Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LIU Chao, WU Fucun, QUE Huayong, ZHANG Guofan
- Relationships of growth and mortality to enzymatic activity, and the relative mRNA expression of cultured scallops Patinopecten yessoensis in the Yellow Sea, China
- Journal of Oceanology and Limnology, 37(4): 1409-1422
- http://dx.doi.org/10.1007/s00343-019-7257-3
Article History
- Received Sep. 6, 2017
- accepted in principle Dec. 3, 2017
- accepted for publication Sep. 14, 2018
2 University of Chinese Academy of Sciences, Beijing 100049, China;
3 Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China;
4 National & Local Joint Engineering Laboratory of Ecological Mariculture, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China
The yesso scallop (Patinopecten yessoensis) is an economically important aquaculture species (Meng et al., 2012). Naturally distributed in the northwest Pacific (Park, 1998), the scallop is commercially cultured using suspended longline and bottom culture in Japan, Korea, and China (Guo et al., 1999). Unlike suspended longline aquaculture, bottom culture does not require buoyancy rafts and therefore yields higher profits at a relatively lower cost (Laing, 2007).However, bottom-cultured scallops are more sensitive to environmental factors and might suffer greater mortality (Liu et al., 2013b). In fact, the marine region in the north of Yellow Sea is a major bottom-culture site of yesso scallops and has experienced serious productivity problems in recent years, including heavy drops in recapture ratios and harvested individual sizes (Li and Xue, 2005).
Many factors have been reported on the success or failure of scallop bottom culture. For instance, predation and scallop migration can reduce the harvest outcome (Cliche et al., 1994; Hatcher et al., 1996; Minchin, 2002). Sea stars, crabs, gastropods, and fish all prey on scallops (Minchin, 1992), with the former two groups considered major predators (Grefsrud et al., 2003; Wong and Barbeau, 2003; Wong et al., 2005). In response to predation pressure, scallops may migrate from ranching areas (Smith et al., 1988; Pohle et al., 1991; Brand, 2016). Migration is also associated with food availability and living space (McMahon and Matter, 2006) as well as environmental factors that influence energy metabolism and immunity, such as temperature, dissolved oxygen levels (Soletchnik et al., 1999, 2007; Berthelin et al., 2000; Xiao et al., 2005). Indeed, several previous reports on bivalve productivity (growth and mortality) have implicated complex interactions between environmental factors and physiological conditions (Yu et al., 2010; Li et al., 2013). Additionally, antioxidant and metabolic enzyme activity, along with relevant gene-expression patterns, were found to be the most direct factors influencing scallop growth and survival (Yu et al., 2010). Despite their apparent importance, however, surprisingly few studies have examined the effects of such enzymes on bottomcultured yesso scallops.
In this study, we focused on the influence of glutamic pyruvic transaminase (GPT) and lactic dehydrogenase (LDH) activity on growth and mortality of bottom-cultured scallops. The former is important in amino acid metabolism (Leach and Taylor, 1982), with links to cell inflammation and cell necrosis (Sultana et al., 2015). In Pacific oyster, Crassostrea gigas, hemolymph GTP levels significantly increase after 7 days of exposure to Cd-treated seawater, a major stressor (Choi et al., 2008). The latter enzyme is important in anaerobic glycolysis, and its activity is frequently used as a metabolic indicator (Houle-Leroy et al., 2000). Some LDH activity in the gill and adductor ranged from 2 and 290 nmol/(min·mg) protein in some bivalves, such as Mytilus edulis (Long et al., 2003), Macoma balthica and Scrobicularia plana, (Livingstone et al., 1983). Compared to crustacean or insects, LDH activity in mollusks is relatively lower, but activities of producing octopine and succinate are higher (Grieshaber and Gäde, 1977; Livingstone et al., 1983; Strahl et al., 2011).
Over-production of reactive oxygen species (ROS) during energy metabolism will cause oxidative damage including DNA breakage, lipid peroxidation, and protein denaturation (Wenming and di Giulio, 1988; Liu et al., 2009; Wang et al., 2012). To mitigate oxidative stress, most organisms have evolved antioxidant defense systems including antioxidant enzymes and non-enzymatic small antioxidant molecules (Labreuche et al., 2006; Cheng et al., 2007). Total antioxidant capacity (TAOC) is the most common measure of antioxidant activity and capability (Zang et al., 2012). Of the known antioxidant enzymes, superoxide dismutase (SOD) is one of the most important enzymes involved in superoxide anion detoxification (Hermes-Lima et al., 1998; Nordberg and Arnér, 2001). In shellfish, several factors such as temperature (Chen et al., 2007), dissolved oxygen (Lu et al., 2015) and pathogen invasion (Wang et al., 2012) all trigger antioxidant response. For example, SOD activity of scallop Chlamys farreri significantly increased before decreasing after a 2-h exposure to air at 25℃ (Chen et al., 2007). Additionally, exposure to low oxygen concentration for 15 weeks resulted in higher TAOC among Mercenaria mercenaria than among oysters Crassostrea virginica (Matoo et al., 2013).
Therefore, in the present study, we investigated seasonal differences in growth and mortality rates of bottom-cultured yesso scallops, as well as how they relate to TAOC, GPT, LDH, and SOD (activity and relative expression). This study is the first to conduct field surveys on bottom-cultured yesso scallops between varying seasons of summer and winter. Based on our results, we propose beneficial suggestions for improving the productivity of scallop bottom aquaculture.
2 MATERIAL AND METHOD 2.1 Study sitesThis study was conducted across four sampling locations (Site 1: 38°54′–38°55′N, 122°47′–122°48′E; Site 2: 38°57′–38°58′N, 122°53′–122°54′E; Site 3: 38°59′–39°00′N, 122°47′–122°48′E; Site 4: 38°58′– 38°59′N, 122°34′–122°35′E) at a scallop farm located in the north of Yellow Sea of China (Fig. 1). All sites had the same area (1.63 km×1.63 km) and water depth (40–45 m). Each site was subdivided into nine (3×3) smaller squares. The central square of the subdivisions was the experimental zone and surrounding squares were buffer areas that minimized outside scallop immigration into the study area. Sites 1 and 4 had muddy sediment, while Sites 2 and 3 were sandybottomed. Hatchery-reared spats (10 months old) with a shell length (SL) > 3 cm were released directly onto the seabed of all four sites in the experimental zone on 12-20-2012. The initial bottom culture densities in Sites 1 to 4 were 4.5×106, 8.25×106, 1.05×107, and 1.35×107 individuals/km2, respectively. Field investigations were conducted from December 2012 to December 2014. These surveys were scheduled consistently as much as possible but also depended on the weather conditions and the vessel availability. Mortality was assessed from the time of release to the study termination. Shell length (SL) and number of survivals of the scallops were investigated on 12-20-2012, 03-28-2013, 06-26-2013, 08-22- 2013, 10-30-2013, 12-28-2013, 02-26-2014, 06-17- 2014, 08-09-2014, and 12-22-2014. The Research and Development Centre of Zhangzidao Fishery Group Co. Ltd., assisted with SL and survivingscallop-count data collection in July and November 2014. Due to experimental-design differences and cost considerations, only adult scallops collected in 2014 were used for the analysis of relationships between enzyme activity/relative expression and growth/survival (see Section 2.2). The study complied with internationally accredited guidelines and ethical regulations on animal research.
