Institute of Oceanology, Chinese Academy of Sciences
Article Information
- DOU Xiang, MENG Fanping, DUAN Weiyan, LIU Qunqun, LI Hao, DU Shuhao, PENG Xiaoling
- Growth inhibition and oxidative stress in two species of marine diatoms exposed to 1-phenylethanol
- Journal of Oceanology and Limnology, 37(4): 1342-1352
- http://dx.doi.org/10.1007/s00343-019-8205-y
Article History
- Received Aug. 15, 2018
- accepted in principle Sep. 6, 2018
- accepted for publication Sep. 26, 2018
2 College of Environmental Science and Engineering, Ocean University of China, Qingdao 266000, China
Fragrances, such as synthetic musk compounds, have been found in the marine environment as one of the major organic contaminants (Bester et al., 1998). They are not biodegradable and can be accumulated in a wide variety of fish, mussels, shrimp, marine mammals and sharks (Rimkus and Wolf, 1995; Bester et al., 1998; Gatermann et al., 2002; Nakata, 2005; Nakata et al., 2007). The ecological risk of fragrances has aroused the concern of researchers.
1-phenylethanol (1-PEA, CAS 98-85-1), also known as styralyl alcohol, with a light gardenia flowery perfume, is widely used as a fragrance additive in cosmetics as well as a flavoring agent for food and beverages (Api et al., 2017). At present, the volume of 1-PEA used in the world is 10–100 metric tons per year (Api et al., 2017). In addition, 1-PEA is also an intermediate product in the petrochemical industry from the coproduction of propylene oxide and styrene monomer (POSM) (Engelhardt, 2006). During the POSM production process, the concentrations of discharged 1-PEA in the wastewater may range from 114–237 mg/L (Dao et al., 2014). In recent decades, more and more POSM plants have been put into operation around the world (Brown, 2000; Lemmer, 2001; Everchem, 2017; Brelsford, 2018). The extensive use of 1-PEA as a fragrance additive and the successful completion of POSM facilities significantly increase the probability of 1-PEA release into the marine environment. The resulting environmental problems cannot be ignored.
Currently, the toxicity of 1-PEA to freshwater organisms has been evaluated. The 96-h median lethal concentration (LC50) for zebrafish (Barchydanio rerio) is 100 mg/L (ECHA, 2017), the 48-h LC50 for golden orfe (Leuciscus idus) is 345 mg/L (Sigma-Aldrich, 2012), the 48-h median effect concentration (EC50) for invertebrates is 45.32 mg/L, and the 72-h EC50 for aquatic algae and cyanobacteria is 200 mg/L (ECHA, 2017). However, little information is available on the influence of 1-PEA on marine organisms, which is highly desirable to understand the environmental risks of 1-PEA.
As important primary producers in the marine ecosystems, microalgae exert important impacts on the structure and function of marine ecosystems. When the growth and diversity of microalgae decrease under chemical stresses, the productivity of high trophic-level species and the balance of the whole marine ecosystem change (Nie et al., 2013). Besides, microalgae often provide one of the first signals of ecosystem impacts due to their short response times (Pavlić et al., 2005). Therefore, further study of the toxic effects of marine pollutants on microalgae species is of significant importance. Where marine algae are under investigation, diatoms are the usual test organisms (EN ISO, 2016), since they are the most sensitive species to the compounds being examined (Hörnström, 1990).
When microalgae are exposed to pollutants, large amounts of reactive oxygen species (ROS) can be produced and accumulated. The increased levels of ROS produce oxidative damage to macromolecules such as proteins, nucleic acids, and lipids, causing cellular metabolic disorders, finally leading to the damage of different cellular organelles (Morelli and Scarano, 2004; Sabatini et al., 2009). In order to scavenge ROS, plant cells have developed a wide range of defense mechanisms involving enzymatic and non-enzymatic antioxidant systems, which is believed to play an important role in cell protection (Wang et al., 2008). Therefore, investigating the responses of antioxidant systems to the pollutants would help to reveal the responding mechanisms of microalgae.
In this study, we tested the acute toxicity of 1-PEA in two marine diatoms, Phaeodactylum tricornutum Bohlin and Skeletonema costatum. Both diatoms are test species designated in the EN ISO 10253 (2016) and OCSPP 850.4500 (USEPA, 2012). Inhibition effects of 1-PEA on algal growth were evaluated. Furthermore, the toxicity mechanism of 1-PEA is explored based on the variations in chlorophyll, reduced glutathione (GSH), malondialdehyde (MDA) and three key antioxidant enzymes (including superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx)). The results of this study can provide a scientific basis and a better understanding of the potential ecological risks of 1-PEA in marine environments.
2 MATERIAL AND METHOD 2.1 Chemicals1-PEA was purchased from the Tokyo Chemical Industry Co. Ltd. (Tokyo, Japan) with a water solubility of 29.0 g per liter at 20 ℃. The chemical structure of 1-PEA is shown in Fig. 1. A stock solution of 5 000 mg/L was prepared in filtered (0.45 μm) natural seawater (with a pH of 7.92 and a salinity of 32.0) which was collected from the coastal area of Shilaoren in Qingdao City, China. The other chemicals used in the study were obtained from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). All chemicals were of analytical or biochemical grade.
