Institute of Oceanology, Chinese Academy of Sciences
Article Information
- Md Reaz CHAKLADER, Ashfaqun NAHAR, Md Abu HANIF, Muhammad A. B. SIDDIK
- Intraspecific morphometric diversity of barramundi (Lates calcarifer Bloch, 1790) in the waters of southern Bangladesh
- Journal of Oceanology and Limnology, 37(4): 1393-1402
- http://dx.doi.org/10.1007/s00343-019-8179-9
Article History
- Received Jun. 27, 2018
- accepted in principle Aug. 20, 2018
- accepted for publication Sep. 25, 2018
2 Department of Marine Fisheries and Oceanography, Patuakhali Science and Technology University, Patuakhali 8602, Bangladesh;
3 Department of Fisheries Biology and Genetics, Patuakhali Science and Technology University, Patuakhali 8602, Bangladesh
The study of morphometric variation among fish population has substantial signifi cance in fisheries science from various perspectives including evolution, optimization of yield, ecology, a design of biological sampling program, water resources management and conservation, and stock assessment (AnvariFar et al., 2011; Chaklader et al., 2016a; Siddik et al., 2016a, b). Investigation of phenotypic characters is very useful for demonstrating the degree of intraspecifi c variation within a population since the evolutionary adaptation of a species to its environment is most easily perceived by fish morphometry (Karakousis et al., 1991; Pinheiro et al., 2005). However, the stock structure can be used as an effective tool for the commencement of management strategies for conserving the fish species, subspecies, stocks and races (Turan et al., 2005). Understanding stock structure is a prerequisite for developing fisheries management plans as poor knowledge of stock structure can lead to a variety of problems, including, loss of genetic diversity (Smith et al., 1991), rapid changes in biological characteristics and productivity of a species (Ricker, 1981), overfishing less productive stocks (Graham, 1982), and inaccurate predictions about the stock could result in recruitment loss and reduced yields (Begg et al., 1999; Hanif et al., 2015). Therefore, over the years, many researchers have conducted a good number of studies on the stock structure of fish using the morphometric characters (Turan et al., 2006; Chaklader et al., 2015; Miyan et al., 2016; Nahar et al., 2018).
The southern coastal rivers and estuaries of Bangladesh are unique and productive ecosystem serving as feeding, breeding and nursery grounds for a variety of fish fauna (Chaklader et al., 2016b, 2018; Siddik et al., 2016d, 2017; Hanif et al., 2017a, b; Nahar et al., 2018). Lates calcarifer, a euryhaline and catadromous food fish (Kungvankij et al., 1986; Yadav, 1999) belonging to family Latidae of order Perciformes is commonly known as 'Barramundi'. It is an important coastal and estuarine fish widely distributed in Indo-West Pacifi c region from the Arabian Gulf to China, Papua New Guinea, and Northern Australia (Nelson, 1994; Yadav, 1999; Yue et al., 2009). This species is famous as a table fish for its delicate fl avored fl esh, nutritional value and for commanding a high, stable price in the Bangladeshi markets (Siddik et al., 2016c; Luna, 2018). Previously, this species was abundantly available in the estuaries of Bangladesh especially in the coastal part of the Bay of Bengal of southern Bangladesh. Nowadays, this diadromous population has experienced a drastic decline over the entire coastal region of Bangladesh possibly due to anthropogenic activities (pollution, damming, indiscriminate and illegal fishing), invasion of exotic fish, habitat loss, and even global climate change consequence (Kungvankij et al., 1986; Siddik et al., 2015).
Although multitude wave of studies have been carried out on wild and cultured population of Asian seabass in different countries to delineate genetic diversity using mitochondrial DNA sequences (Chenoweth et al., 1998; Doup et al., 1999), allozymes (Keenan, 1994), microsatellites (Yu at al., 2002; Zhu et al., 2006), there is a little information on the stock structure of Lates calcariferfrom the coastal region of Bangladesh, despite their enormous economic signifi cance. Hence, the present study was undertaken to investigate the intraspecifi c diversity in morphological characters of Lates calcarifer inhabiting in four coastal rivers of southern Bangladesh.
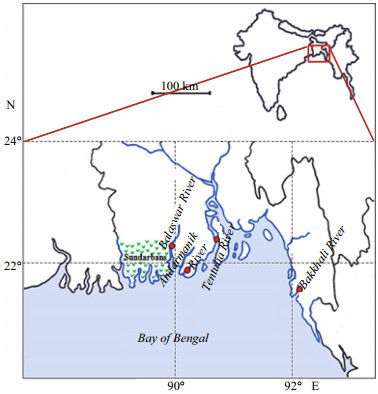
2 MATERIAL AND METHOD 2.1 Sampling of fishA total of 160 samples of Lates calcariferwere obtained from Bakkhali (n=39), Tentulia (n=38), Andarmanik (n=40), and Balaswar (n=43) rivers located in southern Bangladesh which all flow directly into the Bay of Bengal. Fifty samples were targeted at each site as Reist (1985) and Turan et al. (2006)recommended using at least 25 samples for morphological analysis. Samples were caught using commercial fishing nets and the majority of samples were captured with the assistance of municipal harvesters or management authorities. After collection, samples were kept frozen at - 20℃ for transport to the laboratory. The sample size, sampling gear, date of collection and GPS coordination are presented in Table 1 and Fig. 1.