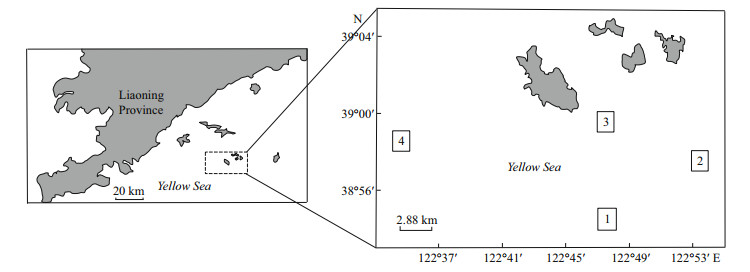
|
| Fig.1 Location of the study sites where the yesso scallops P. yessoensis were bottom-cultured in the north of Yellow Sea of China The boxed areas represent Sites 1, 2, 3 and 4, respectively. All sites had the same area (1.63 km×1.63 km). Sites 1 and 4 had muddy sediment, while Sites 2 and 3 were sandy-bottomed. Hatchery-reared spats (10 months old) were released directly onto the bottom on December 20, 2012. The initial densities in Sites 1 to 4 were 4.5×106, 8.25×106, 1.05×107, and 1.35×107 individuals/km2, respectively. |
Environmental conditions were measured in the bottom seawater (BSW) in 08-22-2013, 12-28-2013, and 08-19-2014, 12-22-2014. Temperature and dissolved oxygen (DO) were measured with an YSI multi-parameter water logger (550A-12). At each site, 1.5 L BSW was collected with a Niskin bottle and then filtered through a 200-μm mesh to remove large particles. Water samples were then transported on ice to the laboratory for measuring suspended particulate matter (SPM) and particulate organic matter (POM). Water samples (500 mL per site) were filtered in triplicate through a GC/C glass-fiber filter membrane (Whatman, Ø0.45 μm) that was ashed in a muffle furnace at 450℃ for 8 h and weighed (0.000 01 g in precision). Immediately post-filtering, 10 mL of distilled water was added to wash seawater from the membrane. Membranes were dried at 60℃ for 24 h until constant weight (0.000 01 g in precision); the dry weight of the remaining material at 60℃ represented the SPM. Then the membranes were combusted in a muffle furnace at 450℃ for 8 h. Once POM was oxidized to gas, its weight (0.000 01 g in precision) was calculated.
2.3 Assessment of scallop growth and mortality ratesBottom cultured scallops were collected with a benthic trawling net (1.6 m×3.0 m). We acclimated 200 scallops per site in recycled seawater on the vessel for about 4 h before moving them to the laboratory on land for growth rate (SL, Eq.1) measurements, using a Vernier caliper (0.01 mm accuracy). The number of living scallops per square kilometer (N) was counted from recordings with an underwater camera, and the mortality rate was calculated with Equation 2.
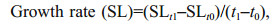 (1)
(1)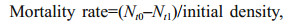 (2)
(2)where t0=initial measurement time of SL or living scallop count, and t1=final measurement time of SL or living scallop count.
Growth and mortality rates of the sampled scallops were calculated with initial data from June and October 2013, as well as July and November 2014, respectively. Scallop mortality curves per site (from the time of release to study termination) were generated from the calculation of mortality rate.
2.4 Metabolic and antioxidant enzymatic activityTo measure enzymatic activity and relative gene expression, we collected 20 more scallops per site for immediate dissection on the vessel. We used a hypodermic needle to aspirate 0.5-mL hemolymph from the antrum cardiacum for centrifugation at 800×g and 4℃ for 10 min. The supernatant was immediately stored in liquid nitrogen. Gills, gonads, and adductor muscles were removed, placed in 1.5- mL Eppendorf tubes, and immediately stored in liquid nitrogen for transport to the laboratory. Samples were stored at -80℃ until needed.
To minimize inter-animal variability when measuring enzyme activity, we separately mixed thawed (on ice) samples of each tissue type (taken equally from five scallops) to reach a final weight of 0.100 g per sample, then added 0.9-mL 1% PBS buffer (137 mmol/L NaCl, 2.7 mmol/L KCl, 10 mmol/L Na2HPO4, 2 mmol/L KH2PO4, pH=7.2). For hemolymph, 0.9 mL of distilled water was added to a 0.1-mL equivalence mixture of five scallop samples. All samples were homogenized in a glass homogenizer and then centrifuged at 800×g and 4℃ for 10 min. The supernatant was collected for use in enzymatic assays.
Protein content was quantified with an assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), following the Bradford method, bovine serum albumin was the standard. Enzyme activity assays were performed in triplicate with a Multiskan GO Microplate Spectrophotometer (Thermo Fisher Scientific, MA, USA).
Glutamic-pyruvic transaminase and LDH were measured in gonads and adductor muscles, while SOD and total antioxidant capacity (TAOC) were measured in hemolymph and gill samples. Enzymatic activity was obtained through colorimetric analysis, following manufacturer protocol in the corresponding detection kits (Nanjing Jiancheng Bio-engineering Institute). Previously published methods (Reitman and Frankel, 1957; Cabaud and Wróblewski, 1958) were used to measure GPT and LDH activity, respectively. The hydroxylamine technique was used for measuring SOD activity, while ferric iron reactions with antioxidants in tissues were used to determine TAOC.
2.5 Relative expression of GPT and SODThe relative expression of GPT and SOD in the hemolymph, gills, adductor muscle, and gonads were determined with real-time PCR. For the analysis, equal amounts of gill, gonad, and adductor-muscle samples from five scallops were mixed separately to reach a final weight of 0.100 g (per tissue). A 1-mL equivalence mixture of hemolymph from five scallops was centrifuged for 1 min at 1.9×104 g and 4℃; the precipitate was then collected for RNA extraction. Reverse transcription to generate cDNA was performed following manufacturer protocol from the PrimeScriptTM RT Reagent Kit and the gDNA Eraser (TaKaRa Bio, Shiga, Japan); the reaction volume was 20.0 μL, containing 1.0 μg total RNA per tissue.
Real-time PCR was conducted with the cDNA template and SYBR Premix Ex Taq (TaKaRa, Japan), following manufacturer protocol. Primers are listed in Table 1. β-actin was selected as the internal standard because of its stable expression in different tissues across time. Relative expression in June 2014 was the control group. The 2-ΔΔCtmethod was used to determine relative expression levels of every gene. The relative expression of SOD combines the total relative expression of Mn-SOD and CuZn-SOD.

|
We used one-way ANOVA and post-hoc Tukey's tests to examine significant monthly differences in growth rate, enzyme activity, as well as GPT and SOD relative expression. All results were expressed as means ± standard deviation. The relationships among growth, mortality, enzyme activity, and relative expression were determined with a stepwise multivariate linear regression. Data were arcsine or natural log transformed to reduce heterogeneous variances and non-normality if necessary. All analyses were performed using IBM SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). Significance was set at P < 0.05.
3 RESULT 3.1 Environmental conditionsThe temperature (Fig. 2a) and dissolved oxygen concentration (Fig. 2b) at all four sites fluctuated greatly from 2013 to the end of 2014. Temperature decreased from August to December and increased from December to August in both years. Dissolved oxygen exhibited the opposite trend. Mean SPM ranged from 0.026 68 g/L (Site 2, August 2014) to 0.044 99 g/L (Site 3, December 2013). Both of the SPM and POM concentrations showed a similar fluctuation among specific sites. The changing trend of POM concentration was similar among Sites 2, 3, and 4. However, Site 2 has lower POM concentration than the other three sites from December 2013 to December 2014.
