Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LIU Song, LI Bing, CHEN Xiaolin, QIN Yukun, LI Pengcheng
- Effect of polysaccharide from Enteromorpha prolifera on maize seedlings under NaCl stress
- Journal of Oceanology and Limnology, 37(4): 1372-1381
- http://dx.doi.org/10.1007/s00343-019-8150-9
Article History
- Received May. 18, 2018
- accepted in principle Aug. 22, 2018
- accepted for publication Oct. 23, 2018
2 Laboratory for Marine Drugs and Bioproducts, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266000, China;
3 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266000, China;
4 Marine Science and Engineering College, Qingdao Agriculture University, Qingdao 266000, China
Due to climate change, the incidence of abiotic stresses has been increased in recent years. On the other hand, the impact of intensive agriculture becomes another main reason that can result in unfavorable conditions for the growth of crop plants. Abiotic stresses account for considerable losses in crop production around the world. Among the abiotic stressors, salt stress is one of the most serious abiotic stresses that can lead to the reduction of agricultural productivity (Zhu, 2000; Wang et al., 2003; Ruiz-Lozano et al., 2012; Battacharyya et al., 2015). Compared with normal growth conditions, high salt concentration can inhibit photosynthesis, destroy the metabolic balance and cellular structures, and directly made crop yield reduce (Munns and Tester, 2008; Zhang et al., 2017a). At present, there are different approaches to response to a salt stress in order to increase the crop yield including development salinealkali-resistant crops, improvement physical and chemical properties of soil and application of exogenous biostimulators (Sharp et al., 1984; Mekhedov and Kende, 1996; Liu et al., 2015). In addition, the utilization of biostimulators was proved an effective and easy way to enhance salt tolerance of plants. Presently, there are different kinds of biostimulators such as salicylic acid, plant harmones, Ca2+, and polysaccharides or oligosaccharides (Abdel-Basset, 1998; Pacholczak et al., 2016; Zhang et al., 2017b, b; Mejía-Espejel et al., 2018; Glosek-Sobieraj et al., 2018). Recently, seaweeds polysaccharides or oligosaccharides such as fucoidan, sodium alginate, and carrageenan have been proved to elicit saltresistant activity for crops (Klarzynski et al., 2003; Luan et al., 2009; Bi et al., 2011). However, although polysaccharides from Enteromorpha prolifera have been shown many biological activities such as antioxidant, immunomodulation, hypoglycaemic, hypolipidemic, antitumor, anti-aging, and antibacterial effects (Wang et al., 1995; Xu et al., 2005; Jiao et al., 2010; Li et al., 2013; Lü et al., 2014), studies in the literature on the agricultural utilization of them are scarce.
Enteromorpha prolifera is an important edible and medicinal alga and widely distributes in China, particularly in the eastern coastal area (Lü et al., 2014). In recent years, it has frequently bloomed in coastal areas in Qingdao, China and caused ecological problems, such as destruction of marine ecosystems, a threat to coastal fisheries, tourism development, obstruction the channel, etc. At present, E . prolifera in Qingdao has been commonly utilized to make fertilizer for plants. However, it was not clear whether EP could promote or stress the growth of plants. Therefore, in this paper, EP was prepared and the components and structure were determined. Then, we investigated its effect on maize seedlings under a NaCl stress. Diff erent parameters of maize seedlings such as membrane permeability, and adjustment of osmotic substances such as soluble protein, soluble, and proline, antioxidant enzymes containing superoxide dismutase (SOD), catalase (CAT), peroxidase (POD), and ascorbate peroxidase (APX)were determined individually. This study could provide a scientifi c basis for developing new biostimulators for plants.
2 MATERIAL AND METHOD 2.1 Preparation of EPEnteromorpha prolifera was collected from the First Bathing Beach of Qingdao, China, in 2011. The alga was washed with tap water, air-dried, and ground into powder. Dry alga powder (100 g) was heated in stirring in distilled water (7 500 mL) at 90℃ for 4 h, fi ltered with a 300-mesh sieve, and the hot liquid supernatant was fi ltered with diatomite. The solution was concentrated by rotary evaporation; and the concentrated liquid was dialyzed against tap water and distilled water for 48 h, respectively. In addition, the solution was concentrated to about 1 000 mL under a reduced pressure. Then, 4 000-mL ethanol was added to the solution to precipitate the polysaccharide. The precipitation was lyophilized to yield a white powdered product that was named EP.
Total carbohydrate content was analyzed with phenol-sulfuric acid method using rhamnose as the standard for EP (Dubois et al., 1956). The sulfated content was measured by the barium chloride gelatin method (Kawai et al., 1969). The molar ratios of monosaccharide composition were measured in reference to Zhang et al. (2009). All monosaccharide reagents were obtained from Sigma Aldrich (St Louis, MO, USA). Fourier Transform Infrared Spectroscopy (FT-IR) of EP was characterized by a Nicolet-360 FTIR spectrometer (360 scans, at a resolution of 6 cm-1)from 400 to 4 000 cm-1. The dried EP sample was mixed with potassium bromide (KBr) and pressed into pellets for measurement (Song et al., 2016).