|
|
| Fig.1 Map of the southern coastal region of Bangladesh showing four sampling rivers, the Balaswar, Andarmanik, Tentulia, and Bakkhali |
Morphometric measurements based on conventional orthogonal methods were taken on the left side of the body between the perpendicular plane and the horizontal plane on which the fish was resting, not following the curve of the body (Fig. 2). All conventional morphometric characters were measured from the undamaged de-frozen samples using a digital slide caliper to the nearest 0.1 cm and total mass was weighed using a digital balance to the nearest 0.1 g (Holčík, 1986; Quilang et al., 2007; Renaud, 2011). All measurements were taken by the same person and within one-month time to ensure similar period between death and freezing that might have the impact on the results of the study (Murta, 2000; Turan et al., 2006).

|
| Fig.2 Morphometric characters measured on L. calcarifer Variables were selected based on the studies of Holčík et al. (1986) and Renaud (2011). LT: total length; LS: standard length; LH: head length; O↓: head depth; B↓: highest body depth; K↓: lowest body depth; UI: preorbital length; EH: postorbital length; IE: eye diameter; LB: 1st pre-dorsal fin length; LM: 2nd pre-dorsal fin length; FS: 1st post-dorsal fin length; NS: 2nd post-dorsal fin length; D1↓: height of 1st dorsal fin; D22↓: height of 2nd dorsal fin; P↓: height of pectoral fin; V↓: height of venal fin; A↓: height of anal fin; BF: 1st dorsal fin base length; MN: 2nd dorsal fin base length; WX: length of pectoral base; QR: length of ventral base; YZ: length of anal base; UJ: upper jaw length; LJ: lower jaw length. |
Discriminate analysis (stepwise insertion of the variable) was applied to identify the combination of variables for separating the population of Lates calcarifer samples. In morphometrics, the infl uence of individual body size can result in heterogeneity of shape without providing information on diff erences in body proportion, and therefore size effect was normalized according to Elliott et al. (1995) equation to eliminate any variation resulting from allometric growth.
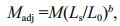
where M is the original morphometric measurement; Madj the size adjusted measurement; Ls the standard length of the fish; L0 is the overall mean of standard length for all fish from all samples in each analysis; and b estimated for each character from the observed data as the slope of the regression of log Mon log L0 using all fish from each group. In the present study, there was no signifi cant correlation between body size and morphometric measurements.
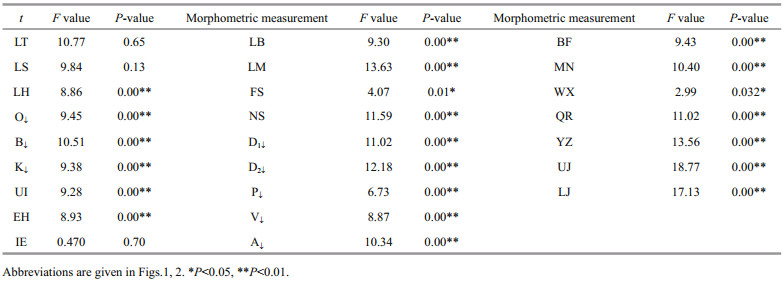
The signifi cant diff erences for each morphometric characters among the four populations were tested by univariate ANOVA. In the present study, 22 sizecorrected morphometric variables were subjected to multivariate analysis comprising discriminate function analysis (DFA), principal component analysis (PCA) and cluster analysis (CA) in order to evaluate interspecifi c morphometric variation among four locations. Principal component analysis aids to lessen morphometric data (Veasey et al., 2001), eliminate redundancy among the variables (Reist, 1985; AnvariFar et al., 2011; Mousavi-Sabet and Anvarifar, 2013) and to identify a number of influential variables for the discrimination of population (Samaee et al., 2009). Kaiser-Meyer-Olkin (KMO) measure was performed to determine whether these variables are suitable for PCA analysis, which varies between 0 and 1. It has been suggested that KMO values > 0.5 are acceptable while the value between 0.5 and 0.7 are mediocre, between 0.7 and 0.8 are good, between 0.8 and 0.9 are superb, reported by Field (2000). The KMO value in the present study was 0.96 for the overall matrix, suggesting that the data used in the study were appropriate for factor analysis.
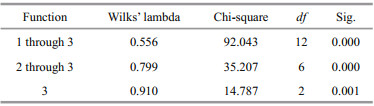
To compare the discrepancies between and among all groups, The Wilks' lambda was also performed. A cross-validation test was applied to determine the ability of the phenotypes to discriminate among populations. The divergence among samples was analyzed based on the percentage of correctly and incorrectly classifi ed fish. The percentage of correctly classifi ed (PCC) and incorrectly fish was calculated by DFA and the expected actual error rates of the classifi cation functions were estimated by a crossvalidation using PCC. The degree of intermingling among the populations was shown by the number of misclassifi ed individuals. Cluster analysis in the form of dendrograms was also performed as a complementary of PCA analysis to determine the phenotypic relationship among the examined population (Veasey et al., 2001).