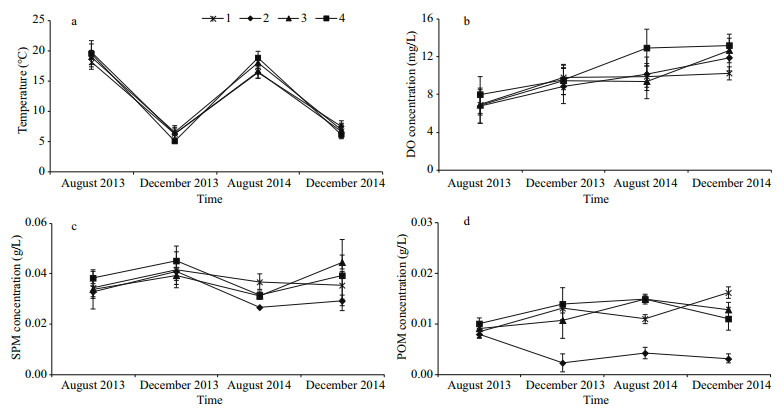
|
| Fig.2 Environmental conditions (a. temperature; b. dissolved oxygen; c. suspended particulate matter, SPM; d. particulate organic matter, POM) in the four sites (1, 2, 3 and 4) in August 2013, December 2013, August 2014 and December 2014 |
As showed in Fig. 3, the mortality rate in the two timeframes (December 2012 to March 2013 and March 2013 to June 2013) were significantly higher than that in other timeframes (P < 0.05) in Sites 2, 3 and 4. In Site 1, the mortality rate from December 2012 to March 2013 was significantly higher than that in other timeframes (P < 0.05). In a word, the mass accumulated mortality across the four sites varied from 82.40% to 93.89% in the first 6 months after release (Fig. 3). Subsequently, the mortality decreased considerably and remained consistently low in all sites.
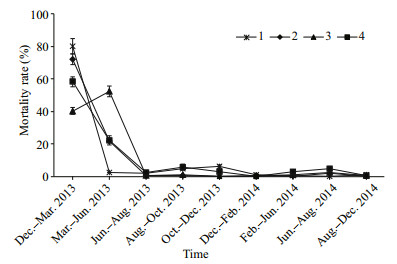
|
| Fig.3 Mortality rates of bottom cultured scallops at Sites 1, 2, 3, and 4 from the release time to the end of the investigation |
Seasonality also appeared to affect scallop growth and mortality rates. Across all four sites in 2013, scallop growth rate (SL) was significantly higher (P < 0.05) in winter (December) than in summer (August) at all sites (Fig. 4a). Similarly, growth rate (SL) during November–December 2014 was significantly higher than in other months (P < 0.05) across all sites (Fig. 4a). Discounting the mass postrelease mortality, 2013 mortality rate was significantly higher in summer than in winter for Sites 2 and 3 (P < 0.05, Fig. 4b). Mortality rates of 2014 were significantly higher in summer than in winter for all sites except Site 1 (P < 0.05, Fig. 4b).
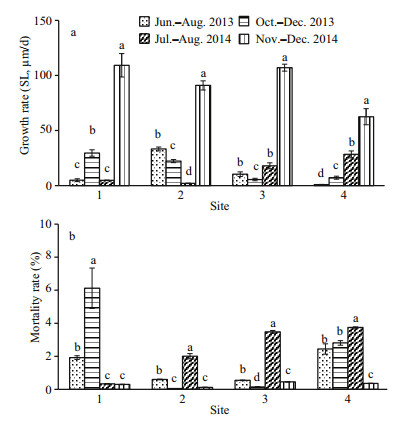
|
| Fig.4 The growth rate (SL) (a) and mortality rate (b) of bottom cultured yesso scallops at four sites in August and December of 2013 and 2014 Data are expressed as mean±S.D. In this figure, a, b, c, and d refer to the difference between months at the same site. The same letters in each set of columns indicate no significant difference (P > 0.05), whereas different letters indicate a significant difference (P < 0.05). |
Activities of GPT and LDH in different tissues showed significant seasonal and site-related variations. In general, gonad GPT activity decreased significantly during August and December 2014 at all four sites (P < 0.05, Fig. 5a). In contrast, adductormuscle GPT activity increased significantly only in Site 2. From August to December in both years, adductor-muscle LDH activity increased significantly at Sites 2 and 4 (P < 0.05), but did not change significantly at Site 1 and decreased significantly at Site 3 (Fig. 5b). Gonad LDH activity decreased significantly in Sites 2 and 3 but did not differ at Sites 1 and 4.
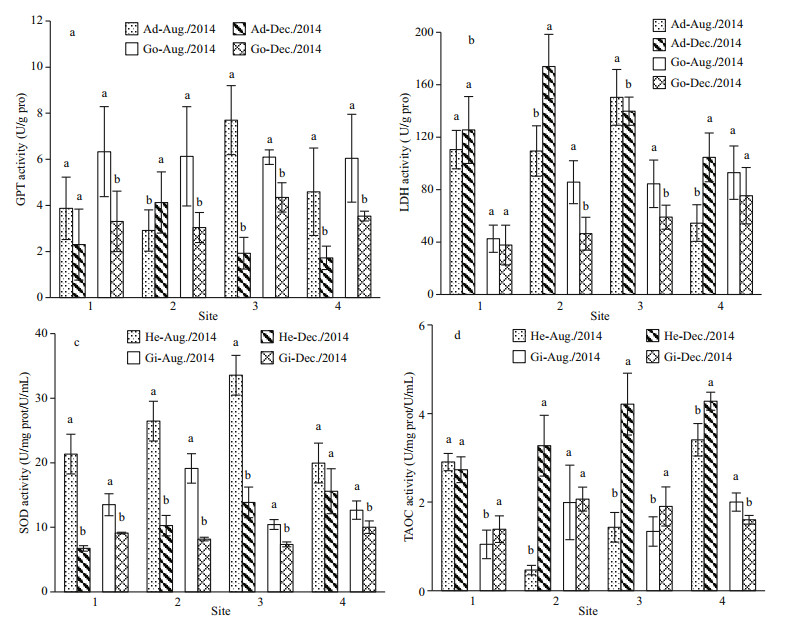
|
| Fig.5 The activity of enzymes involved in antioxidant and energy metabolism from August to December Enzymes involved in energy metabolism included glutamic pyruvic transaminase (GPT) (a), lactic dehydrogenase (LDH) (b), and their activities were measured in the adductor muscle (Ad) and gonad tissue (Go). Enzymes involved in antioxidant function included superoxide dismutase (SOD) (c) and total antioxidant capacity (TAOC) (d), and their activities were measured in hemolymph (He) and gills (Gi). The unit of SOD and TAOC activity in hemolymph was U/mL, and its unit in gill was U/mg prot. Data are expressed as means±S.D. Each group consists of 15 scallops (n=15). In this figure, a and b refer to the difference between months at the same site. The same letters in each set of columns indicate no significant difference (P > 0.05), whereas different letters indicate a significant difference (P < 0.05). |
Superoxide dismutase activity and TAOC in tissues exhibited variation similar to GPT and LDH. Hemolymph and gill SOD activities (Fig. 5c) decreased significantly at Sites 1, 2, and 3 (P < 0.05). At Site 4, gill but not hemolymph SOD activity differed significantly between months (P > 0.05). Haemolymph TAOC (Fig. 5d) increased significantly from August to December at Sites 2, 3, and 4 (P < 0.05), but did not change at Site 1. Gill TAOC increased significantly at Sites 1 and 3, decreased significantly at Site 4, and did not differ at Site 2.