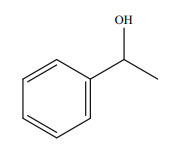
|
| Fig.1 The chemical structure of 1-PEA |
Two diatoms, i.e., P. tricornutum and S. costatum, were obtained from the Algal Culture Collection at Ocean University of China (OUC), and the Institute of Oceanology, Chinese Academy of Sciences, respectively. Each species of diatom was cultured in MAA medium for more than two weeks at 20 ℃ in an artificial incubator with an illumination of 60 μmol/(m2·s) (provided by white fluorescent lamps) under a 14 h/10 h light/dark cycle.
2.3 The 96-h algal growth inhibition testTests were performed in 500 mL Erlenmeyer flasks. Algae cells in the exponential growth phase, at initial cell densities of 1×104 cells/mL were exposed to various concentrations of 1-PEA in 250 mL MAA (150, 185, 227, 279, and 344 mg/Lfor P. tricornutum; and 100, 111, 123, 136, and 151 mg/Lfor S. costatum). These dosing levels were selected using an equal logarithmic interval method based on results from a range-finding test. MAA media without 1-PEA was used as a control. All treatments and controls were conducted in quadruplicate and incubated under the conditions described above for 96 h. Suspension cultures were shaken by hand twice daily. To avoid loss of 1-PEA due to volatilization, cultures were always maintained in a closed state except for sampling during cell density measurements. The cell density (×104 cells/mL) was measured every 24 h by counting with a hemocytometer under a light microscope (YS2-H, Nikon, Japan).
For each species during the exponential growth phase, the specific growth rates (r) and the generation time (Td) were calculated with the following equations (USEPA, 2012):
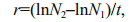
where N2 and N1 represent cell densities (cells/mL) at times t2 and t1, respectively and t is the time interval from t1 to t2 in hour.
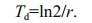
The median effective concentration values at 96 h (96-h EC50) were calculated using probit analysis (Finney, 1971). The concentration obtained at 5.0 of probit was the value of 96-h EC50 (USEPA, 2012).
2.4 Determination of SOD, CAT, GSH, GPx, and MDAAt the end of the 96-h exposure, 200 mL of treated or control algae were harvested by centrifugation (5 000×g for 10 min) (TGL 20M, Kaida Scientific Instruments Co. Ltd., China) at 4 ℃. The pellet was rinsed twice with double distilled water. Subsequently, the pellet was re-suspended in 2 mL of phosphate buffer (PBS, pH 7.8, 0.1 mol/L). Using the ultrasonic cell disruptor (power 120 W, intermittent 5 s, working 5 s) (JY92-Ⅱ, Ningbo Xingzhi Biotechnology Co. Ltd., China), P. tricornutum and S. costatum were sonicated in an ice bath for 15 min and 30 min, respectively. The sonicated suspension was then centrifuged at 10 000×g for 10 min at 4 ℃. The supernatant was used for the determination of SOD, CAT, GSH, GPx and MDA using a series of testing kits (serial numbers of A001, A007, A006, A005, and A003, Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions. The absorbance of five chromogenic reactions was read at 550, 405, 420, 412, and 532 nm, respectively, using a UV-vis spectrophotometer (TU-1800, Persee, China). The level of each biochemical parameter was calculated based on the difference in absorbance between the sample tube and the blank tube. The results of SOD, CAT, GPx were expressed as U/106 cells. The results of GSH and MDA were expressed as mg/106 cells and nmol/106 cells, respectively. One SOD unit was defined as the enzyme amount necessary to the inhibition rate of 50% in the 1mL reaction solution. One CAT unit was defined as the enzyme amount that transformed 1 μmol of H2O2 per second. One GPx unit was defined as the enzyme amount that decreased the GSH concentration in the reaction system by 1 μmol/L per minute (excluding the effect of non-enzymatic reaction).
2.5 Determination of chlorophyll contentChlorophylls were extracted using 90% (v/v) acetone at 4 ℃ and analyzed according to the protocol of Jeffrey and Humphrey (1975). Briefly, 5 mL of algae cultures were centrifuged at 4 000×g, 5 mL of 90% (v/v) acetone was added to the pellet which was then incubated at 4 ℃ for 24 h, before being centrifuged for 15 min at 4 000×g. The absorbance values of the supernatant were measured at 664 and 630 nm in a UV/vis spectrophotometer (TU-1800, Persee, China). Chlorophyll a (Chl a) and chlorophyll c (Chl c1+c2) contents were calculated using the following formulae and expressed as mg of chlorophyll per 106 cells,
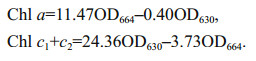
Data were expressed as the mean±standard deviation (SD) (n=4). Statistical analysis was performed by one-way ANOVA using the SPSS 18.0 software package. Experimental means were considered significantly different from the controls at a level of P < 0.05.
3 RESULT 3.1 Algal growth and 96-h EC50Under the treatment concentrations designed, the growth of two diatoms was obviously inhibited by 1-PEA which was shown to increase with treatment time and the concentration of 1-PEA (Fig. 2). At the end of 96 h exposure time, the cell density of P. tricornutum in the highest concentration group (33.88×104 cells/mL) was only 23% that of the control group (144.63×104 cells/mL). Also, the specific growth rate (r) of the microalgae decreased significantly in the treatment group at 1-PEA concentrations of more than 185 mg/L, whilst the density doubling time (Td) was significantly prolonged (Table 1). After exposure for 96 h, the cell density of S. costatum in the highest concentration group (36.25×104 cells/mL) was only 32% that of the control group (114.88×104 cells/mL). The r value decreased significantly and the Td value was significantly prolonged in the treatment group at 1-PEA concentrations of more than 136 mg/L(Table 1). The 96-h EC50 values of P. tricornutum and S. costatum were 257.14 mg/L and 126.46 mg/L respectively (Table 2).