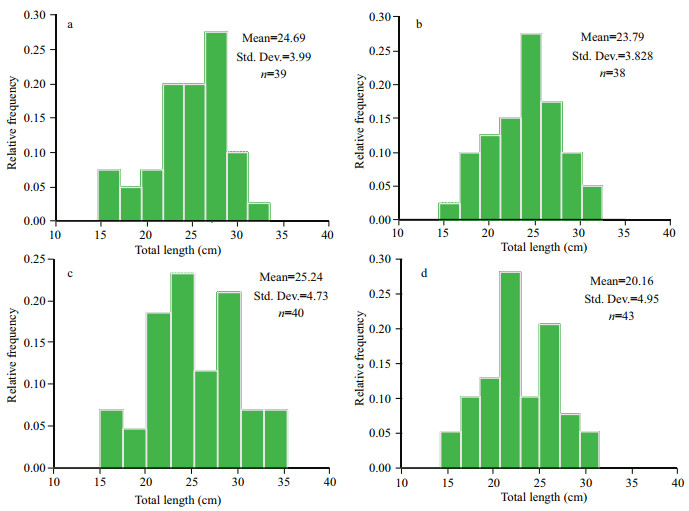
3 RESULTTwenty-two out of 25 morphometric variables revealed a signifi cant diff erence (univariate ANOVA, P < 0.001) among the four samples of L. calcarifer (Table 2). Size distribution based on total length is presented in Fig. 3 where the mean value of Balaswar is 24.69 cm (size range 15.70–32.50 cm), 23.79 cm in Andarmanick (size range 15.10–31.50 cm), 25.24 cm in Bakkhali (size range 15.70–34.40 cm) and 20.16 cm in Tentulia River (size range 15.70–30.50 cm). The Wilks' lambda test indicated highly signifi cant diff erences in all function for morphometric variables of all populations (P < 0.001) (Table 3).
|
|
| Fig.3 Total length dependent size distribution of L. calcarifer, sampled from Bakkhali (a), Tentulia (b), Andarmanick (c) and Baleswer (d) River in southern Bangladesh |
|
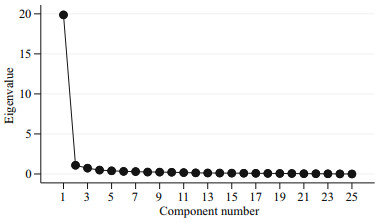
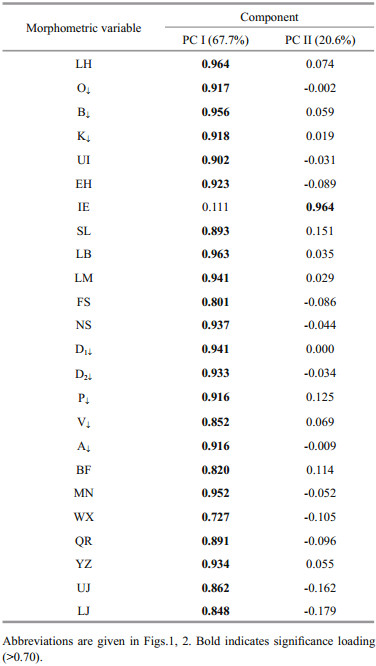
Principal component analyses (PCA) were undertaken for 22 morphometric variables and eigenvalues of greater than 1 are considered signifi cant descriptor (Fig. 4) according to Kaiser's rule. Therefore, only the first two PCs were used for this study, explaining 82.62% of the variability in the data set where first principle component (PC Ⅰ) contributed 78.03% and the second (PC Ⅱ) contributed 4.59% (Table 4). PC Ⅰ composed of LH, O↓, B↓, , K↓, , UI, EH, LB, LM, FS, NS, D1↓, , D2↓, , P↓, , V↓, , A↓, , BF, MN, WX, QR, YZ, UJ, LJ, and only IE was signifi cant on PC Ⅱ (Table 5). In the present study, variables loaded both positively and negatively with different magnitudes suggest that morphometric characters of the examined population were free from size effect. It is worthy to mention that a factor loading > 0.30 is considered signifi cant, 0.40 is considered more crucial, and 0.5 or greater is considered more very signifi cant (Nimalathasan et al., 2009). Factor loading above 0.7 was considered signifi cant in the present study (Mousavi-Sabet and Anvarifar, 2013). The visual inspection of PC Ⅰ on PC Ⅱ showed temporal variation and spatial variation forming two groups where Bakkhali River in one group and Andarmanick, Baleswar and Tentulia River in the other group (Fig. 5).