2.2 Plant material and treatmentsMaize (Brassica rapa L.) seeds were surface sterilized with 1% sodium hypochlorite solution. Then, they were cleaned with tap water. After the seeds germinated at 25℃ for 48 h in the dark, good germination seeds were transplanted into Petri dishes (5 cm in diameter). Then Hoagland solution was put into the dishes and the seeds were cultured in a light growth chamber at 28/22℃ and 14 h:10 h light/dark scheme. The relative humidity was 75%, and the strength of illumination was 400 μmol/(m2·s). After the first leaf fully grew up, Hoagland solution was replaced by 1/2 Hoagland solution. When the second leaf of every seed fully developed, the maize seedlings were randomLy divided into four groups in triplicate (CK (control), with 1/2 Hoagland), NaCl (1/2 Hoagland+100 mmol/L NaCl), EP (1/2 Hoagland +1 mg/mL EP), and EP+NaCl (1/2 Hoagland+100 mmol/L NaCl+1 mg/mL EP). Due to the high Mw of EP, we dissolved it in 60℃ water. The nutrient solution was renewed every other day. After 10 days of treatment, the leaves of maize seedlings were used to measure the physiological parameters.
2.3 Growth parametersAfter treatment, maize seedlings of each group were harvested for determination of shoot length and rootstock length. Dry weight was determined after the samples was dried at 105℃ for 2 h.
2.4 Influence on membrane permeabilityThe infl uence on membrane permeability was expressed by relative conductivity (L). The leaves were rinsed with deionized water and cut into 1-mm piece at the same part of the leaves. Put the pieces into tubes respectively, and add some deionized water into the tube. The tube was vacuumed for 15 min. Then the air was slowly put in so that leaves were in the bottom of the tubes. The plug was covered and the tube was placed for 1 h. The conductivity (S1) was determined. Then, every tube was sealed tightly and placed in the boiling water for 15 min. When the tube was cooled down into room temperature, the conductivity (S2) was determined, and L=S1/S2.
2.5 Soluble protein, soluble sugar, and proline contentsThe content of soluble protein was determined by Bradford method (Bradford, 1976). The content of soluble sugar was measured by the anthrone method (Sánchez et al., 1998). The proline content was measured by the ninhydrin acid reagent method (Bates, 1973). The L-proline used was standard.
2.6 Lipid peroxidation degreesMalondialdehyde (MDA) content was determined by thiobarbituric acid (TBA) reaction method reported by Heath and Packer (Heath and Packer, 1968). Samples (0.2 g) were homogenized in 10% trichloroacetic acid (TCA) and centrifuged for 10 min at 4 000×g . An amount of 2-mL supernatant was mixed with the same volume of 0.6%-TBA and bathed in boiling water for 30 min. The mixture was cooled immediately afterwards. Next, the cooled reaction liquid was centrifuged at 10 000×g for 15 min and the supernatant was used to determine the MDA content.
2.7 Antioxidant enzyme activitiesThe second fully-grown leaf samples (0.2 g) of maize seedlings were homogenized in liquid nitrogen and it was used for enzyme extraction. Superoxide dismutase (SOD) activity was measured according to the Beauchamp method (Beauchamp and Fridovich, 1971). Catalase (CAT) activity of the leaf samples was measured according to the method of Lu et al. (2007) at 240 nm with a UV spectrophotometer. Peroxidase (POD) activity was followed by the method used by Seckin et al. (2009). The absorbance values at 470 nm were read to determine the POD activities. Ascorbate peroxidase (APX) was measured according to the procedure of Dzung et al. (2011). The APX activity was calculated from the decline in absorbance at 290 nm.
2.8 Chlorophyll contentsAfter 10-day treatment with NaCl, the contents of chlorophyll a (Chl a ), chlorophyll b (Chl b ), and the total chlorophyll (Chl a +Chl b ) of the seedlings were determined by the method of Mittal et al. (2012).
2.9 Statistical analysisAll data were expressed in means±standard deviation (SD). ANOVA was utilized to evaluate statistical diff erences among the experimental groups; P < 0.05 was considered statistically signifi cant diff erence.