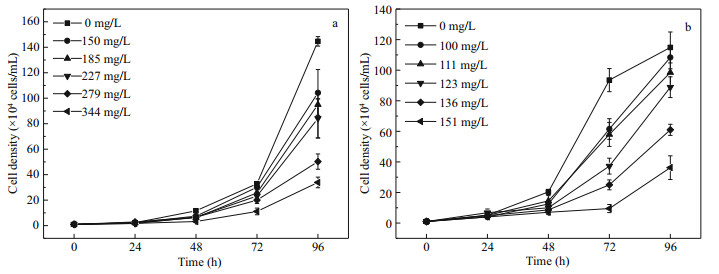
|
| Fig.2 Growth curves of P. tricornutum (a) and S. costatum (b) cultured in media containing different 1-PEA concentrations after 96-h exposure |

|
The contents of Chl a were 8.42 and 13.35 times higher than Chl c in diatoms P. tricornutum and S. costatum respectively, without exposure to 1-PEA. However, in all treatments, P. tricornutum did not show an evident change in both Chl a and Chl c contents after 96-h exposure (Fig. 3a). In contrast, a marked decline in the two chlorophylls of S. costatum (17% and 58% of those in the controls, P < 0.05) was observed for 1-PEA treatments of ≥111 mg/L. Chl a content was also shown to decrease with increasing concentration of 1-PEA (Fig. 3b).
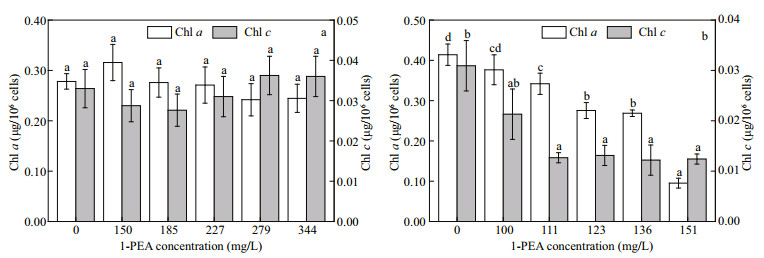
|
| Fig.3 Contents of Chl a and Chl c in P. tricornutum (a) and S. costatum (b) at different 1-PEA concentrations after 96 h exposure Data are expressed as the mean±SD of four independent experiments. Those with different letters on error bar are significantly different (P < 0.05). |
The activities of three antioxidant enzymes (SOD, CAT, and GPx) and GSH content were evaluated in diatom cells after exposure to 1-PEA for 96 h (Fig. 4). When exposed to lower concentrations of 1-PEA (between 150 and 227 mg/L), all four parameters in P. tricornutum did not show significant differences compared to controls, while a significant increase was observed at concentrations of 279 mg/L and 344 mg/L of 1-PEA (114% and 152% for SOD, 251% and 758% for CAT, 245% and 358% for GPx, 215% and 346% for GSH, P < 0.05) (Fig. 4a, c, e, g).
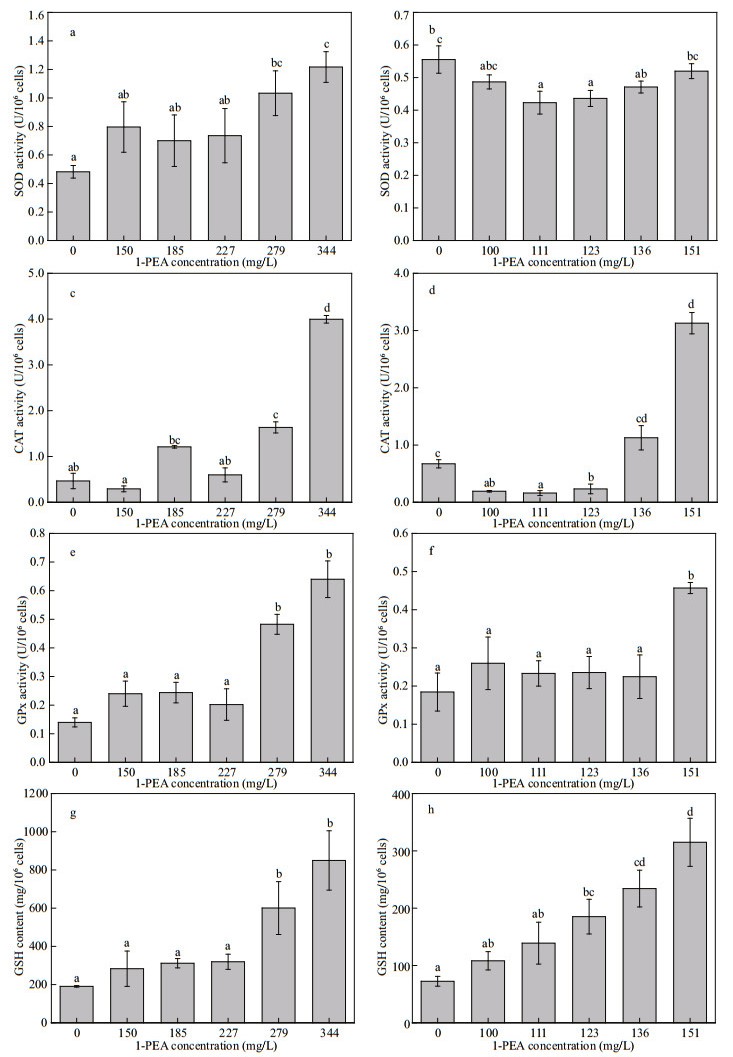
|
| Fig.4 Effects of 1-PEA on SOD, CAT, GPx, and GSH in P. tricornutum (a, c, e, g) and S. costatum (b, d, f, h) after 96 h exposure Data are expressed as the mean±SD of four independent experiments. Those with different letters on error bar are significantly different (P < 0.05). |
In S. costatum exposed to 1-PEA, the four parameters showed differential trends in response. SOD and CAT showed an evident decrease in activity (P < 0.05) when algal cells were exposed to concentrations between 111 and 136 mg/L, and between 100 and 123 mg/L of 1-PEA, respectively. This was followed by a recovery or strong induction (365% for CAT, P < 0.05) in their activities at the highest concentration (151 mg/L). GPx activity showed a significant increase compared to control (148%, P < 0.05) at 151 mg/L of 1-PEA. GSH levels in all treatment groups (100–151 mg/L) increased after 96-h exposure, which was significant (P < 0.05) when algal cells were exposed to 1-PEA at 123 mg/L or more (Fig. 4b, d, f, h).