|
| Fig.4 Results of the principal component showing the scree plot for L. calcarifer based on morphometric measurement in four coastal rivers directly connected with the Bay of Bengal |

|
|
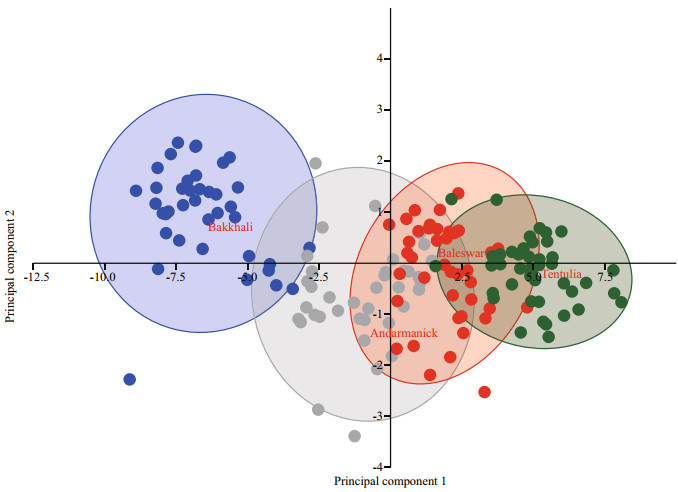
|
| Fig.5 Scatterplot of 95% confidence ellipse on the first two principal components for the four groups of L. calcarifer, based on morphometric characters |
The dendrogram derived from cluster analysis revealed the clear isolation of Bkkahlai River population from other river populations, which supports the result highlighted by principal component analysis (Fig. 6). In this analysis, the four river populations resulted in two main distinct groups: the first group comprised only Bakkhali population, while the second group included Balaswar, Tentulia, and Andarmanik populations. The strong morphometric similarity was observed between the population of Lates calcariferfrom Balaswar and Andarmanik when compared to other river populations.

|
| Fig.6 Dendrogram of the dissimilarity of populations among the four coastal rivers in southern Bangladesh based on morphometric characters |
Invalidation of morphometric discriminate analysis, 141 out of 160 (88.1%) individuals were correctly classifi ed into their original population (Table 6). The classifi cation rate varied between 83.7% and 92.3% with high reclassifi cation rate registered for Bakkhlai (92.3%) and Tentulia (92.1%)population, the most clearly isolated groups. The highest proportion of misclassifi ed individuals was evident in Baleswar (16.3%) followed by Andarmanick (15%).
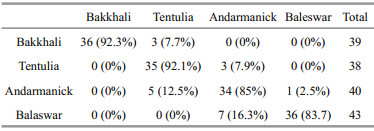
|
The results of the present study revealed that L . calcariferfrom different rivers in the southern coastal waters of Bangladesh exhibited morphometric variability forming two morphological types: Bakkhali population was separated as one group from the Andarmanick, Baleswar, and Tentulia populations, which together formed a separate group. Generally, teleost fishes show a greater degree of disparity within and between populations when compared to other vertebrates and are more prone to morphological variation induced by different environmental factors such as temperature, turbidity, habitat condition, feeding condition, pH (Wimberger, 1992). Among fish populations, notable intraspecifi c phenotypic and genetic dimorphism may have appeared due to the degree of geographic distance and limited movement among the populations, which is the basis for separation and management of distinct populations (Miyan et al., 2016; Sarker et al., 2016). Such dimorphism can be attributed by the different process such as reproductive isolation due to homing unlike spawning areas (Hourston, 1982) and hydrographic characters (tide, current, and wave) which hinder migration from one place to other (Iles and Sinclair, 1982; Mir et al., 2013). Therefore fail to understand the complexity of stock management can lead to genetic erosion and other unknown ecological consequence (Begg et al., 1999).
The result of between-group PCA analyses led to the identifi cation of two phenotypically distinct local populations. Variation of the two morphotypes was statistically confi rmed by the high percentage of correct classifi cation (88.1%). The result derived from the PCA analysis in the study was further confi rmed by the cluster analysis based on Euclidian square distance, which demonstrated that, among the four river populations, three showed overlapping and the remaining one was clearly distinct. The differentiation may suggest a relationship between the extent of phenotypic divergence and geographic distance, indicating that migration of population from Bakkhali River to Andarmanick, Baleswar, and Tentulia River may be limited. The populations of Balaswar, Tentulia, and Andarmanik were morphologically overlapped to each other in PCA, demonstrating low phenotypic divergence among the populations and geographic distance is not a barrier to migration between populations. The overlapping distribution may be enough mixing due to comprehensive migration among these locations and may support temporal and spatial residence of these populations in these areas. It may also suggest that if fish resided in a particular region for most of their life cycle, they would show lower morphometric divergence within the populations. Conversely, the marked separation of Bakkhali population indicates that there may be a resident population, self-recruiting population and sub-species of sea bass sample in Bakkhali River. Geographic structure and high salinity may be responsible for the isolation of the population. According to Miyan et al. (2016), the geographic hurdle plays a crucial role in obstructing gene flow between populations, which allow the distinction among them. In addition, Konan et al. (2010) reported a considerable morphologically different population of freshwater shrimp Macrobrachium vollenhovenii collected from Cote d'Ivoire Rivers of different geographical locations. In other words, morphometrics investigations are important to delineate various stocks over species geographical range (Tzeng, 2004; Almeida et al., 2008; Akbarzadeh et al., 2009).