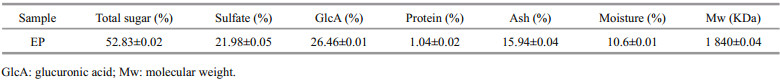
3 RESULT AND DISCUSSION 3.1 Preparation of EPThe yield of EP from E . prolifera was 18.75%. The chemical components of EP are given in Table 1. As shown in Table 1, EP is a sulfated polysaccharide with total sugar 52.83%, sulfate 21.98%, GlcA 26.46%, and few proteins (1.04%). The ash content was 15.94%, which might be due to metal ion such as Na+, K+ or Mg2+ that combined with sulfate in EP (Michalak et al., 2015, 2016, 2017). Moreover, the molecular weight (Mw) of EP is 1 840 KDa, which is higher than that reported by Xu et al. (2015) due to different extraction methods. Monosaccharide composition analysis (Table 2) reveals that rhamnose is the major sugar in the EP, which is agreeable with that reported by Cho et al. (2010). However, the content of glucose is higher, which might be due to different harvested locations.
The FT-IR spectrum of EP is shown in Fig. 1. Typical signals of polysaccharide at about 3 400, 2 934, 1 642, 1 408, 1 248, and 1 039 cm-1 are clear. The absorption peaks at 3 400 and 2 934 cm-1 correspond to O-H stretching vibrations and C-H stretching vibrations, respectively. The peaks of 1 642 and 1 408 cm-1 refl ect the carbonyl C=O antisymmetric and symmetric vibrations in the uronic acid of EP. The 1 248-cm-1 spike belongs to S=O asymmetric stretching vibration of sulfate group and that of 1 039 cm -1 to C-O-H in glucosidal bond or C-O-C stretching vibrations in the ring. In addition, the EP shows a band at about 800 cm-1 indicating a symmetrical C-O-S vibration. These results show that the EP was a sulfated polysaccharide.
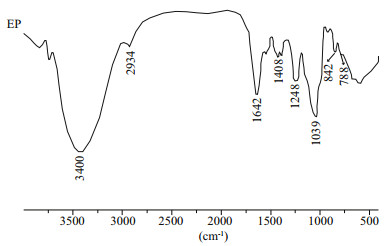
|
| Fig.1 FTIR of EP |
As shown in Fig. 2, compared with CK, EP could increase the plant height by 8.05%, rootstock height by 21.07%, dry weight of leaves by 2.41%, and weight of roots by 17.49%. Treatment in 100-mmol/L NaCl inhibited significantly the growth of maize seedlings by decreasing plant height, rootstock height, dry weight of leaves, and dry weight of roots. However, when EP was utilized for maize, it could obviously alleviate salt stress on maize by increasing plant height, rootstock height, dry weight of leaves, and dry weight of roots.

|
| Fig.2 Influence of EP on plant height (a), rootstock height (b), the dry weight of leaves (c), and dry weight of roots (d) of maize seedlings The means designed with different letters are significantly different at P < 0.05. |
The roots and leaves of a plant are the weights of roots and leaves, which are important indices to the growth of maize seedling and stress tolerance. Therefore, we calculated the roots/leaves of maize seedlings with different treatments as shown in Table 3. Without NaCl stress, EP could boost the roots/leaves of maize seedlings by 14.69% compared with the control. NaCl treatment significantly reduced the roots/leaves of maize seedlings, which showed that the effect of NaCl on roots was greater than that on leaves. The use of EP could reduce the reduction of roots/leaves. From the above-mentioned results of maize seedling growth and biomass accumulation, EP had the effect of alleviating the negative effect of NaCl stress on maize growth, so we inferred that EP could enhance the water absorption of roots to reduce the growth inhibition of salt stress.
As shown in Fig. 3, the membrane permeability of maize leaves of the EP group was 97% of the control, which demonstrated that EP did no harm to the crop. When 100-mmol/L NaCl was utilized, the membrane system was seriously destroyed and the membrane permeability increased by 78.9% compared to the control. While EP and NaCl were used, the membrane permeability increased only by 52.9%. Commonly, the membrane system was the main site of plant damaged by salt. The damage degree of the membrane for the plant could be refl ected by the membrane permeability. The value of the membrane permeability was greater, showing that the membrane damage of the plant was greater. When cell membrane was destroyed, a large number of intracellular organic matters leaked. The membrane permeability could be determined by measuring the leakage rate of intracellular organic matters, which could refl ect the damage degree of the membrane system (Rodriguez et al., 1997; Volkmar et al., 1998). Therefore, these results show that EP could harm the leaf system of maize but reduce the salt stress on the cell permeability in the role of a protective film system.