3.4 Lipid peroxidationThe MDA level in P. tricornutum was significantly induced in the treatment group at 344 mg/L, with an increment of 172% (P < 0.05) (Fig. 5a). In contrast, there was a significant decrease in the MDA content (50%, P < 0.05) in S. costatum exposed to the highest concentration (151 mg/L) of 1-PEA (Fig. 5b).

|
| Fig.5 MDA levels in P. tricornutum (a) and S. costatum (b) after 96 h exposure to 1-PEA Data are expressed as the mean±SD of four independent experiments. Those with different letters on error bar are significantly different (P < 0.05). |
The result presented in this study suggested that 1-PEA is toxic to the two marine diatoms and therefore has the potential to threaten marine ecosystems. During the growth inhibition tests for 96 h, P. tricornutum and S. costatum showed different sensitivity to 1-PEA, with 96-h EC50 values of 257.14 mg/L and 126.46 mg/L, respectively. In comparison with other organic chemicals (Table 3), 1-PEA showed an inhibitory effect on the growth of P. tricornutum which is similar to two aromatic compounds (salicylic acid and paracetamol), and one alcohol compound (triethanolamine). In addition, 1-PEA was shown to have a higher toxic effect than perfluorooctanoic acid (PFOA, an alcohol compound which has been used extensively in industry and consumer products). For S. costatum, 1-PEA was less toxic than most of the aromatic compounds (with a range of 72 h or 96-h EC50 of 9.65–78 mg/L) except for chlorobenzene but had a higher level of inhibition compared to aliphatic compounds such as 1, 2-dichloroethane, tetrachloroethylene, dichloromethane. Generally, the toxicity of 1-PEA to P. tricornutum is lower than those of the majority of organic pollutants, while toxicity in S. costatum was found to lie between that of aromatic and aliphatic compounds.

|
Organic xenobiotics-induced toxicity in aquatic organisms is considered to be partly attributed to the excess accumulation of ROS, such as superoxide anion (O2·−) and hydroxyl radicals (·OH), as well as non-radical molecules including hydrogen peroxide (H2O2) and singlet oxygen (1O2) (Ames et al., 1993; Livingstone, 2001). ROS may impair the structure and intended function of biological macromolecules such as carbohydrates, proteins and nucleic acids due to their high reactivity (Blokhina and Fagerstedt, 2010). During normal cellular metabolism, ROS can be effectively controlled within a low to moderate level by the antioxidant defense systems including non-enzymatic scavengers (such as GSH) and antioxidant enzymes (such as SOD, CAT, and GPx). Amongst the antioxidant enzymes, SOD is considered the only eukaryotic antioxidant enzyme can disproportionate O2·− to H2O2 which is subsequently metabolized by CAT into H2O and O2. GPx catalyzes the reduction of both H2O2 and hydroperoxides to H2O and alcohols, respectively, at the expense of GSH (Ali et al., 2005; Roy et al., 2005; Van Raamsdonk and Hekimi, 2012). The imbalance between the production and elimination of ROS in living organisms under adverse environmental conditions is often called "oxidative stress" (Davies, 1995). As a final product formed during lipid peroxidation caused by oxidative stress, MDA levels can be used as an important index of ROS-dependent tissue damage (Valavanidis et al., 2006).
Our results indicate that both enzymatic and non-enzymatic antioxidants levels changed dramatically in diatom cells exposed to 1-PEA for 96 h with significant differences observed between the two species. For P. tricornutum, when the 1-PEA concentrations were higher than 96-h EC50 value (i.e. 279 and 344 mg/L), significant increases in SOD, CAT and GPx activities and GSH levels (Fig. 4a, c, e, g) were observed, suggesting the production of O2·−, H2O2 and hydroperoxides had increased to promote stress-related antioxidant responses. However, the antioxidant responses were not sufficient to prevent oxidative damage at a 1-PEA concentration of 344 mg/L, as evidenced by a high level of MDA (Fig. 5a).