Environmental features, such as biotic factors (food items and availability of foods) and abiotic factors (salinity, turbidity, alkalinity, temperatures, current pattern, and land-use pattern) infl uences the morphometric variability and homogeneity of fish (Siddik et al., 2016b) as fish phenotypes show high plasticity in response to environmental infl uences compared to other vertebrates. Environmental parameters, especially salinity, are notably different in Bakkhali (approximately 24) (Sarker et al., 2016)compared to Andarmanick (19.8), Baleswar (8.5) and Tentulia (3.5) (Dasgupta et al., 2014). These results are in agreement with the fi ndings of Boussou et al. (2010) and Quilang et al. (2007) who reported morphological variances among the species due to uncommon physicochemical parameters of the water, which were geographically close to each other. In the present study, the distinct separation of Bakkhali population with other stocks may be attributed to hydrological conditions, a wide geographical distance between them, and the selection of the sampling site. This disparity is commonly found in the morphology of fish due to the isolation of portions of a population within local habitat conditions.
These intraspecifi c morphological variations may be due to variation in body shape but not to the effect of size since it was normalized successfully by allometric transformation. Moreover, traits related to size infl uence the morphometric analysis, and errors may occur in the result if the data is not being adjusted for statistical analysis (Tzeng, 2004; Vatandoust et al., 2015). In the present study, the allometric transformation had been applied successfully to remove the effect of size and the detected signifi cant phenotypic discreteness among the populations were based on the measurements which are particularly body and eye shape when it was tested by ANOVA and multivariate analysis. Moreover, the size average of studied species in four rivers was almost similar, indicating that fishing gear was almost the same as fish samples were procured from commercial capture. Sometimes it is often quite diffi cult to explain the causes of morphological diff erences between populations (Poulet et al., 2004), but it has been suggested that genetic, environment and the interaction between them govern the morphological attributes of fish (Swain and Foote, 1999; Poulet et al., 2004; Pinheiro et al., 2005). Although genetic components may contribute to the variation in fish stock, phenotypic diversity between stocks in some circumstances may be entirely governed by environmental infl uences (Begg et al., 1999). For instance, an individual's phenotype is more infl uenced by environmental factors especially the early development stage of fish, which are of particular importance (Pinheiro et al., 2005). Morphometric characters are infl uenced by different environmental parameters well discussed by several authors in the course of fish population segregation (Swain and Foote, 1999). The size range of fish measured in the present study represents adolescent fish and hence, there is the possibility that the detected different morphotypes among the four populations of barramundi may be due to diff erences in environmental parameters.
5 CONCLUSIONThe result obtained from morphometric analysis revealed a clear separation of Bakkhali stock, suggesting the requirement of strategic assessment and management of the population separately, whereas intermingling relationship between the Balaswar, Tentulia, and Andarmanik population suggested employing uniform management strategies to use the stock sustainably for future. Further detailed research based on molecular and biochemical methods can be warranted to validate the present fi ndings unambiguously. However, in addition to morphometric studies, molecular markers based methods (e.g. mtDNA, microsatellite) would be quite useful in the delineation of the different stock structure of A. chacunda in the waters off southern Bangladesh. The fi ndings of the study will provide baseline biological information, which may be utilized to formulate scientifi cally sound fisheries management in relation to optimal harvest and monitoring strategies for the distinct stocks of Lates calcarifer populations.
6 DATA AVAILABILITY STATEMENTAlthough all data generated or analyzed during this study have been presented in the forms of fi gures and tables in this paper but are also available on reasonable request from the corresponding author.
Akbarzadeh A, Farahmand H, Shabani A A, Karami M, Kaboli M, Abbasi K, Rafiee G R. 2009. Morphological variation of the pikeperch Sander lucioperca (L.) in the southern Caspian Sea, using a truss system. Journal of Applied Ichthyology, 25(5): 576-582.
DOI:10.1111/jai.2009.25.issue-5 |
Almeida P R, Tomaz G, Andrade N O, Quintella B R. 2008. Morphological analysis of geographic variation of sea lamprey ammocoetes in Portuguese river basins. In:Dufour S, Prévost E, Rochard E, Williot P eds. Fish and Diadromy in Europe (Ecology, Management, Conservation). Springer, Dordrecht, 200: 47-59.
|
AnvariFar H, Khyabani A, Farahmand H, Vatandoust S, AnvariFar H, Jahageerdar S. 2011. Detection of morphometric differentiation between isolated up- and downstream populations of Siah Mahi (Capoeta capoeta gracilis) (Pisces:Cyprinidae) in the Tajan River (Iran). Hydrobiologia, 673(1): 41-52.
DOI:10.1007/s10750-011-0748-7 |
Begg G A, Friedland K D, Pearce J B. 1999. Stock identification and its role in stock assessment and fisheries management:an overview. Fisheries Research, 43(1-3): 1-8.
DOI:10.1016/S0165-7836(99)00062-4 |
Boussou C K, Konan F K, Edia O E, Ouattara M, Bony Y K, Ouattara A, Gourene G. 2010. Morphometric analysis of populations of Chromidotilapia guntheri (Sauvage, 1882)(Cichlidae, perciformes) in four coastal rivers of Côte d'Ivoire (West Africa). Pan-American Journal of Aquatic Sciences, 5: 387-400.
|
Chaklader M R, Siddik M A B, Hanif M A, Nahar A, Islam M A. 2018. Length-weight relationships of Acanthopagrus longispinnis (Valenciennes, 1830), Raconda russeliana(Gray, 1831) and Coilia neglecta (Whitehead, 1967) from the Bay of Bengal coast, Bangladesh. Journal of Applied Ichthyology, 34(4): 1 091-1 093.