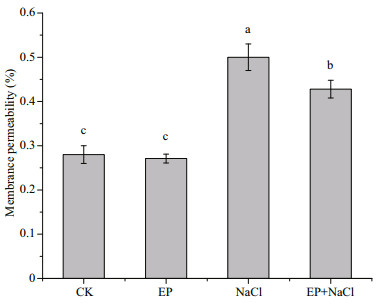
|
| Fig.3 Influence of EP on membrane permeability of maize seedlings The means designed with different letters are significantly different at P < 0.05. |
As shown in Fig. 4, when EP was used to culture maize, the soluble protein content, soluble sugar content and proline content of the maize seedling leave obviously increased by 33.3%, 26.9%, and 12.8% respectively, compared to the control. When maize was treated with 100-mmol/L NaCl, all the indices were boosted. However, when EP and NaCl were simultaneously utilized, the contents of soluble protein, soluble sugar, and proline were increased additionally by 15.3%, 9.8%, and 33.7%, indicating that these matters played as osmotic agents that could improve osmotic adjustment of maize seedling leaves under a salt stress. Having reported previously, soluble protein, soluble sugar, and proline are important osmotic matters in plant cells, and their contents can aff ect directly the osmotic potential of plant cells; higher concentration of them could maintain lower osmotic potential and help plant resist against the damage brought by salt stress, and maintain the membrane integrity and protein stability (Khatkar and Kuhad, 2000; Szabados and Savouré, 2010; Farhangi-Abriz and Torabian, 2017). Our results are in agreement with these studies and it is indicated that EP could regulate the contents of compatible solutes of maize seedling leaves under a salt stress.
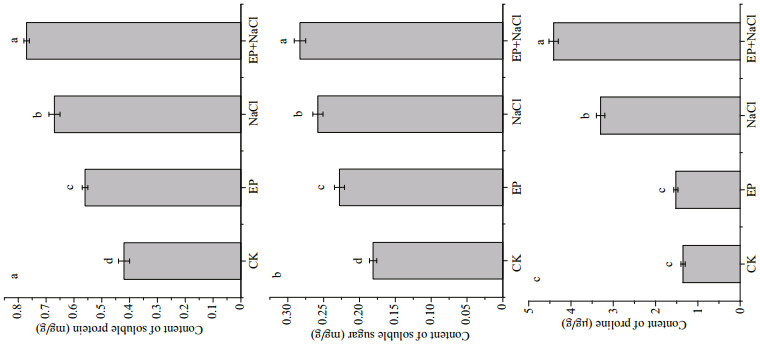
|
| Fig.4 Effects of EP on soluble protein, soluble sugar, and proline contents in maize seedling leaves The means designed with different letters are significantly different at P < 0.05. |
In Fig. 5, the addition of EP for the culture of maize did not aff ect the content of MDA. While in NaCltreated maize, the content of MDA of the maize seedling leaves was significantly increased (by 1.2 times compared to CK). While EP was also utilized, the content of MDA decreased obviously compared with NaCl (by 15.6%). The salt stress could lead to membrane impairment in plants and mostly mediated through membrane lipid peroxidation such as O2- ∙, H2O2, and ∙OH, etc. Lipid peroxidation could cause the increase of MDA content. The accumulation of MDA in plant cells could destroy membrane protein and cause intracellular ROS metabolism imbalance. Therefore, it could lead to membrane damage and thus inhibit the growth of plants (Meloni et al., 2003; Gunes et al., 2007). Our results indicated that the use of EP did not cause a lipid peroxidation damage of seedling membrane but reduce the accumulation of MDA under the salt stress in the role of a protective film system.
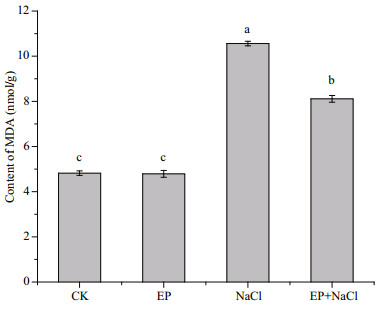
|
| Fig.5 Effect of EP on the content of MDA in maize seedling leaves The means designed with different letters are significantly different at P < 0.05. |
In a salt-stress environment, the plant can produce a large number of ROS and free radicals, which may injure cell membrane structure and intracellular biological macromolecules. Therefore, the scavenging ability of ROS and free radicals is an important parameter of plant salt tolerance. There are two types of antioxidant protection systems for scavenging ROS and free radicals in the plant. One is the enzyme reaction protection system such as SOD, CAT, POD, and APX, etc., and another is the non-enzymatic reaction protection system such as ascorbic acid and reduced glutathione. The enzyme reaction protection system plays an important role in scavenging ROS and free radicals in the plant. Thus, SOD, CAT, POD, and APX are commonly determined to characterize the salt stress of plant (Sudhakar et al., 2001; Foyer and Noctor, 2005).
SOD is a metal enzyme in plants and could disproportionate oxygen-free radicals. It cooperates with other oxygen-free radical scavenging enzymes to protect the cell membrane system and avoid damage by ROS and free radicals. Figure 6a shows the effect of EP on SOD of maize seedlings under the salt stress. Without NaCl stress, the SOD activity of leaves treated with 1 mg/mL EP did not change significantly compared to CK; and 100 mmol/L NaCl treatment reduced significantly SOD activity by 44.6% compared with CK. For EP and NaCl cotreatment groups, SOD activity decreased by 25.5% only. Thus, EP can inhibit the decrease of SOD activity under a salt stress and reduce the oxidative damage to cell membrane of maize seedling leaves.