Michiels et al. (1994) proposed that the sensitivity of cells to ROS is dependent on the equilibrium between the formation of H2O2 from O2·− in the dismutation reaction catalyzed by SOD, and its degradation by GPx and CAT, rather than depending on the activities of individual antioxidant enzymes. SOD/(CAT+GPx) ratio was applied in some studies (Sahoo and Chainy, 1997). In this ratio, SOD can consume O2·− to form H2O2, while CAT+GPx indicates the consumption of H2O2. Ruas et al. (2008) used this ratio to reflect the balances between the production and the removal of free radicals in the blood of three cichlid fish. However, there have been few studies on the response of SOD/(CAT+GPx) ratio to contaminants in microalgae. According to our results (Table 4), the SOD/(CAT+GPx) ratio generally decreased with increasing 1-PEA concentration with the lowest value of 0.26 detected at 344 mg/L. The relatively low SOD activity might lead to insufficient neutralization of O2·−, resulting in the generation of ·OH by the reaction of O2·−+2e-→·OH. It has been reported that •OH is the most dangerous free radical in cells which attacks lipid membranes to initiate the most devastating effects of oxidative stress, i. e. membrane lipid peroxidation (Lobo et al., 2010). This may account for the phenomenon that a significant elevation of MDA occurred and the enzymatic and non-enzymatic antioxidants in P. tricornutum were strongly induced. Therefore, the balanced functioning between SOD, CAT, and GPx is very important for proper neutralization of continuously generated ROS.

|
In S. costatum, an obvious stimulation in GPx activity and GSH content appeared at higher concentrations of 1-PEA. However, unlike P. tricornutum, SOD and CAT activities were inhibited at relatively low concentrations of pollutant (111–136 mg/L and 100–123 mg/L, respectively) followed by a rise at high pollutant concentrations, reaching a level close to that of the control or higher. The decreased activities of SOD and CAT may be due to the inhibition of their syntheses in this species induced by low levels of 1-PEA. When 1-PEA concentrations reached 136 and 151 mg/L, the SOD/(CAT+GPx) ratio dropped to only 0.35 and 0.14, respectively (Table 4), indicating the generation and accumulation of ·OH because of the weak activity of SOD. In addition, the MDA level showed a decrease rather than an increase under this condition. Similar results have been previously reported by other investigators. In a study into the antioxidant responses of different micro-algal species to nonylphenol (NP)-induced oxidative stress, MDA content declined gradually with increasing NP concentrations, particularly in Chlorella vulgaris and Chlorella sp. (Gao et al., 2017). These observations may be explained by three possible reasons. Firstly, the antioxidant defense system could efficiently scavenge ROS and thus prevent lipid peroxidation in cells exposed to pollutants (Gao et al., 2017). Secondly, peroxidation is occurring but producing other end products outside of MDA (Janero, 1990; Esterbauer et al., 1991) that are not detected by the analytical method used for MDA measurements (i.e. TBARS method). In a study by de Zwart et al. (1997), using gas chromatography with electron capture detection, eight lipid peroxidation degradation products (e.g., acetaldehyde, butanal, and hexanal) were simultaneously detected in the urine of rats treated with carbon tetrachloride. Thirdly, MDA, generating from peroxidation due to 1-PEA exposure, may crosslink DNA and protein in algal cells resulting in a decline of its level. According to some medical reports, MDA is an endogenous crosslinking agent that crosslinks DNA via two exo-cyclic guanine amino groups (Niedernhofer et al., 2003) and is also one of the most significant sources of endogenous DNA-protein crosslinks (Voitkun and Zhitkovich, 1999). The first case is incompatible with severe growth inhibition and chlorophyll decreases at a 1-PEA concentration of 151 mg/L (Figs. 2, 3, and Table 1). In contrast, the latter two mechanisms can explain the damage in algal cells exposed to 1-PEA at higher concentrations, supporting the presence of lipid peroxidation induced by 1-PEA.
Chl a and Chl c are important pigments in the lighth-arvesting complex in diatom chloroplasts. Of these, Chl a can transfer photons to the reaction center (P680) in PSⅡ, and Chl c is the accessory pigment that absorbs light in the blue-green region and transfers energy to Chl a (Veith and Büchel, 2007; Yamano et al., 2018). Therefore, they can be used to indicate the effects of pollutants on the photosynthetic activity and growth status of diatoms. Chl a and Chl c in S. costatum showed high sensitivity to 1-PEA compared to those in P. tricornutum, in which the contents decreased significantly even at concentrations as low as 111 mg/L (Fig. 3). These data indicated that the chlorophyll molecules in S. costatum are vulnerable to the effects of ROS resulting from the metabolism of 1-PEA. This may be because MDA easily accumulates in the chloroplasts of this species, and then crosslinks with proteins and nucleic acids to form insoluble compounds such as lipofuscin which lead to the alterations in the integrity and function of proteins, enzymes and DNA molecules as well as the leakage of electrolytes (Chen, 1991). Environmental pollutants such as benzene, toluene, ethylbenzene, and xylenes have been reported to stimulate lipofuscin formation in Euglena gracilis (Euglenophyta), accompanied by a decline in algal chlorophyll (Cheng et al., 2013, 2015). Compared to Chl c which has a C-C single bond instead of C=C double bond, the Chl a molecule is vulnerable to the attack by ROS as it contains more unsaturated double bonds (Fawley, 1989) which may explain the more obvious decline in Chl a content observed at most of the concentrations of 1-PEA.