DOI:10.1111/jai.13728 |
Chaklader M R, Siddik M A B, Hanif M A, Nahar A, Mahmud S, Piria M. 2016a. Morphometric and meristic variation of endangered pabda catfish, Ompok pabda (HamiltonBuchanan, 1822) from southern coastal waters of Bangladesh. Pakistan Journal of Zoology, 48(3): 681-687.
|
Chaklader M R, Siddik M A B, Hanif M A, Nahar A. 2016b. Size structure of finescale razorbelly minnow, Salmostoma phulo (Cyprinidae) inhabiting a coastal river of Bangladesh. Iranian Journal of Fisheries Sciences, 15(4): 1 348-1 361.
|
Chaklader M R, Siddik M A B, Nahar A. 2015. Taxonomic diversity of paradise threadfin Polynemus paradiseus(Linnaeus, 1758) inhabiting southern coastal rivers in Bangladesh. Sains Malaysiana, 44(9): 1 241-1 248.
DOI:10.17576/jsm |
Chenoweth S F, Hughes J M, Keenan C P, Lavery S. 1998. Concordance between dispersal and mitochondrial gene flow:isolation by distance in a tropical teleost, Lates calcarifer (Australian barramundi). Heredity, 80(2): 187-197.
DOI:10.1046/j.1365-2540.1998.00292.x |
Dasgupta S, Kamal F A, Khan Z H, Choudhury S, Nishat A. 2014. River Salinity and Climate Change: Evidence from Coastal Bangladesh. Policy Research working paper; no.WPS 6817. Washington, DC: World Bank Group. http://documents.worldbank.org/curated/en/522091468209055387/River-salinity-and-climate-change-evidence-from-coastalBangladesh.
|
Doup R G, Horwitz P, Lymbery A J. 1999. Mitochondrial genealogy of Western Australian barramundi:applications of inbreeding coefficients and coalescent analysis for separating temporal population processes. Journal of Fish Biology, 54(6): 1 197-1 209.
DOI:10.1111/jfb.1999.54.issue-6 |
Elliott N G, Haskard K, Koslo J A. 1995. Morphometric analysis of orange roughy (Hoplostethus atlanticus) off the continental slope of Southern Australia. Journal of Fish Biology, 46(2): 202-220.
DOI:10.1111/jfb.1995.46.issue-2 |
Field A. 2000. Discovering Statistics using SPSS for Windows.Sage, London.
|
Graham, J J.. 1982. Production of larval herring, Clupea harengus, along the Maine coast, 1964-1978. Journal of Northwest Atlantic Fishery Science, 3: 63-85.
DOI:10.2960/J.v3.a6 |
Hanif M A, Siddik M A B, Chaklader M R, Pham H D, Kleindienst R. 2017a. Length-weight relationships of three catfish species from a tributary of the Dhaleshwari River, Bangladesh. Journal of Applied Ichthyology, 33(6): 1 261-1 262.
DOI:10.1111/jai.13448 |
Hanif M A, Siddik M A B, Chaklader M R. 2015. Fish diversity in the southern coastal waters of Bangladesh:present status, threats and conservation perspectives. Croatian Journal of Fisheries, 73: 251-274.
|
Hanif M A, Siddik M A B, Nahar A, Chaklader M R, Fotedar R. 2017b. A new distribution of the Buffon's river garfish, Zenarchopterus buffonis (Valenciennes, 1847) in the southern coastal rivers of Bangladesh. Journal of Applied Ichthyology, 33(6): 1 211-1 214.
DOI:10.1111/jai.13462 |
Holčík J. 1986. The Freshwater Fishes of Europe. Vol 1:Part I, Petromyzontidae. AULA-Verlag, Wiesbaden: 117-140.
|
Hourston A S. 1982. Homing by Canada's west coast herring to management units and divisions as indicated by tag recoveries. Canadian Journal of Fisheries and Aquatic Sciences, 39(10): 1 414-1 422.
DOI:10.1139/f82-190 |
Iles T D, Sinclair M. 1982. Atlantic herring:stock discreteness and abundance. Science, 215(4533): 627-633.
DOI:10.1126/science.215.4533.627 |
Karakousis Y, Triantaphyllidis C, Economidis P S. 1991. Morphological variability among seven populations of brown trout, Salmo trutta L., in Greece. Journal of Fish Biology, 38(6): 807-817.
DOI:10.1111/jfb.1991.38.issue-6 |
Keenan C P. 1994. Recent evolution of population structure in Australian barramundi, Lates calcarifer (Bloch):an example of isolation by distance in one dimension. Australian Journal of Marine and Freshwater Research, 45(7): 1 123-1 148.
DOI:10.1071/MF9941123 |
Konan K M, Adépo-Gourène A B, Ouattara A, Nyingy W D, Gourene G. 2010. Morphometric variation among male populations of freshwater shrimp Macrobrachium vollenhovenii Herklots, 1851 from Côte d'Ivoire Rivers. Fisheries Research, 103(1-3): 1-8.