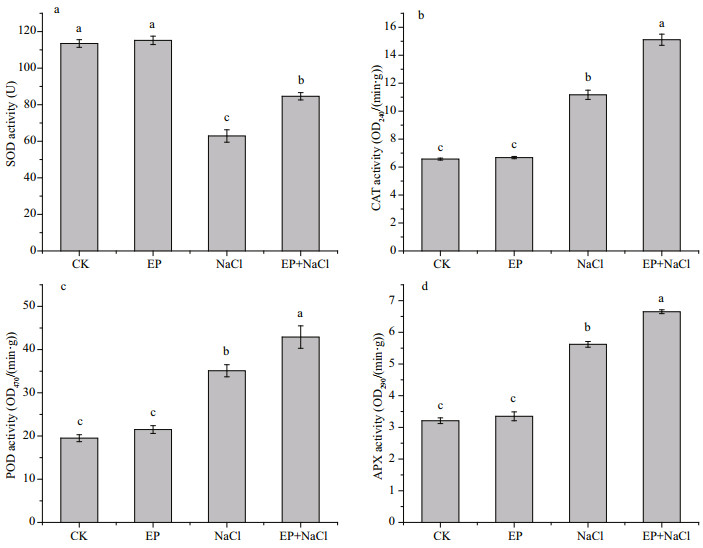
|
| Fig.6 Effects of EP on SOD (a), CAT (b), POD (c), and APX (d) in maize seedling leaves The means designed with different letters are significantly different at P<0.05. |
CAT is also one of the important enzymes for active oxygen scavenging systems in plant and can specially remove H2O2. Similar to the role of SOD, the EP treatment did not increase the activity of CAT compared with CK. For 100 mmol/L NaCl stress, the CAT of maize seedling leaves improved by 70% compared with CK, but CAT increased by 2.3 times compared with CK, which is obviously higher than that of NaCl stress. Therefore, EP could increase CAT activity in the plant, and enhance H2O2 scavenging ability in maize seedling leaves.
POD also played a crucial role in ROS detoxifi cation especially for reducing membrane lipid peroxidation and protecting the membrane integrity of plants. Treatment of 1 mg/mL EP increased CAT activity by 10% compared with CK. Treatment of 100 mmol/L NaCl improved the POD activity for 1.8 times, but the treatment of NaCl+EP improved the POD activity for 2.2 times. These results show that EP could enhance the activity of POD in maize seedling leaves regardless of the presence of NaCl stress, and it could enhance the scavenging ability of ROS in plants and protect the membrane.
APX is an important component of the active oxygen scavenging enzyme system and it is mainly present in plant chloroplasts and cytoplasm. Ascorbic acid is the main electron donor for the specifi c peroxidase (Mittler, 2002). The results are similar to ours of SOD and CAT. The EP treatment did not change the APX activity in maize seedling leaves compared with CK. In the treatment of 100 mmol/L NaCl, adding EP could significantly increase the APX activity by 18.4% compared with that of sole NaCl stress. Thus, EP could improve the ROS scavenging capacity by increasing the APX activity of plants.
3.7 Chlorophyll contentsFigure 7 shows the infl uence on contents of chlorophyll including Chl a and b in different treatments. The results indicate that the 1-mg/mL EP treatment significantly increased the contents of Chl a and b by 20.8% and 9.8%, respectively compared with CK. Treatment of 100-mmol/L NaCl obviously reduced the contents of Chl a and b to 46.3% and 65.6% of the control, respectively. However, when EP and NaCl were co-used, the reduction in the contents of chlorophyll was alleviated significantly. Therefore, EP could improve the contents of Chl a and Chl b and reduce the chlorophyll degradation caused by the salt stress, and alleviate the growth inhibition by improving photosynthesis of maize seedlings.
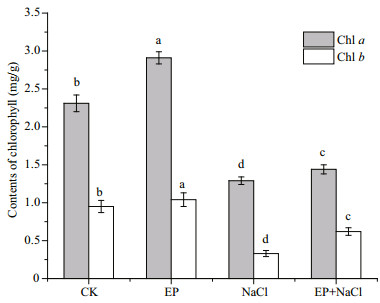
|
| Fig.7 Influence on contents of chlorophyll with different treatment The means designed with different letter are significantly different at P<0.05. |
Alga polysaccharides feature many important biological activities against plant disease (Jaulneau et al., 2010) and abiotic stresses. Sodium alginate has been proved that it could increase survival rate, chlorophyll content, and SOD activity so did the adaptability of Helianthus annuus L. seedling to a salt stress (Yang and Guo, 2010). Similarly, sodium alginate could increase salt-tolerance adaptability if Raphanus sativus seedlings and relief the harm from NaCl stress (Yang and Guo, 2011). Zou et al. (2018)showed that polysaccharide could protect wheat seedlings against salt stress by regulating antioxidant enzyme activities and modulating intracellular ion concentration. In this paper, we have indicated that EP could increase the resistance to salt stress not only by regulating SOD, CAT, POD, and APX activities but also by regulating membrane permeability. However, the specifi c mechanism needs further research.