5 CONCLUSION1-PEA was shown to significantly inhibit the growth of two diatoms. Correspondingly, two diatoms were shown to have differential biochemical responses to 1-PEA exposure. In P. tricornutum, all the four antioxidant parameters were not induced until 1-PEA concentration was close to or greater than the 96-h EC50 value. However, the activities of SOD and CAT, and the synthesis of two chlorophylls were inhibited in S. costatum exposed to 1-PEA at the 96-h EC50 value. Based on this study, the discharge of 1-PEA in coastal areas has the potential to raise ecological risks due to adverse effects on marine organisms like diatoms. Further investigations of growth inhibition and oxidative stress in two diatoms over long-term exposure exposed to 1-PEA are required to provide long-term effects of 1-PEA on marine organisms.
6 DATA AVAILABILITY STATEMENTThe data generated or analyzed during the current study are available from the corresponding author on reasonable request.
Ali M B, Yu K W, Hahn E J, Paek K Y. 2005. Differential responses of anti-oxidants enzymes, lipoxygenase activity, Ascorbate content and the production of saponins in tissue-cultured root of mountain Panax ginseng C. A. Mayer and Panax quinquefolium L. in bioreactor subjected to methyl jasmonate stress. Plant Science, 169(1): 83-92.
|
Ames B N, Shigenaga M K, Hagen T M. 1993. Oxidants, antioxidants, and the degenerative diseases of aging. Proceedings of the National Academy of Sciences of the United States of America, 90(17): 7 915-7 922.
DOI:10.1073/pnas.90.17.7915 |
Api A M, Belsito D, Botelho D, et al. 2017. RIFM fragrance ingredient safety assessment, α-methylbenzyl alcohol, CAS registry number 98-85-1. Food and Chemical Toxicology, 110: S569-S576.
DOI:10.1016/j.fct.2017.09.045 |
Bester K, Hühnerfuss H, Lange W, Rimkus G G, Theobald N. 1998. Results of non target screening of lipophilic organic pollutants in the German Bight Ⅱ:polycyclic musk fragrances. Water Research, 32(6): 1 857-1 863.
DOI:10.1016/S0043-1354(97)00424-7 |
Blokhina O, Fagerstedt K V. 2010. Reactive oxygen species and nitric oxide in plant mitochondria:origin and redundant regulatory systems. Physiologia Plantarum, 138(4): 447-462.
|
Brelsford R. 2018. CNOOC-Shell JV commission Nanhai ethylene expansion. (2018-05-02). https://www.ogj.com/articles/2018/05/cnooc-shell-jv-commission-nanhai-ethylene-expansion.html.
|
Brown R. 2000. Dow to build world-scale POSM plant. (2000-07-16). https://www.icis.com/resources/news/2000/07/17/117555/dow-to-build-world-scale-posm-plant/.
|
Chen S Y. 1991. Injury of membrane lipid peroxidation to plant cell. Plant Physiology Communications, 27(2): 84-90.
|
Cheng P, Arthur D M, Sichani H T, Xia Q, Ng J C. 2013. Assessing benzene-induced toxicity on wild type Euglena gracilis Z and its mutant strain SMZ. Chemosphere, 93(10): 2 381-2 389.
DOI:10.1016/j.chemosphere.2013.08.037 |
Cheng P, Lee J W, Sichani H T, Ng J C. 2015. Toxic effects of individual and combined effects of BTEX on Euglena gracilis. Journal of Hazardous Materials, 284: 10-18.
DOI:10.1016/j.jhazmat.2014.10.024 |
Claessens M, Vanhaecke L, Wille K, Janssen C R. 2013. Janssen C R. Emerging contaminants in Belgian marine waters:Single toxicant and mixture risks of pharmaceuticals. Marine Pollution Bulletin, 71(1-2): 41-50.
|
Dao L, Grigoryeva T, Laikov A, Devjatijarov R, Ilinskaya O. 2014. Full-scale bioreactor pretreatment of highly toxic wastewater from styrene and propylene oxide production. Ecotoxicology and Environmental Safety, 108: 195-202.
DOI:10.1016/j.ecoenv.2014.07.012 |
Davies K J A. 1995. Oxidative stress:the paradox of aerobic life. Biochemical Society Symposia, 61: 1-31.
DOI:10.1042/bss0610001 |
De Zwart L L, Venhorst J, Groot M, Commandeur J N M, Hermanns R C A, Meerman J H N, Van Baar B L M, Vermeulen N P E. 1997. Simultaneous determination of eight lipid peroxidation degradation products in urine of rats treated with carbon tetrachloride using gas chromatography with electron-capture detection. Journal of Chromatography B:Biomedical Sciences and Applications, 694(2): 277-287.
DOI:10.1016/S0378-4347(97)00144-8 |
Duan W Y, Meng F P, Lin Y F, Wang G S. 2017. Toxicological effects of phenol on four marine microalgae. Environmental Toxicology and Pharmacology, 52: 170-176.
DOI:10.1016/j.etap.2017.04.006 |
ECOTOX. 2018a. ECOTOXicology database: nitrobenzen.Environmental Protection Agency, U. S. https://cfpub.epa.gov/ecotox/search.cfm.
|
ECOTOX. 2018b. ECOTOXicology database: tetrachloroethylene. Environmental Protection Agency, U. S. https://cfpub.epa.gov/ecotox/search.cfm.
|
ECOTOX. 2018c. ECOTOXicology database: dichloromethane. Environmental Protection Agency, U.S. https://cfpub.epa.gov/ecotox/search.cfm.
|
ECOTOX. 2018d. ECOTOXicology database: 2-butanone. Environmental Protection Agency, U. S. https://cfpub.epa.gov/ecotox/search.cfm.
|
EN International Organization for Standardization (EN ISO). 2016. ISO 10253: 2016. Water quality-marine algal growth inhibition test with Skeletonema sp. and Phaeodactylum tricornutum.
|
Engelhardt G. 2006. In vivo micronucleus test in mice with 1-phenylethanol. Archives of Toxicology, 80(12): 868-872.