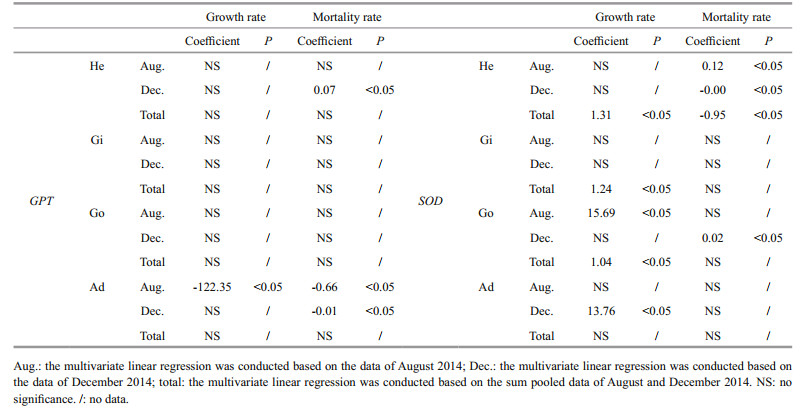
In addition, stepwise multivariate linear regression indicated that enzymatic activities significantly affected scallop growth and mortality rates in specific months (P < 0.05, Table 2). During August, scallop growth rate was negatively correlated to gonad GPT and gill SOD activities but positively related to hemolymph TAOC. In contrast, during in December, adductor-muscle LDH, gonadal LDH activities, and gill SOD activities were negatively correlated with scallop growth rate. When August and December data were pooled, scallop growth rate was negatively correlated with gonad GPT and LDH activities, as well as with gill SOD activity, but positively correlated with gill and hemolymph TAOC.
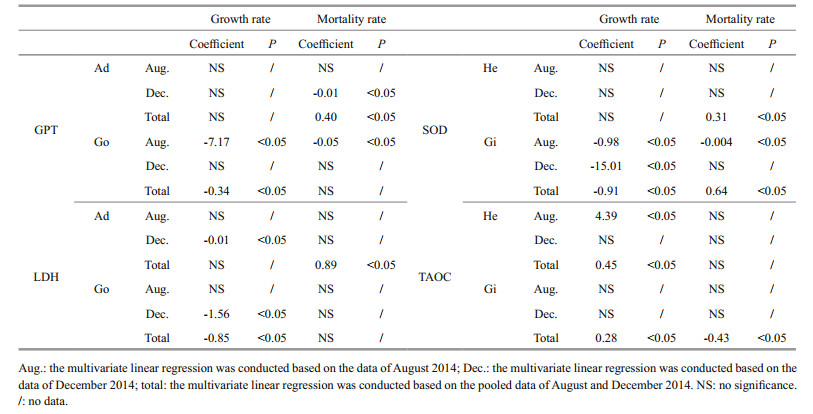
|
In August, scallop mortality rate was negatively correlated with gonad GPT and gill SOD activities. In December, the mortality rate was negatively correlated with adductor-muscle GPT activity. Pooled data from August and December indicated that mortality rates were positively correlated with adductor-muscle GPT and LDH activities, as well as hemolymph SOD activity. However, scallop mortality rate and gill TAOC was negatively correlated.
3.4 Relative expression of GPT and SODFrom August to December, GPT relative expression in hemolymph and gill increased significantly at Site 1 but did not change at any other Sites (Table 3). In gonad tissue, GPT relative expression increased significantly only at Site 4, whereas in the adductor muscle, it increased significantly at Sites 2, 3, and 4 (P < 0.05).

|
In hemolymph, gill, and adductor-muscle tissues, SOD relative expression increased significantly across all sites from August to December (P < 0.05, Table 3). In comparison, gonad SOD relative expression decreased significantly at Sites 1, 2, and 4 (P < 0.05), however, no seasonal change occurred at Site 3.
Relative expression of SOD and GPT exhibited some significant relationships with scallop growth and mortality. During December, hemolymph GPT relative expression was positively correlated with the mortality rate, while adductor muscle GPT expression was negatively correlated (P < 0.05, Table 4). In August, adductor muscle GPT relative expression was negatively correlated with both growth and mortality rates.
|
During August, SOD relative expression levels in hemolymph and gonads were positively correlated with mortality rate and growth rate, respectively (P < 0.05, Table 4). During December, SOD relative expression levels in adductor muscles were positively correlated with growth rate (P < 0.05). Additionally, hemolymph and gonad SOD expression levels were negatively and positively correlated with the mortality rate, respectively. Based on pooled data from August and December, SOD relative expression in hemolymph, gill, and gonad positively affected growth rate, whereas hemolymph SOD expression negatively influenced mortality rate.
4 DISCUSSIONWe conducted field surveys to investigate possible relationships between growth, mortality, enzyme activity, and related gene expression in bottom cultured yesso scallops. Preliminary results showed that scallop mass mortality mainly occurred during the first 6 months after release, confirming existing data from other bivalves showing a serious productivity problem (Bell et al., 2008). Major causes of post-release mortality in aquaculture are generally predation, dispersal, and acclimation effects, although mortality magnitude varies between species (Richardson, 1992). Besides, the acute temperature and fluctuation will result directly or indirectly in the mortality of scallops (Dickie, 1958). We reported a > 80% mortality rate in yesso scallops during the first 6 months post-release. Because of the limitation of survey tools and experimental zone area, it seems difficult to get the precise causes of the mass mortality. The severity of this die-off was unexpected but indicated the need for strategies focusing on the immediate month's pre- and post-release, aimed at reducing mortality. Previous reports on bivalves suggest that predator removal and acclimation in shallow sea bottom before being released into deep waters may be viable solutions (Morel and Bossy, 2001; Grefsrud et al., 2003). In the bottom culture of yesso scallops, these methods probably reduce the mortality in the first 6 months after being released.
Apart from the occurrence of mass mortality in the first several months after release, scallop mortality and growth varied seasonally (Fig. 3). Summer mortality has been reported in some aquaculture species, such as Pacific oysters (Soletchnik et al., 2005) and Bay scallops (Lu and Blake, 1997). Similarly, our data demonstrated that bottom-cultured yesso scallops experienced less growth and higher mortality in summer than in winter, especially during 2014. Summer mass mortality is typically attributed to complex interactions between environmental factors (e.g. temperature, dissolved oxygen, and primary productivity), physiological condition (e.g. immunological and metabolism function), and pathogens (Patrick et al., 2006). All the three of these also separately influence scallop growth rate (Li et al., 2013).