4 CONCLUSIONThe polysaccharide extract of Enteromorpha prolifera is a sulfated polysaccharide, and its molecular weight is high (1 840 KDa); thus it is a hereopolysaccharide and the main monosaccharide is rhamnose. Moreover, the extract could promote the growth of maize seedlings under salt stress and able to increase salt-tolerance by regulating membrane permeability, adjustment of osmotic substances, and antioxidant enzymes activities. Therefore, the extract can be explored as a salt-resistance substance for plants.
5 DATA AVAILABILITY STATEMENTThe datasets in the current study are not publicly available due to author request. It can be available from the corresponding author on reasonable request.
Abdel-Basset R. 1998. Calcium channels and membrane disorders induced by drought stress in Vicia faba plants supplemented with calcium. Acta Physiol. Plant., 20(2): 149-153.
DOI:10.1007/s11738-998-0006-4 |
Bates L S. 1973. Rapid determination of free proline for waterstress studies. Plant Soil, 39(1): 205-207.
DOI:10.1007/BF00018060 |
Battacharyya D, Babgohari M Z, Rathor P, Prithiviraj B. 2015. Seaweed extracts as biostimulants in horticulture. Sci.Hortic., 196: 39-48.
DOI:10.1016/j.scienta.2015.09.012 |
Beauchamp C, Fridovich I. 1971. Superoxide dismutase:improved assays and an assay applicable to acrylamide gels. Anal. Biochem., 44(1): 276-287.
|
Bi F, Iqbal S, Arman M, Ali A, Hassan M U. 2011. Carrageenan as an elicitor of induced secondary metabolites and its effects on various growth characters of chickpea and maize plants. J. Saudi Chem. Soc., 15(3): 269-273.
DOI:10.1016/j.jscs.2010.10.003 |
Bradford M M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72(1-2): 248-254.
DOI:10.1016/0003-2697(76)90527-3 |
Cho M, Yang C, Kim Y S, You S G. 2010. Molecular characterization and biological activities of watersoluble sulfated polysaccharides from Enteromorpha prolifera. Food Sci. Biotechnol., 19(2): 525-533.
DOI:10.1007/s10068-010-0073-3 |
DuBois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem., 28(3): 350-356.
DOI:10.1021/ac60111a017 |
Dzung N A, Khanh V T P, Dzung T T. 2011. Research on impact of chitosan oligomers on biophysical characteristics, growth, development and drought resistance of coffee. Carbohydr. Polym., 84(2): 751-755.
DOI:10.1016/j.carbpol.2010.07.066 |
Farhangi-Abriz S, Torabian S. 2017. Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotox. Environ. Saf., 137: 64-70.
DOI:10.1016/j.ecoenv.2016.11.029 |
Foyer C H, Noctor G. 2005. Oxidant and antioxidant signalling in plants:a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ., 28(8): 1 056-1 071.
DOI:10.1111/pce.2005.28.issue-8 |
Glosek-Sobieraj M, Cwalina-Ambroziak B, Hamouz K. 2018. The effect of growth regulators and a biostimulator on the health status, yield and yield components of potatoes(Solanum tuberosum L.). Gesunde Pflanz., 70(1): 1-11.
DOI:10.1007/s10343-017-0407-7 |
Gunes A, Inal A, Alpaslan M, Eraslan F, Bagci E G, Cicek N. 2007. Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.)grown under salinity. J. Plant Physiol., 164(6): 728-736.
DOI:10.1016/j.jplph.2005.12.009 |
Heath R L, Packer L. 1968. Photoperoxidation in isolated chloroplasts:I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys., 125(1): 189-198.
DOI:10.1016/0003-9861(68)90654-1 |
Jaulneau V, Lafitte C, Jacquet C, Fournier S, Salamagne S, Briand X, Esquerré-Tugayé M T, Dumas B. 2010. Ulvan, a sulfated polysaccharide from green algae, activates plant immunity through the jasmonic acid signaling pathway. J. Biomed. Biotechnol., 2010: 525 291.
DOI:10.1155/2010/525291 |
Jiao L L, Jiang P, Zhang L P, Wu M J. 2010. Antitumor and immunomodulating activity of polysaccharides from Enteromorpha intestinalis. Biotechnol. Bioproc. Eng., 15: 421-428.
DOI:10.1007/s12257-008-0269-z |
Kawai Y, Seno N, Anno K. 1969. A modified method for chondrosulfatase assay. Anal. Biochem., 32(2): 314-321.
|
Khatkar D, Kuhad M S. 2000. Short-term salinity induced changes in two wheat cultivars at different growth stages. Biol. Plant., 43(4): 629-632.