DOI:10.1007/s00204-006-0122-0 |
Esterbauer H, Schaur R J, Zollner H. 1991. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radical Biology and Medicine, 11(1): 81-128.
|
European Chemicals Agency (ECHA). 2017. Brief Profile: 1-phenylethanol. (2017-09-09). https://echa.europa.eu/brief-profile/-/briefprofile/100.002.461.
|
Everchem. 2017. Repsol licenses POSM technology repsol licences PO and styrene technology to Tianjin Bohua.(2017-01-10). https://everchem.com/repsol-licensesposm-technology.
|
Fawley M W. 1989. A new form of chlorophyll c involved in light-harvesting. Plant Physiology, 91(2): 727-732.
DOI:10.1104/pp.91.2.727 |
Finney D J. 1971. Probit Analysis. 3rd edn. Cambridge University Press, Cambridge.
|
Gao Q T, Wong Y S, Tam N F. 2017. Antioxidant responses of different microalgal species to nonylphenol-induced oxidative stress. Journal of Applied Phycology, 29(3): 1 317-1 329.
DOI:10.1007/s10811-017-1065-y |
Gatermann R, Biselli S, Hühnerfuss H, Rimkus G G, Hecker M, Karbe L. 2002. Synthetic musks in the environment.Part 1:species-dependent bioaccumulation of polycyclic and nitro musk fragrances in freshwater fish and mussels. Archives of Environmental Contamination and Toxicology, 42(4): 437-446.
DOI:10.1007/s00244-001-0041-2 |
Hörnström E. 1990. Toxicity test with algae-a discussion on the batch method. Ecotoxicology and Environmental Safety, 20(3): 343-353.
DOI:10.1016/0147-6513(90)90011-S |
Janero D R. 1990. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radical Biology and Medicine, 9(6): 515-540.
DOI:10.1016/0891-5849(90)90131-2 |
Jeffrey S W, Humphrey G F. 1975. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochemie Und Physiologie der Pflanzen, 167(2): 191-194.
DOI:10.1016/S0015-3796(17)30778-3 |
Lemmer S, Klomp R, Ruemekorf R, Scholz R. 2001. Preconcentration of wastewater through the Niro freeze concentration process. Chemical Engineering & Technology, 24(5): 485-488.
|
Libralato G, Ghirardini A V, Avezzù F. 2010. Seawater ecotoxicity of monoethanolamine, diethanolamine and triethanolamine. Journal of Hazardous Materials, 176(1-3): 535-539.
DOI:10.1016/j.jhazmat.2009.11.062 |
Livingstone D R. 2001. Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Marine Pollution Bulletin, 42(8): 656-666.
DOI:10.1016/S0025-326X(01)00060-1 |
Lobo V, Patil A, Phatak A, Chandra N. 2010. Free radicals, antioxidants and functional foods:impact on human health. Pharmacognosy Reviews, 4(8): 118.
DOI:10.4103/0973-7847.70902 |
Michiels C, Raes M, Toussaint O, Remacle J. 1994. Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radical Biology and Medicine, 17(3): 235-248.
DOI:10.1016/0891-5849(94)90079-5 |
Morelli E, Scarano G. 2004. Copper-induced changes of non-protein thiols and antioxidant enzymes in the marine microalga Phaeodactylum tricornutum. Plant Science, 167(2): 289-296.
DOI:10.1016/j.plantsci.2004.04.001 |
Nakata H, Sasaki H, Takemura A, Yoshioka M, Tanabe S, Kannan K. 2007. Bioaccumulation, temporal trend, and geographical distribution of synthetic musks in the marine environment. Environmental Science & Technology, 41(7): 2 216-2 222.
|
Nakata H. 2005. Occurrence of synthetic musk fragrances in marine mammals and sharks from Japanese coastal waters. Environmental Science & Technology, 39(10): 3 430-3 434.
|
Nie X P, Liu B Y, Yu H J, Liu W Q, Yang Y F. 2013. Toxic effects of erythromycin, ciprofloxacin and sulfamethoxazole exposure to the antioxidant system in Pseudokirchneriella subcapitata. Environmental Pollution, 172: 23-32.
DOI:10.1016/j.envpol.2012.08.013 |
Niedernhofer L J, Daniels J S, Rouzer C A, Greene R E, Marnett L J. 2003. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. Journal of Biological Chemistry, 278(33): 31 426-31 433.
DOI:10.1074/jbc.M212549200 |
Pavlić Ž, Vidaković-Cifrek Ž, Puntarić D. 2005. Toxicity of surfactants to green microalgae Pseudokirchneriella subcapitata and Scenedesmus subspicatus and to marine diatoms Phaeodactylum tricornutum and Skeletonema costatum. Chemosphere, 61(8): 1 061-1 068.
DOI:10.1016/j.chemosphere.2005.03.051 |
Pesticide Action Network (PAN). 2018a. PAN Pesticide Database: 3, 4-Dichloraniline. Pesticide Action Network North America, US. http://www.pesticideinfo.org/List_AquireAll.jsp?Rec_Id=PRI567&Taxa_Group=Phytoplankton.
|
Pesticide Action Network (PAN). 2018b. PAN Pesticide Database: Styrene. Pesticide Action Network North America, US. http://www.pesticideinfo.org/List_AquireAll.jsp?Rec_Id=PRI36223&Taxa_Group=Phytoplankton.
|
Rimkus G G, Wolf M. 1995. Nitro musk fragrances in biota from freshwater and marine environment. Chemosphere, 30(4): 641-651.