Water temperature is likely to be the most important factor affecting between-season variation in scallops growth (Yu et al., 2010), having been demonstrated to significantly affect behaviour, growth, and survival of marine organisms (Konstantinov et al., 2003; Lin et al., 2005; Brucet et al., 2012). The yesso scallop is a cold-water species (Park, 1998), suggesting that winter temperatures are probably more favorable for its physiological status consistent with our observed growth and mortality trends in 2014. Variation in dissolved oxygen and POM concentration also explained growth and mortality patterns during 2014. We noted that in contrast to other sites, scallop growth rates in Sites 2 and 3 were higher during summer than winter of 2013 (Fig. 4a). The difference is likely attributed to sediment variety, which determines food quality and abundance (Grizzle and Lutz, 1989; Lenihan, 1999). Thus, POM composition in Sites 2 and 3 may be more beneficial for scallop growth during summer than during winter. Overall, our study demonstrated that a combination of environmental factors influenced growth and mortality, with the effect of each factor changing across seasons. To understand which of these factors exerted the strongest effect on growth and survival, we require more environmental data and statistical analysis.
Environmental factors are critical to determining scallop growth and mortality, through influencing physiological status (Malham et al., 2009; Cotter et al., 2010). Thus, we also examined how metabolic and immunological (antioxidant) enzyme activity and relative mRNA expression correlated with on the scallops growth and mortality. Previous research has indicated that the increased GPT activity is correlated with cell inflammation and cell necrosis (Sultana et al., 2015). In this study, lower growth rate and higher mortality rate during summer was likely due to increased inflammation, implying that GPT activity was also elevated. Furthermore, GPT is involved in protein oxygenolysis, promoting amino-acid transdeamination (Kader et al., 2011). Thus, decreased protein concentration because of higher GPT activity likely hampered of scallop growth rate.
The metabolic enzyme LDH is important to anaerobic respiration, and here we observed that a significant enhancement of gonad LDH activity in August, but not in December (Fig. 5b). This outcome is likely due to the lower dissolved oxygen in the ocean during summer. As a result, anaerobic respiration is forced to increase, leading to heightened LDH levels (Livingstone et al., 1981; Strahl et al., 2011), accelerated glycogen consumption, and ultimately, insufficient energy supply (Pörtner, 2002). Cell damage and viral infections associated with summer die-offs also promote LDH activity (Wang et al., 2001). These observations explain elevated LDH activity during a season of hampered growth and elevated mortality in scallops. Interestingly, we observed that LDH activity increased in adductor muscles but decreased in gonads at three Sites (1, 2, 4) from August to December (Fig. 5b). This difference is likely due to the distinct functions of the two organs and their responses to the external environment, but more research is necessary to test this hypothesis.
Stressors such as high temperature or low dissolved oxygen will increase ROS and antioxidant activity in marine invertebrates will increase (Dyrynda et al., 1998; Abele and Puntarulo, 2004). The antioxidant level is one of the important factors evaluating the health status of scallops (Lauzon-Guay et al., 2005). In this research, SOD activity was higher in summer than in winter, implying that the antioxidant system was upregulated to eliminate ROS and repair oxidative damage during summer. The need to allocate energetic resources toward antioxidant responses lead to the less energy for processes essential to growth and survival. Indeed, our regression analysis revealed that SOD activity in gill was negatively correlated with growth rate and SOD activity in hemolymph was positively correlated with mortality rate. However, TAOC was positively correlated with growth rate and negatively correlated with mortality rate. These opposing patterns are likely because TAOC reflects the activity of the overall antioxidant system, including non-enzymatic aspects that may be associated with other survival-enhancing metabolic activities (di Giulio et al., 1989; Abele and Puntarulo, 2004). For instance, vitamin C and vitamin E can regulate steroid synthesis to promote gland maturation and growth (Hilton et al., 1979). Future studies should aim to collect more data on the relative importance of enzymatic versus non-enzymatic antioxidants across different seasons and environmental conditions.
Seasonal environmental and tissue-function variation (Jaenisch and Bird, 2003) likely explained observed differences in SOD relative expression. SOD expression can either decrease (under strong, sustained stimulation) (Jo et al., 2008) or increase (under low or infrequent stimulation) (Zhen et al., 2014). In the present study, SOD relative expression was lower at higher temperatures (i.e. in August), meaning that SOD expression was also positively correlated with growth and negatively correlated with mortality. Because mRNA expression responds to environmental stimuli more rapidly than does enzyme activity (Zhen et al., 2014), the former is frequently used as a biomarker for pollutants (Shao et al., 2012; Liu et al., 2013a). Therefore, SOD mRNA expression may be useful as a prediction index of scallop growth and mortality.
In conclusion, seasonal variation in the growth and mortality of bottom-cultured yesso scallops results from harsher environmental conditions during summer, specifically high temperature and low dissolved oxygen. In response, energy consumption and antioxidant response increased, depleting energetic resources that are also needed for other essential processes. As a result, growth, and survival were extremely hampered. In previous reports, GPT (Syuichi and Yasuo, 1978; Burton and Feldman, 1983; Ji et al., 2017; Yuan et al., 2017) and LDH (Hsieh et al., 1955; Rong et al., 2013) are often detected to explore their relationships to growth or mortality, while the SOD (Yin et al., 2015; Xiu et al., 2016) and TAOC (Bedaiwy et al., 2004; Guerra et al., 2012; Hao et al., 2015) are often seen as indices of the antioxidant status and health status. In this study, although we demonstrated overall correlations pattern between metabolic and antioxidant enzyme activities, associated gene expressions, and scallop growth/ survival performance statistically (Tables 2 and 4), more enzymes involved in energy metabolic and antioxidant status should be considered for predicting or fitting the growth and mortality performance of bottom cultured scallops. Future studies on bottomcultured yesso scallops might pay more attention to the gonadal GPT and LDH, gill SOD and TAOC, as well as relative expression of adductor-muscle GPT, hemolymph SOD, and gonadal SOD.
Overall, the present study clearly indicated that the activity and relative expression of these enzymes both respond to and affect yesso-scallop growth and mortality. Further confirmation of the observed relationships would greatly contribute to the improvement of management practices in aquaculture. In particular, given the high cost of field surveys and the presence of considerable biological and environmental variation, we recommend further development of laboratory-based methods that detect relevant enzyme activity and relative mRNA expression, for use as accurate performance estimations in the bottom-culture system.
5 DATA AVAILABILITY STATEMENTThe datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abele D, Puntarulo S. 2004. Formation of reactive species and induction of antioxidant defence systems in polar and temperate marine invertebrates and fish. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology, 138(4): 405-415.
|
Bedaiwy M A, Agarwal A, Said T M, Worley S, Thornton J, Falcone T. 2004. Differential growth of human embryos in vitro:role of total antioxidant capacity. Fertility and Sterility, 82(S2): S195-S196.
|
Bell J D, Leber K M, Blankenship H L, Loneragan N R, Masuda R. 2008. A new era for restocking, stock enhancement and sea ranching of coastal fisheries resources. Reviews in Fisheries Science, 16(1-3): 1-9.
DOI:10.1080/10641260701776951 |
Berthelin C, Kellner K, Mathieu M. 2000. Storage metabolism in the Pacific oyster (Crassostrea gigas) in relation to summer mortalities and reproductive cycle (West Coast of France). Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology, 125(3): 359-369.
DOI:10.1016/S0305-0491(99)00187-X |
Brand A R. 2016. Scallop ecology:distributions and behaviour. Developments in Aquaculture and Fisheries Science, 40: 469-533.
DOI:10.1016/B978-0-444-62710-0.00011-0 |
Brucet S, Boix D, Nathansen L W, Quintana X D, Jensen E, Balayla D, Meerhoff M, Jeppesen E. 2012. Effects of temperature, salinity and fish in structuring the macroinvertebrate community in shallow lakes: implications for effects of climate change. PLoS One, 7(2): e30877.
