry M J, Larsen P R. 2002. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocrine Reviews, 23(1): 38-89.
|
Brown C L, Urbinati E C, Zhang W M, Brown S B, McCombKobza M. 2014. Maternal thyroid and glucocorticoid hormone interactions in larval fish development, and their applications in aquaculture. Reviews in Fisheries Science & Aquaculture, 22(3): 207-220.
|
Carosa E, Fanelli A, Ulisse S, Di Lauro R, Rall J E, Jannini E A. 1998. Ciona intestinalis nuclear receptor 1:a member of steroid/thyroid hormone receptor family. Proceedings of the National Academy of Sciences of the United States of America, 95(19): 11 152-11 157.
DOI:10.1073/pnas.95.19.11152 |
Fukazawa H, Hirai H, Hori H, Roberts R D, Nukaya H, Ishida H, Tsuji K. 2001. Induction of abalone larval metamorphosis by thyroid hormones. Fisheries Science, 67(5): 985-988.
DOI:10.1046/j.1444-2906.2001.00351.x |
Heyland A, Price D A, Bodnarova-Buganova M, Moroz L L. 2006. Thyroid hormone metabolism and peroxidase function in two non-chordate animals. Journal of Experimental Zoology. Part B, Molecular and Developmental Evolution, 306B(6): 551-556.
DOI:10.1002/jez.b.v306b:6 |
Huang L Y, Specker J L, Bengtson D A. 1996. Effect of triiodothyronine on the growth and survival of larval striped bass (Morone saxatilis). Fish Physiology and Biochemistry, 15(1): 57-64.
|
Huang W, Xu F, Qu T, Li L, Que H Y, Zhang G F. 2015a. Iodothyronine deiodinase gene analysis of the Pacific oyster Crassostrea gigas reveals possible conservation of thyroid hormone feedback regulation mechanism in mollusks. Chinese Journal of Oceanology and Limnology, 33(4): 997-1.
DOI:10.1007/s00343-015-4300-x |
Huang W, Xu F, Qu T, Zhang R, Li L, Que H Y, Zhang G F. 2015b. Identification of thyroid hormones and functional characterization of thyroid hormone receptor in the Pacific oyster Crassostrea gigas provide insight into evolution of the thyroid hormone system. PLoS ONE, 10(12): e0144991.
DOI:10.1371/journal.pone.0144991 |
Jakobs T C, Schmutzler C, Meissner J, Köhrle J. 1997. The promoter of the human type Ⅰ 5'-deiodinase gene-mapping of the transcription start site and identification of a DR+4 thyroid-hormone-responsive element. European Journal of Biochemistry, 247(1): 288-297.
DOI:10.1111/ejb.1997.247.issue-1 |
Landines M A, Sanabria A I, Senhorini J A, Urbinati E C. 2010. The influence of triiodothyronine (T3) on the early development of piracanjuba (Brycon orbignyanus). Fish Physiology and Biochemistry, 36(4): 1 291-1 296.
DOI:10.1007/s10695-010-9410-y |
Lustrino D, Silva A C M, Araujo I G, Tunholi V M, TunholiAlves V M, Castro R N, Carvalho D P, Pinheiro J, Marassi M P. 2017. Evidence of the presence of thyroid hormones in Achatina fulica snails. Anais da Academia Brasileira de Ciencias, 89(3): 2 181-2 188.
|
Morvan-Dubois G, Demeneix B A, Sachs L M. 2008. Xenopus laevis as a model for studying thyroid hormone signalling:from development to metamorphosis. Molecular and Cellular Endocrinology, 293(1-2): 71-79.
DOI:10.1016/j.mce.2008.06.012 |
Paris M, Escriva H, Schubert M, Brunet F, Brtko J, Ciesielski F, Roecklin D, Vivat-Hannah V, Jamin E L, Cravedi J P, Scanlan T S, Renaud J P, Holland N D, Laudet V. 2008. Amphioxus postembryonic development reveals the homology of chordate metamorphosis. Current Biology, 18(11): 825-830.