DOI:10.1023/A:1002868519779 |
Klarzynski O, Descamps V, Plesse B, Yvin J C, Kloareg B, Fritig B. 2003. Sulfated fucan oligosaccharides elicit defense responses in tobacco and local and systemic resistance against tobacco mosaic virus. Mol. Plant Microbe Int., 16(2): 115-122.
DOI:10.1094/MPMI.2003.16.2.115 |
Li B, Liu S, Xing R E, Li K C, Li R F, Qin Y K, Wang X Q, Wei Z H, Li P C. 2013. Degradation of sulfated polysaccharides from Enteromorpha prolifera and their antioxidant activities. Carbohydr. Polym., 92(2): 1 991-1 996.
DOI:10.1016/j.carbpol.2012.11.088 |
Liu L P, Long X H, Shao H B, Liu Z P, Tao Y, Zhou Q S, Zong J Q. 2015. Ameliorants improve saline-alkaline soils on a large scale in northern Jiangsu Province, China. Ecol.Eng., 81: 328-334.
DOI:10.1016/j.ecoleng.2015.04.032 |
Lü H T, Gao Y J, Shan H, Lin Y T. 2014. Preparation and antibacterial activity studies of degraded polysaccharide selenide from Enteromorpha prolifera. Carbohydr.Polym., 107: 98-102.
DOI:10.1016/j.carbpol.2014.02.045 |
Lu Z Q, Liu D L, Liu S K. 2007. Two rice cytosolic ascorbate peroxidases differentially improve salt tolerance in transgenic Arabidopsis. Plant Cell Rep., 26(10): 1 909-1 917.
DOI:10.1007/s00299-007-0395-7 |
Luan L Q, Nagasawa N, Ha V T T, Hien N Q, Nakanishi T M. 2009. Enhancement of plant growth stimulation activity of irradiated alginate by fractionation. Radiat. Phys.Chem., 78(9): 796-799.
DOI:10.1016/j.radphyschem.2009.05.001 |
Mejía-Espejel L, Robledo-Paz A, Lozoya-Gloria E, PeñaValdivia C B, Carrillo-Salazar J A. 2018. Elicitors on steviosides production in Stevia rebaudiana Bertoni calli. Sci. Hortic., 242: 95-102.
DOI:10.1016/j.scienta.2018.07.023 |
Mekhedov S L, Kende H. 1996. Submergence enhances expression of a gene encoding 1-aminocyclopropane-1-carboxylate oxidase in deepwater rice. Plant Cell Physiol., 37(4): 531-537.
DOI:10.1093/oxfordjournals.pcp.a028976 |
Meloni D A, Oliva M A, Martinez C A, Cambraia J. 2003. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot., 49(1): 69-76.
DOI:10.1016/S0098-8472(02)00058-8 |
Michalak I, Dmytryk A, Śmieszek A, Marycz K. 2017. Chemical characterization of Enteromorpha prolifera extract obtained by enzyme-assisted extraction and its influence on the metabolic activity of Caco-2. Int. J. Mol.Sci., 18(3): 479.
DOI:10.3390/ijms18030479 |
Michalak I, Górka B, Wieczorek P P, Rój E, Lipok J, Łęska B, Messyasz B, Wilk R, Schroeder G, Dobrzyńska-Inger A, Chojnacka K. 2016. Supercritical fluid extraction of algae enhances levels of biologically active compounds promoting plant growth. Eur. J. Phycol., 51(3): 243-252.
DOI:10.1080/09670262.2015.1134813 |
Michalak I, Tuhy Ł, Chojnacka K. 2015. Seaweed extract by microwave assisted extraction as plant growth biostimulant. Open Chem., 13(1): 1 183-1 195.
|
Mittal S, Kumari N, Sharma V. 2012. Differential response of salt stress on Brassica juncea:photosynthetic performance, pigment, proline, D1 and antioxidant enzymes. Plant Physiol. Biochem., 54: 17-26.
DOI:10.1016/j.plaphy.2012.02.003 |
Mittler R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci., 7(9): 405-410.
DOI:10.1016/S1360-1385(02)02312-9 |
Munns R, Tester M. 2008. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol., 59: 651-681.
DOI:10.1146/annurev.arplant.59.032607.092911 |
Pacholczak A, Nowakowska K, Mika N, Borkowska M. 2016. The effect of the biostimulator Goteo on the rooting of ninebark stem cuttings. Folia Hortic., 28(2): 109-116.
DOI:10.1515/fhort-2016-0013 |
Rodriguez H G, Roberts J K M, Jordan W R, Drew M C. 1997. Growth, water relations, and accumulation of organic and inorganic solutes in roots of maize seedlings during salt stress. Plant Physiol., 113(3): 881-893.
DOI:10.1104/pp.113.3.881 |
Ruiz-Lozano J M, Porcel R, Azcón C, Aroca R. 2012. Regulation by arbuscular mycorrhizae of the integrated physiological response to salinity in plants:new challenges in physiological and molecular studies. J. Exp.Bot., 63(11): 4 033-4 044.