DOI:10.1016/j.fishres.2010.01.005 |
Kungvankij P, Tiro J R Jr, Pudadera B J Jr, Potestas I O. 1986.Biology and Culture of Sea Bass (Lates calcarifer).Network of Aquaculture Centres in Asia, Bangkok, Thailand. 70p.
|
Luna S. 2008. Lates calcarifer, Barramundi: fisheries, aquaculture, gamefish, aquarium. FishBase. http://fishbase.sinica.edu.tw/summary/speciessummary.php?genusname=Lates&speciesname=calcarifer. Accessed on April 2, 2008.
|
Mir J I, Sarkar U K, Dwivedi A K, Gusain O P, Jena J K. 2013. Stock structure analysis of Labeo rohita (Hamilton, 1822)across the Ganga basin (India) using a truss network system. Journal of Applied Ichthyology, 29(5): 1 097-1 103.
DOI:10.1111/jai.2013.29.issue-5 |
Miyan K, Khan M A, Patel D K, Khan S, Ansari N G. 2016. Truss morphometry and otolith microchemistry reveal stock discrimination in Clarias batrachus (Linnaeus, 1758) inhabiting the Gangetic river system. Fisheries Research, 173: 294-302.
DOI:10.1016/j.fishres.2015.10.024 |
Mousavi-Sabet H, Anvarifar H. 2013. Landmark-based morphometric variation between Cobitis keyvani and Cobitis faridpaki (Pisces:Cobitidae), with new habitat for C. faridpaki in the southern Caspian Sea basin. Folia Zoology, 62(3): 167-175.
DOI:10.25225/fozo.v62.i3.a1.2013 |
Murta A G. 2000. Morphological variation of horse mackerel(Trachurus trachurus) in the Iberian and North African Atlantic:implications for stock identification. ICES Journal of Marine Science, 57(4): 1 240-1 248.
DOI:10.1006/jmsc.2000.0810 |
Nahar A, Chaklader M R, Siddi M A B, Ilham I, Pham H D, Munilkumar S. 2017. Stock structure of the critically endangered Clupisoma garua (Hamilton, 1822):an investigation based on discriminant analysis approach. Journal of Aquaculture Research and Development, 8(2): 1000470.
|
Nahar A, Hanif M A, Siddik M A B, Chaklader M R, Islam M A. 2018. Length-weight and length-length relationships of four endemic fish species caught from Payra River, Southern Bangladesh. Journal of Applied Ichthyology, 34(3): 785-787.
DOI:10.1111/jai.13601 |
Nelson J S. 1994. Fishes of the World. John Wiley and Sons, New York.
|
Nimalathasan B. 2009. Determinants of key performance indicators (KPIs) of private sector banks in Sri Lanka:an application of exploratory factor analysis. The Annals of The "Ştefan cel Mare" University of Suceava. Fascicle of The Faculty of Economics and Public Administration, 9(2): 9-17.
|
Pinheiro A, Teixeira C M, Rego A L, Marques J F, Cabral H N. 2005. Genetic and morphological variation of Solea lascaris (Risso, 1810) along the Portuguese coast. Fisheries Research, 73(1-2): 67-78.
DOI:10.1016/j.fishres.2005.01.004 |
Poulet N, Berrebi P, Crivelli A J, Lek S, Argillier C. 2004. Genetic and morphometric variations in the pikeperch(Sander lucioperca L.) of a fragmented delta. Archiv für Hydrobiologie, 159(4): 531-554.
DOI:10.1127/0003-9136/2004/0159-0531 |
Quilang J P, Basiao Z U, Pagulayan R C, Roderos R R, Barrios E B. 2007. Meristic and morphometric variation in the silver perch, Leiopotherapon plumbeus (Kner, 1864), from three lakes in the Philippines. Journal of Applied Ichthyology, 23(5): 561-567.
DOI:10.1111/jai.2007.23.issue-5 |
Reist J D. 1985. An empirical evaluation of several univariate methods that adjust for size variation in morphometric data. Canadian Journal of Zoology, 63(6): 1 429-1 439.
DOI:10.1139/z85-213 |
Renaud C B. 2011. Lampreys of the World. An Annotated and Illustrated Catalogue of Lamprey Species Known to Date, FAO Species Catalogue for Fishery Purposes. No. 5.FAO, Rome. 109p.
|
Ricker W E. 1981. Changes in the average size and average age of pacific salmon. Canadian Journal of Fisheries and Aquatic Science, 38(12): 1 636-1 656.
DOI:10.1139/f81-213 |
Samaee S M, Patzner R A, Mansour N. 2009. Morphological differentiation within the population of Siah mahi, Capoeta capoeta gracilis, (Cyprinidae, Teleostei) in a river of the south Caspian Sea basin:a pilot study. Journal of Applied Ichthyology, 25(5): 583-590.
DOI:10.1111/jai.2009.25.issue-5 |
Sarker J, Patwary S A, Uddin A M M B, Hasan M, Tanmay M H, Kanungo I, Parvej M R. 2016. Macrobenthic community structure-an approach to assess coastal water pollution in Bangladesh. Fisheries and Aquaculture Journal, 7(1): 1 000 157.
DOI:10.4172/2150-3508.1000157 |
Siddik M A B, Chaklader M R, Hanif M A, Islam M A, Fotedar R. 2016d. Length-weight relationships of four fish species from a coastal artisanal fishery, southern Bangladesh. Journal of Applied Ichthyology, 32(6): 1 300-1 302.