DOI:10.1016/j.cub.2008.04.078 |
Power D M, Llewellyn L, Faustino M, Nowell M A, Bjornsson B T, Einarsdottir I E, Canario A V M, Sweeney G E. 2001. Thyroid hormones in growth and development of fish. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 130(4): 447-459.
|
Qiu C H, Liu S F, Hong Y, Fu Z Q, Wei M M, Ai D Z, Lin J J. 2012. Molecular characterization of thyroid hormone receptor beta from Schistosoma japonicum and assessment of its potential as a vaccine candidate antigen against schistosomiasis in BALB/c mice. Parasites & Vectors, 5: 172.
|
Qu T, Huang B Y, Zhang L L, Li L, Xu F, Huang W, Li C Y, Du Y S, Zhang G F. 2014. Identification and functional characterization of two executioner caspases in Crassostrea gigas. PLoS One, 9(2): e89040.
DOI:10.1371/journal.pone.0089040 |
Song Y, Miao J J, Pan L Q, Wang X. 2016. Exposure to 2, 2', 4, 4'-tetrabromodiphenyl ether (BDE-47) alters thyroid hormone levels and thyroid hormone-regulated gene transcription in Manila clam Ruditapes philippinarum. Chemosphere, 152: 10-16.
DOI:10.1016/j.chemosphere.2016.02.049 |
Umesono K, Murakami K K, Thompson C C, Evans R M. 1991. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell, 65(7): 1 255-1 266.
DOI:10.1016/0092-8674(91)90020-Y |
Wu T T, Shi X W, Zhou Z, Wang L L, Wang M Q, Wang L L, Huang M M, Yang C Y, Song L S. 2012. An iodothyronine deiodinase from Chlamys farreri and its induced mRNA expression after LPS stimulation. Fish & Shellfish Immunology, 33(2): 286-293.
|
Wu W J, Niles E G, LoVerde P T. 2007. Thyroid hormone receptor orthologues from invertebrate species with emphasis on Schistosoma mansoni. BMC Evolutionary Biology, 7: 150.
DOI:10.1186/1471-2148-7-150 |
Yen P M. 2001. Physiological and molecular basis of thyroid hormone action. Physiological Reviews, 81(3): 1 097-1 142.
DOI:10.1152/physrev.2001.81.3.1097 |
Zavacki A M, Ying H, Christoffolete M A, Aerts G, So E, Harney J W, Cheng S Y, Larsen P R, Bianco A C. 2005. Type 1 iodothyronine deiodinase is a sensitive marker of peripheral thyroid status in the mouse. Endocrinology, 146(3): 1 568-1 575.
DOI:10.1210/en.2004-1392 |
Zhang G F, Fang X D, Guo X M, Li L, Luo R B, Xu F, Yang P C, Zhang L L, Wang X T, Qi H G, Xiong Z Q, Que H Y, Xie Y L, Holland P W H, Paps J, Zhu Y B, Wu F C, Chen Y X, Wang J F, Peng C F, Meng J, Yang L, Liu J, Wen B, Zhang N, Huang Z Y, Zhu Q H, Feng Y, Mount A, Hedgecock D, Xu Z, Liu Y J, Domazet-Lošo T, Du Y S, Sun X Q, Zhang S D, Liu B H, Cheng P Z, Jiang X T, Li J, Fan D D, Wang W, Fu W J, Wang T, Wang B, Zhang J B, Peng Z Y, Li Y X, Li N, Wang J P, Chen M S, He Y, Tan F J, Song X R, Zheng Q M, Huang R L, Yang H L, Du X D, Chen L, Yang M, Gaffney P M, Wang S, Luo L H, She Z C, Ming Y, Huang W, Zhang S, Huang B Y, Zhang Y, Qu T, Ni P X, Miao G Y, Wang J Y, Wang Q, Steinberg C E W, Wang H Y, Li N, Qian L M, Zhang G J, Li Y R, Yang H M, Liu X, Wang J, Yin Y, Wang J. 2012. The oyster genome reveals stress adaptation and complexity of shell formation. Nature, 490(7418): 49-54.
DOI:10.1038/nature11413 |
Zhang J S, Lazar M A. 2000. The mechanism of action of thyroid hormones. Annual Review of Physiology, 62: 439-466.
DOI:10.1146/annurev.physiol.62.1.439 |
 2019, Vol. 37
2019, Vol. 37