DOI:10.1371/journal.pone.0030877 |
Burton R S, Feldman M W. 1983. Physiological effects of an allozyme polymorphism:Glutamate-pyruvate transaminase and response to hyperosmotic stress in the copepod Tigriopus californicus. Biochemical Genetics, 21(3-4): 239-251.
DOI:10.1007/BF00499136 |
Cabaud P G, Wróblewski F. 1958. Colorimetric measurement of lactic dehydrogenase activity of body fluids. American Journal of Clinical Pathology, 30(3): 234-236.
DOI:10.1093/ajcp/30.3.234 |
Chen M Y, Yang H S, Delaporte M, Zhao S J. 2007. Immune condition of Chlamys farreri in response to acute temperature challenge. Aquaculture, 271(1-4): 479-487.
DOI:10.1016/j.aquaculture.2007.04.051 |
Cheng P Z, Liu X, Zhang G F, He J G. 2007. Cloning and expression analysis of a HSP70 gene from Pacific abalone (Haliotis discus hannai). Fish & Shellfish Immunology, 22(1-2): 77-87.
|
Choi Y K, Jo P G, Choi C Y. 2008. Cadmium affects the expression of heat shock protein 90 and metallothionein mRNA in the Pacific oyster, Crassostrea gigas. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 147(3): 286-292.
|
Cliche G, Giguère M, Vigneau S. 1994. Dispersal and mortality of sea scallops, Placopecten magellanicus (Gmelin 1791), seeded on the sea bottom off les-de-la-Madeleine. Journal of Shellfish Research, 13(2): 565-570.
|
Cotter E, Malham S K, O'Keeffe S, Lynch S A, Latchford J W, King J W, Beaumont A R, Culloty S C. 2010. Summer mortality of the Pacific oyster, Crassostrea gigas, in the Irish Sea:the influence of growth, biochemistry and gametogenesis. Aquaculture, 303(1-4): 8-21.
DOI:10.1016/j.aquaculture.2010.02.030 |
di Giulio R T, Washburn P C, Wenning R J, Winston G W, Jewell C S. 1989. Biochemical responses in aquatic animals:a review of determinants of oxidative stress. Environmental Toxicology and Chemistry, 8(12): 1103-1123.
DOI:10.1002/etc.v8:12 |
Dickie L M. 1958. Effects of high temperature on survival of the Giant Scallop. Journal of the Fisheries Research Board of Canada, 15(6): 1189-1211.
DOI:10.1139/f58-063 |
Dyrynda E A, Pipe R K, Burt G R, Ratcliffe N A. 1998. Modulations in the immune defences of mussels (Mytilus edulis) from contaminated sites in the UK. Aquatic Toxicology, 42(3): 169-185.
DOI:10.1016/S0166-445X(97)00095-7 |
Grefsrud E S, Strand , Haugum G A. 2003. Handling time and predation behaviour by the crab, Cancer pagurus, preying on cultured scallop, Pecten maximus. Aquaculture Research, 34(13): 1191-1200.
DOI:10.1046/j.1365-2109.2003.00927.x |
Grieshaber M, Gäde G. 1977. Energy supply and the formation of octopine in the adductor muscle of the scallop, Pecten jacobaeus (Lamarck). Comparative Biochemistry and Physiology Part B:Comparative Biochemistry, 58(3): 249-252.
|
Grizzle R E, Lutz R A. 1989. A statistical model relating horizontal seston fluxes and bottom sediment characteristics to growth of Mercenaria mercenaria. Marine Biology, 102(1): 95-105.
|
Guerra C, Zenteno-Savín T, Maeda-Martínez A N, Philipp E E R, Abele D. 2012. Changes in oxidative stress parameters in relation to age, growth and reproduction in the shortlived catarina scallop Argopecten ventricosus reared in its natural environment. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology, 162(4): 421-430.
|
Guo X M, Ford S E, Zhang F S. 1999. Molluscan aquaculture in China. Journal of Shellfish Research, 18(1): 19-31.
|
Hao Z L, Liu J Z, Tang X J, Zhan Y Y, Tian Y, Ding J, Yang L M, Chang Y Q. 2015. A comparative study of survival, metabolism, immune indi-cators and HSP70 expression in three kinds of shell colors Japanese scallop Mizuhopecten yessoensis under high tem-perature stress. Marine Sciences, 39(11): 108-115.
(in Chinese with English abstract) |
Hatcher B G, Scheibling R E, Barbeau M A, Hennigar A W, Taylor L H, Windust A J. 1996. Dispersion and mortality of a population of sea scallop (Placopecten magellanicus) seeded in a tidal channel. Canadian Journal of Fisheries and Aquatic Sciences, 53(1): 38-54.
(in Chinese with English abstract) DOI:10.1139/f95-170 |
Hermes-Lima M, Storey J M, Storey K B. 1998. Antioxidant defenses and metabolic depression.The hypothesis of preparation for oxidative stress in land snails. Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology, 120(3): 437-448.
DOI:10.1016/S0305-0491(98)10053-6 |
Hilton J W, Cho C Y, Brown R G, Slinger S J. 1979. The synthesis, half-life and distribution of ascorbic acid in rainbow trout. Comparative Biochemistry and Physiology Part A:Physiology, 63(3): 447-453.
DOI:10.1016/0300-9629(79)90615-7 |
Houle-Leroy P, Garland Jr T, Swallow J G, Guderley H. 2000. Effects of voluntary activity and genetic selection on muscle metabolic capacities in house mice Mus domesticus. Journal of Applied Physiology, 89(4): 1608-1616.
DOI:10.1152/jappl.2000.89.4.1608 |
Hsieh K M, Suntzeff V, Cowdry E V. 1955. Serum lactic dehydrogenase activity as indication of neoplastic growth and regression. Experimental Biology and Medicine, 89(4): 627-629.
DOI:10.3181/00379727-89-21898 |
Jaenisch R, Bird A. 2003. Epigenetic regulation of gene expression:how the genome integrates intrinsic and environmental signals. Nature Genetics, 33(3S): 245-254.
|
Ji L Q, Sun G X, Li J, Wang Y, Du Y S, Li X, Liu Y. 2017. Effect of dietary β-glucan on growth, survival and regulation of immune processes in rainbow trout (Oncorhynchus mykiss) infected by Aeromonas salmonicida. Fish & Shellfish Immunology, 64: 56-67.
|
Jo P G, Choi Y K, Choi C Y. 2008. Cloning and mRNA expression of antioxidant enzymes in the Pacific oyster, Crassostrea gigas in response to cadmium exposure. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 147(4): 460-469.
|
Kader M A, Koshio S, Ishikawa M, Yokoyama S, Bulbul M, Honda Y, Mamauag R E, Laining A. 2011. Growth, nutrient utilization, oxidative condition, and element composition of juvenile red sea bream Pagrus major fed with fermented soybean meal and scallop by-product blend as fishmeal replacement. Fisheries Science, 77(1): 119-128.
DOI:10.1007/s12562-010-0312-9 |
Konstantinov A S, Pushkar V Y, Aver Yanova O V. 2003. Effects of fluctuations of abiotic factors on the metabolism of some hydrobionts. Biology Bulletin of the Russian Academy of Sciences, 30(6): 610-616.