DOI:10.1111/jai.13181 |
Siddik M A B, Chaklader R, Hanif A, Nahar A, Ilham I, Cole A, Fotedar R. 2017. Variation in the life-history traits of a Schilbid catfish, Clupisoma garua (Hamilton, 1822) in the coastal waters of southern Bangladesh. Chinese Journal of Oceanology and Limnology, 35(5): 1 189-1 196.
DOI:10.1007/s00343-017-6008-6 |
Siddik M A B, Hanif A, Chaklader R, Nahar A, Fotedar R. 2016b. A multivariate morphometric investigation to delineate stock structure of gangetic whiting, Sillaginopsis panijus (Teleostei:Sillaginidae). SpringerPlus, 5: 520.
DOI:10.1186/s40064-016-2143-3 |
Siddik M A B, Hanif A, Chaklader R, Nahar A, Mahmud S. 2015. Fishery biology of gangetic whiting Sillaginopsis panijus (Hamilton, 1822) endemic to Ganges delta, Bangladesh. Egyptian Journal of Aquatic Research, 41(4): 307-313.
DOI:10.1016/j.ejar.2015.11.001 |
Siddik M A B, Islam M A, Hanif M A, Chaklader M R, Kleindienst R. 2016c. Barramundi, Lates calcarifer(Bloch, 1790):a new dimension to the fish farming in coastal Bangladesh. Journal of Aquaculture Research & Development, 7(12): 1 000 461.
|
Siddik M, Chaklader M, Hanif M, Islam M, Sharker M, Rahman M. 2016a. Stock identification of critically endangered olive barb, Puntius sarana (Hamilton, 1822)with emphasis on management implications. Journal of Aquaculture Research & Development, 7(2): 1 000 411.
|
Smith P J, Francis R I C C, McVeagh M. 1991. Loss of genetic diversity due to fishing pressure. Fisheries Research, 10(3-4): 309-316.
DOI:10.1016/0165-7836(91)90082-Q |
Swain D P, Foote C J. 1999. Stocks and chameleons:the use of phenotypic variation in stock identification. Fisheries Research, 43(1-3): 113-128.
DOI:10.1016/S0165-7836(99)00069-7 |
Turan C, Oral M, Öztürk B, Düzgüneş E. 2006. Morphometric and meristic variation between stocks of bluefish(Pomatomus saltatrix) in the black, Marmara, Aegean and northeastern Mediterranean seas. Fisheries Research, 79(1-2): 139-147.
DOI:10.1016/j.fishres.2006.01.015 |
Turan C, Yalcin S, Turan F, Okur E, Akyurt I. 2005. Morphometric comparisons of African catfish, Clarias gariepinus, populations in Turkey. Folia Zoology, 54(1-2): 165-172.
|
Tzeng T D. 2004. Morphological variation between populations of spotted mackerel (Scomber australasicus) off Taiwan. Fisheries Research, 68(1-3): 45-55.
DOI:10.1016/j.fishres.2004.02.011 |
Vatandoust S, Mousavi-Sabet H, Razeghi-Mansour M, AnvariFar H, Heidari A. 2015. Morphometric variation of the endangered Caspian lamprey, Caspiomyzon wagneri(Pisces:Petromyzontidae), from migrating stocks of two rivers along the southern Caspian Sea. Zoological Studies, 54: 56.
DOI:10.1186/s40555-015-0133-8 |
Veasey E A, Schammass E A, Vencovsky R, Martins P S, Bandel G. 2001. Germplasm characterization of Sesbania accessions based on multivariate analyses. Genetic Resources and Crop Evolution, 48: 79-90.
DOI:10.1023/A:1011238320630 |
Wimberger P H. 1992. Plasticity of fish body shape. The effects of diet, development, family and age in two species of Geophagus (Pisces:Cichlidae). Biological Journal of the Linnean Society, 45(3): 197-218.
DOI:10.1111/bij.1992.45.issue-3 |
Yadav B N. 1999. Fish and Fisheries. Daya Publishing House, Delhi, India. 323p.
|
Yu H T, Lee Y J, Huang S W, Chiu T S. 2002. Genetic analysis of the populations of Japanese anchovy (Engraulidae:Engraulis japonicus) using microsatellite DNA. Marine Biotechnology, 4(5): 471-479.
DOI:10.1007/s10126-002-0035-8 |
Yue G H, Zhu Z Y, Lo L C, Wang C M, Lin G, Feng F, Pang H Y, Li G, Gong P, Li H M, Tan J, Chou R, Lim H, Orban L. 2009. Genetic variation and population structure of Asian seabass (Lates calcarifer) in the Asia-Pacific region. Aquaculture, 293(1-2): 22-28.
DOI:10.1016/j.aquaculture.2009.03.053 |
Zhu Z Y, Lin G, Lo L C, Xu Y X, Feng F, Chou R, Yue G H. 2006. Genetic analyses of Asian seabass stocks using novel polymorphic microsatellites. Aquaculture, 256(1-4): 167-173.
DOI:10.1016/j.aquaculture.2006.02.033 |
 2019, Vol. 37
2019, Vol. 37