DOI:10.1093/jxb/ers126 |
Sánchez F J, Manzanares M, De Andres E F, Tenorio J L, Ayerbe L. 1998. Turgor maintenance, osmotic adjustment and soluble sugar and proline accumulation in 49 pea cultivars in response to water stress. Field Crops Res., 59(3): 225-235.
DOI:10.1016/S0378-4290(98)00125-7 |
Seckin B, Sekmen A H, Türkan I. 2009. An enhancing effect of exogenous mannitol on the antioxidant enzyme activities in roots of wheat under salt stress. J. Plant Growth Regul., 28(1): 12-20.
|
Sharp J K, Valent B, Albersheim P. 1984. Purification and partial characterization of a β-glucan fragment that elicits phytoalexin accumulation in soybean. J. Biol. Chem., 259(18): 11 312-11 320.
|
Song L, Chen X L, Liu X D, Zhang F B, Hu L F, Yue Y, Li K C, Li P C. 2016. Characterization and comparison of the structural features, immune-modulatory and anti-avian influenza virus activities conferred by three algal sulfated polysaccharides. Mar. Drugs, 14(1): 4.
|
Sudhakar C, Lakshmi A, Giridarakumar S. 2001. Changes in the antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci., 161(3): 613-619.
DOI:10.1016/S0168-9452(01)00450-2 |
Szabados L, Savouré A. 2010. Proline:a multifunctional amino acid. Trends Plant Sci., 15(2): 89-97.
|
Volkmar K M, Hu Y, Steppuhn H. 1998. Physiological responses of plants to salinity:a review. Can. J. Plant Sci., 78(1): 19-27.
DOI:10.4141/P97-020 |
Wang Z L, Pote J, Huang B R. 2003. Responses of cytokinins, antioxidant enzymes, and lipid peroxidation in shoots of creeping bentgrass to high root-zone temperatures. J. Am.Soc.. Hortic. Sci., 128: 648-655.
DOI:10.21273/JASHS.128.5.0648 |
Xu D L, Huang X C, Ou C R, Xue C H, Yang W G, Wang H H. 2005. In vitro study on polysaccharides in Enteromorpha with non-specific immunity. Food Sci., 26: 232-235.
|
Xu J, Xu L L, Zhou Q W, Hao S X, Zhou T, Xie H J. 2015. Isolation, purification, and antioxidant activities of degraded polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol., 81: 1 026-1 030.
DOI:10.1016/j.ijbiomac.2015.09.055 |
Yang X L, Guo J Y. 2010. Effect of sodium alginate on H. annuus L. seedling to salt-tolerance. Northern Hortic, (23): 37-39.
(in Chinese with English abstract) |
Yang X L, Guo Y D. 2011. Effect of sodium alginate on Raphanus sativus L. seedlings in adaptation to salttolerance. Chin. Veget., 1(2): 81-84.
(in Chinese with English abstract) |
Zhang J J, Zhang Q B, Wang J, Shi X L, Zhang Z S. 2009. Analysis of the monosaccharide composition of fucoidan by precolumn derivation HPLC. Chin. J. Oceanol.Limnol., 27(3): 578-582.
DOI:10.1007/s00343-009-9205-0 |
Zhang X Q, Li K C, Liu S, Zou P, Xing R E, Yu H H, Chen X L, Qin Y K, Li P C. 2017a. Relationship between the degree of polymerization of chitooligomers and their activity affecting the growth of wheat seedlings under salt stress. J. Agric. Food Chem., 65(2): 501-509.
DOI:10.1021/acs.jafc.6b03665 |
Zhang X Q, Li K C, Xing R E, Liu S, Chen X L, Yang H Y, Li P C. 2017b. miRNA and mRNA expression profiles reveal insight into chitosan-mediated regulation of plant growth. J. Agric. Food Chem., 66(15): 3 810-3 822.
|
Zhou H P, Jiang X T, Wang S R, Chen Q H. 1995. Effect of polysaccharide from Enteromorpha prolifera on lipemia, SOD activity and LPO content. Chin. J. Biochem. Mol.Biol., 11(2): 161-165.
(in Chinese with English abstract) |
Zhu J K. 2000. Genetic analysis of plant salt tolerance using Arabidopsis. Plant Physiol., 124(3): 941-948.
DOI:10.1104/pp.124.3.941 |
Zou P, Lu X L, Jing C L, Yuan Y, Lu Y, Zhang C S, Meng L, Zhao H T, Li Y Q. 2018. Low-molecular-weight polysaccharides from Pyropia yezoensis enhance tolerance of wheat seedlings(Triticum aestivum L.) to salt stress. Front. Plant Sci., 9: 427.
DOI:10.3389/fpls.2018.00427 |
 2019, Vol. 37
2019, Vol. 37