DOI:10.1023/B:BIBU.0000007719.82974.44 |
Labreuche Y, Lambert C, Soudant P, Boulo V, Huvet A, Nicolas J L. 2006. Cellular and molecular hemocyte responses of the Pacific oyster, Crassostrea gigas, following bacterial infection with Vibrio aestuarianus strain 01/32. Microbes and Infection, 8(12-13): 2715-2724.
DOI:10.1016/j.micinf.2006.07.020 |
Laing I. 2007. Scallop farming-2nd edition by David Hardy. Aquaculture Research, 38(6): 668-669.
DOI:10.1111/are.2007.38.issue-6 |
Lauzon-Guay J S, Dionne M, Barbeau M A, Hamilton D J. 2005. Effects of seed size and density on growth, tissueto-shell ratio and survival of cultivated mussels (Mytilus edulis) in Prince Edward Island, Canada. Aquaculture, 250(3-4): 652-665.
DOI:10.1016/j.aquaculture.2005.03.049 |
Leach G J, Taylor M H. 1982. The effects of cortisol treatment on carbohydrate and protein metabolism in fundulus heteroclitus. General and Comparative Endocrinology, 48(1): 76-83.
|
Lenihan H S. 1999. Physical-biological coupling on oyster reefs:how habitat structure influences individual performance. Ecological Monographs, 69(3): 251-275.
|
Li Q, Zhao X L, Kong L F, Yu H. 2013. Transcriptomic response to stress in marine bivalves. ISJ-Invertebrate Survival Journal, 10(1): 84-93.
|
Li W J, Xue Z F. 2005. Healthy sustainable proliferation & cultivation of scallop Patinopecten yessoensis. Fisheries Science, 24(9): 49-51.
(in Chinese with English abstract) |
Lin C, Ning X, Su J, Lin Y, Xu B. 2005. Environmental changes and the responses of the ecosystems of the Yellow Sea during 1976-2000. Journal of Marine Systems, 55(3-4): 223-234.
DOI:10.1016/j.jmarsys.2004.08.001 |
Liu J, Pan L Q, Zhang L, Miao J J, Wang J. 2009. Immune responses, ROS generation and the haemocyte damage of scallop Chlamys farreri exposed to Aroclor 1254. Fish & Shellfish Immunology, 26(3): 422-428.
|
Liu Q, Basu N, Goetz G, Jiang N, Hutz R J, Tonellato P J, Carvan Ⅲ M J. 2013a. Differential gene expression associated with dietary methylmercury (MeHg) exposure in rainbow trout (Oncorhynchus mykiss) and zebrafish (Danio rerio). Ecotoxicology, 22(4): 740-751.
DOI:10.1007/s10646-013-1066-9 |
Liu R, Qiu L M, Yu Z A, Zi J, Yue F, Wang L L, Zhang H, Teng W M, Liu X F, Song L S. 2013b. Identification and characterisation of pathogenic Vibrio splendidus from Yesso scallop (Patinopecten yessoensis) cultured in a low temperature environment. Journal of Invertebrate Pathology, 114(2): 144-150.
DOI:10.1016/j.jip.2013.07.005 |
Livingstone D R, de Zwaan A, Leopold M, Marteijn E. 1983. Studies on the phylogenetic distribution of pyruvate oxidoreductases. Biochemical Systematics and Ecology, 11(4): 415-425.
DOI:10.1016/0305-1978(83)90047-9 |
Livingstone D R, de Zwaan A, Thompson R J. 1981. Aerobic metabolism, octopine production and phosphoarginine as sources of energy in the phasic and catch adductor muscles of the giant scallop placopecten magellanicus during swimming and the subsequent recovery period. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 70(1): 35-44.
|
Long S M, Ryder K J, Holdway D A. 2003. The use of respiratory enzymes as biomarkers of petroleum hydrocarbon exposure in Mytilus edulis planulatus. Ecotoxicology and Environmental Safety, 55(3): 261-270.
DOI:10.1016/S0147-6513(02)00137-9 |
Lu X, Wang C, Liu B Z. 2015. The role of Cu/Zn-SOD and Mn-SOD in the immune response to oxidative stress and pathogen challenge in the clam Meretrix meretrix. Fish & Shellfish Immunology, 42(1): 58-65.
|
Lu Y T, Blake N J. 1997. The culture of the southern bay scallop in Tampa Bay, an urban Florida estuary. Aquaculture International, 5(5): 439-450.
DOI:10.1023/A:1018336828796 |
Malham S K, Cotter E, O'Keeffe S, Lynch S, CUlloty S C, King J W, Latchford J W, Beaumont A R. 2009. Summer mortality of the Pacific oyster, Crassostrea gigas, in the Irish Sea:the influence of temperature and nutrients on health and survival. Aquaculture, 287(1-2): 128-138.
DOI:10.1016/j.aquaculture.2008.10.006 |
Matoo O B, Ivanina A V, Ullstad C, Beniash E, Sokolova I M. 2013. Interactive effects of elevated temperature and CO2 levels on metabolism and oxidative stress in two common marine bivalves (Crassostrea virginica and Mercenaria mercenaria). Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology, 164(4): 545-553.
|
McMahon T E, Matter W J. 2006. Linking habitat selection, emigration and population dynamics of freshwater fishes: a synthesis of ideas and approaches. Ecology of Freshwater Fish, 15(2): 200-210.
DOI:10.1111/eff.2006.15.issue-2 |
Meng Q L, Bao Z M, Wang Z P, Wang S, Hu J J, Hu X L, Huang X T. 2012. Growth and reproductive performance of triploid yesso scallops (Patinopecten yessoensis) induced by hypotonic shock. Journal of Shellfish Research, 31(4): 1113-1123.
DOI:10.2983/035.031.0422 |
Minchin D. 1992. Biological observations on young scallops, Pecten maximus. Journal of the Marine Biological Association of the United Kingdom, 72(4): 807-819.
DOI:10.1017/S0025315400060057 |
Minchin D. 2002.The potential for ranching the scallop, Pecten maximus-past, present, and future: problems and opportunities.-Proceedings of ICES Marine Science Symposia 215: 416-423
|
Morel G M, Bossy S F. 2001. A seeding experiment of juvenile great scallops (Pecten maximus (L.)) off the Island of Jersey. Aquaculture International, 9(5): 367-377.
DOI:10.1023/A:1020561909788 |
Nordberg J, Arnér E S J. 2001. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radical Biology and Medicine, 31(11): 1287-1312.
DOI:10.1016/S0891-5849(01)00724-9 |
Park Y J. 1998.Biological Studies on Aquaculture of the Scallop, Patinopecten Yessoensis (Jay). Cheju National University, Korea.
|
Patrick S, Faury N, Goulletquer P. 2006. Seasonal changes in carbohydrate metabolism and its relationship with summer mortality of pacific oyster Crassostrea gigas (Thunberg) in Marennes-Oléron bay (France). Aquaculture, 252(2-4): 328-338.
DOI:10.1016/j.aquaculture.2005.07.008 |
Pohle D G, Bricelj V M, García-Esquivel Z. 1991. The eelgrass canopy an above-bottom refuge from benthic predators for juvenile bay scallops Argopecten irradians. Marine Ecology Progress Series, 74: 47-59.