DOI:10.1016/0045-6535(94)00430-3 |
Roy G, Sarma B K, Phadnis P P, Mugesh G. 2005. Selenium-containing enzymes in mammals:Chemical perspectives. Journal of Chemical Sciences, 117(4): 287-303.
DOI:10.1007/BF02708441 |
Ruas C B G, Carvalho C S D, De Araújo H S S, Espíndola E L G, Fernandes M N. 2008. Oxidative stress biomarkers of exposure in the blood of cichlid species from a metal-contaminated river. Ecotoxicology and Environmental Safety, 71(1): 86-93.
DOI:10.1016/j.ecoenv.2007.08.018 |
Sabatini S E, Juárez A B, Eppis M R, Bianchi L, Luquet C M, De Molina M D C R. 2009. Oxidative stress and antioxidant defenses in two green microalgae exposed to copper. Ecotoxicology and Environmental Safety, 72(4): 1 200-1 206.
DOI:10.1016/j.ecoenv.2009.01.003 |
Sahoo A, Chainy G B N. 1997. Alterations in the activities of cerebral antioxidant enzymes of rat are related to aging. International Journal of Developmental Neuroscience, 15(8): 939-948.
DOI:10.1016/S0736-5748(97)00049-X |
SIDS Initial Assessment Profile (SIAM). 2003. OECD Existing Chemicals Database. https://hpvchemicals.oecd.org/UI/handler.axd?id=7f6b4807-5217-4626-b47c-e139327a412b.
|
Sigma-Aldrich. 2012. Material Safety Data Sheet: 1-Phenylethanol. https://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=CN&language=EN-generic&productNumber=P13800&brand=ALDRICH&PageToGoToURL=https%3A%2F%2Fwww.igmaaldrich.com%2Fcatalog%2Fsearch%3Fterm%3D98-85-1%26interface%3DCAS%2520No.%26N%3D0%26mode%3Dmatch%2520partialmax%26lang%3Dzh-%26region%3DCN%26focus%3Dproduct.
|
Toxicology Data Network (TOXNET). 2018a. Hazardous Substances Data Bank: 1, 2-dichloroethane. U.S. National Library of Medicine. https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/f?./temp/~oAmG1L:1.
|
Toxicology Data Network (TOXNET). 2018b. Hazardous Substances Data Bank: 1, 1-dichloroethylene. U.S.National Library of Medicine. https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/f?./temp/~C9RvhX:1.
|
U.S. Environmental Protection Agency (USEPA). 1980.Ambient Water Quality Criteria for Chlorinated Benzenes.(EPA-440/5-80-028).
|
U.S. Environmental Protection Agency (USEPA). 2012.OCSPP 850.4500: Algal Toxicity (EPA 712-C-006).
|
Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M. 2006. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicology and Environmental Safety, 64(2): 178-189.
DOI:10.1016/j.ecoenv.2005.03.013 |
Van Raamsdonk J M, Hekimi S. 2012. Superoxide dismutase is dispensable for normal animal lifespan. Proceedings of the National Academy of Sciences of Sciences of the United States of America, 109(15): 5 785-5 790.
DOI:10.1073/pnas.1116158109 |
Veith T, Büchel C. 2007. The monomeric photosystem Ⅰ-complex of the diatom Phaeodactylum tricornutum binds specific fucoxanthin chlorophyll proteins (FCPs) as light-harvesting complexes. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1767(12): 1 428-1 435.
DOI:10.1016/j.bbabio.2007.09.004 |
Voitkun V, Zhitkovich A. 1999. Analysis of DNA-protein crosslinking activity of malondialdehyde in vitro. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis, 424(1-2): 97-106.
DOI:10.1016/S0027-5107(99)00011-1 |
Wang H Y, Zeng X B, Guo S Y, Li Z T. 2008. Effects of magnetic field on the antioxidant defense system of recirculation-cultured Chlorella vulgaris. Bioelectromagnetics, 29(1): 39-46.
DOI:10.1002/(ISSN)1521-186X |
World Health Organization (WHO). 1987. Environmental Health Criteria. 71: Pentachlorophenol. World Health Organization, Geneva. 460p.
|
Yamano N, Mizoguchi T, Fujii R. 2018. The pH-dependent photophysical properties of chlorophyll-c bound to the light-harvesting complex from a diatom, Chaetoceros calcitrans. Journal of Photochemistry and Photobiology A:Chemistry, 358: 379-385.
DOI:10.1016/j.jphotochem.2017.09.047 |
Zhou J Y, Duan S S. 2016. Joint toxic effect of perfluorooctanoic acid and perfluorononanoic acid on two marine algae. Ecological Science, 35(6): 84-90.
(in Chinese with English abstract) |
Zhou J, Yang C Y, Wang J H, Sun P C, Fan P, Tian K, Liu S J, Xia C H. 2013. Toxic effects of environment-friendly antifoulant nonivamide on Phaeodactylum tricornutum. Environmental Toxicology and Chemistry, 32(4): 802-809.
DOI:10.1002/etc.2132 |
 2019, Vol. 37
2019, Vol. 37