DOI:10.3354/meps074047 |
Pörtner H O. 2002. Climate variations and the physiological basis of temperature dependent biogeography:systemic to molecular hierarchy of thermal tolerance in animals. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 132(4): 739-761.
|
Reitman S, Frankel S. 1957. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. American Journal of Clinical Pathology, 28(1): 56-63.
DOI:10.1093/ajcp/28.1.56 |
Richardson C A. 1992.Scallops: biology, ecology and aquaculture: edited by S.E. Shumway; Elsevier; 1991; 116 pp.; NLG 300.00; ISBN 0-444-88954-X. Journal of Experimental Marine Biology and Ecology, 164(2): 280- 281.
|
Rong Y F, Wu W C, Ni X L, Kuang T T, Jin D Y, Wang D S, Lou W H. 2013. Lactate dehydrogenase A is overexpressed in pancreatic cancer and promotes the growth of pancreatic cancer cells. Tumor Biology, 34(3): 1523-1530.
DOI:10.1007/s13277-013-0679-1 |
Sakamoto S, Yone Y. 1978. Effect of starvation on hematological characteristics, and the contents of chemical components and activities of enzymes in blood serum of red sea bream. Journal of the Faculty of Agriculture, Kyushu University, 23(1-2): 63-69.
|
Shao B, Zhu L S, Dong M, Wang J, Wang J H, Xie H, Zhang Q M, Du Z K, Zhu S Y. 2012. DNA damage and oxidative stress induced by endosulfan exposure in zebrafish (Danio rerio). Ecotoxicology, 21(5): 1533-1540.
DOI:10.1007/s10646-012-0907-2 |
Smith I, Fonseca M S, Rivera J A, Rittmaster K A. 1988. Habitat value of natural versus recently transplanted eelgrass, Zostera marina, for the bay scallop, Argopecten irradians. Fishery Bulletin, 87: 189-196.
|
Soletchnik P, Lambert C, Costil K. 2005. Summer mortality of crassostrea gigas (Thunberg) in relation to environmental rearing conditions. Journal of Shellfish Research, 24(1): 197-208.
DOI:10.2983/0730-8000(2005)24[197:SMOCGT]2.0.CO;2 |
Soletchnik P, Le Moine O, Faury N, Razet D, Geairon P, Goulletquer P. 1999. Summer mortality of the oyster in the Bay Marennes-Oléron:spatial variability of environment and biology using a geographical information system (GIS). Aquatic Living Resources, 12(2): 131-143.
DOI:10.1016/S0990-7440(99)80022-9 |
Soletchnik P, Ropert M, Mazurié J, Gildas Fleury P, Le Coz F. 2007. Relationships between oyster mortality patterns and environmental data from monitoring databases along the coasts of France. Aquaculture, 271(1-4): 384-400.
DOI:10.1016/j.aquaculture.2007.02.049 |
Strahl J, Dringen R, Schmidt M M, Hardenberg S, Abele D. 2011. Metabolic and physiological responses in tissues of the long-lived bivalve Arctica islandica to oxygen deficiency. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology, 158(4): 513-519.
|
Sultana M S, Koshio S, Ishikawa M, Yokoyama S, Ikemura T. 2015. Effects on dietary supplement of Shochu Distillery By-Product (SDBP) on the growth of red sea bream. Aquaculture Science, 61(1): 47-54.
|
Wang J, Green P S, Simpkins J W. 2001. Estradiol protects against ATP depletion, mitochondrial membrane potential decline and the generation of reactive oxygen species induced by 3-nitroproprionic acid in SK-N-SH human neuroblastoma cells. Journal of Neurochemistry, 77(3): 804-811.
DOI:10.1046/j.1471-4159.2001.00271.x |
Wang X Q, Wang L L, Yao C, Qiu L M, Zhang H, Zhi Z, Song L S. 2012. Alternation of immune parameters and cellular energy allocation of Chlamys farreri under ammonia-N exposure and Vibrio anguillarum challenge. Fish & Shellfish Immunology, 32(5): 741-749.
|
Wenming R J, di Giulio R T. 1988. Microsomal enzyme activities, superoxide production, and antioxidant defenses in ribbed mussels (Geukensia demissa) and wedge clams (Rangia cuneata). Comparative Biochemistry and Physiology Part C:Comparative Pharmacology, 90(1): 21-28.
DOI:10.1016/0742-8413(88)90092-8 |
Wong M C, Barbeau M A, Hennigar A W, Robinson S M. 2005. Protective refuges for seeded juvenile scallops (Placopecten magellanicus) from sea star (Asterias spp.) and crab (Cancer irroratus and Carcinus maenas) predation. Canadian Journal of Fisheries and Aquatic Sciences, 62(8): 1766-1781.
DOI:10.1139/f05-092 |
Wong M C, Barbeau M A. 2003. Effects of substrate on interactions between juvenile sea scallops (Placopecten magellanicus Gmelin) and predatory sea stars (Asterias vulgaris Verrill) and rock crabs (Cancer irroratus Say). Journal of Experimental Marine Biology and Ecology, 287(2): 155-178.
DOI:10.1016/S0022-0981(02)00551-8 |
Xiao J, Ford S E, Yang H S, Zhang G F, Zhang F S, Guo X M. 2005. Studies on mass summer mortality of cultured Zhikong scallops (Chlamys farreri Jones et Preston) in China. Aquaculture, 250(3-4): 602-615.
DOI:10.1016/j.aquaculture.2005.05.002 |
Xiu M, Pan L Q, Jin Q. 2016. Toxic effects upon exposure to polycyclic aromatic hydrocarbon (chrysene) in scallop Chlamys farreri during the reproduction period. Environmental Toxicology and Pharmacology, 44: 75-83.
DOI:10.1016/j.etap.2016.04.001 |
Yin H C, Huang J, Yang D H, Zhao H Y, Jia F, Zhang Y. 2015. Effects of yeast nucleotide on growth performance, serum immune index and muscle composition of Ancherythroculter nigrocauda Yih & Wu. Iranian Journal of fisheries Sciences, 14(3): 646-659.
|
Yu Z H, Yang H S, Liu B Z, Xing K, Zhang L B, Xu Q. 2010. Survival, growth and immune activity of scallop Chlamys farreri cultured at different depths in Haizhou Bay (Yellow Sea, China) during hot season. Chinese Journal of Oceanology and Limnology, 28(3): 498-507.
DOI:10.1007/s00343-010-9025-2 |
Yuan L X, Li M, Meng F X, Gong Y F, Qian Y X, Shi G, Wang R X. 2017. Growth, blood health, antioxidant status, immune response and resistance to Aeromonas hydrophila of juvenile yellow catfish exposed to di-2-ethylhexyl phthalate (DEHP). Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 202: 79-84.
|
Zang Y Q, Tian X L, Dong S L, Dong Y W. 2012. Growth, metabolism and immune responses to evisceration and the regeneration of viscera in sea cucumber, Apostichopus japonicus. Aquaculture, 358-359: 50-60.
DOI:10.1016/j.aquaculture.2012.06.007 |
Zhen H, Wen M, Yang Y, Can Z, Hui G, Li X, Liu D L. 2014. Toxic effects of HgCl2 on activities of SOD, AchE and relative expression of SOD, AChE, CYP1A1 of zebrafish. Ecotoxicology, 23(10): 1842-1845.
DOI:10.1007/s10646-014-1350-3 |
 2019, Vol. 37
2019, Vol. 37


