Institute of Oceanology, Chinese Academy of Sciences
Article Information
- JIANG Yiqian, ZHAN GChi, YE Zhenjiang, TIAN Yongjun
- Analyses of egg size, otolith shape, and growth revealed two components of small yellow croaker in Haizhou Bay spawning stock
- Journal of Oceanology and Limnology, 37(4): 1423-1429
- http://dx.doi.org/10.1007/s00343-019-8105-1
Article History
- Received Apr. 20, 2018
- accepted in principle Sep. 13, 2018
- accepted for publication Oct. 5, 2018
2 Laboratory for Marine Fisheries Science and Food Production Processes, Pilot National Laboratory for Marine Science and Technology, Aoshanwei, Qingdao 266071, China
The small yellow croaker (Larimichthys polyactis) is a benthopelagic Sciaenidae fish that inhabits coastal waters and estuaries. This species widely distributed in the Bohai Sea, Yellow Sea and the East China Sea and supports an important demersal fishery in China, Korea and Japan (Liu, 1962; Kim et al., 2010). It is an asynchronous and multiple spawner from late spring to early summer, spawning pelagic eggs in estuaries and coastal waters. The small yellow croaker demonstrates seasonal migration and complex population structure with multiple spawning stocks (Xu and Chen, 2009), these characteristics bring big challenges to the stock assessment and fishery management. Previous studies, using morphometric and meristic characteristics, roughly divided the population of small yellow croaker into three stocks, but did not draw a consistent conclusion (Liu, 1962; Lin et al., 1964). Recent studies introduced new methods, such as otolith internal growth and shape, microsatellites and mitochondrial DNA, and truss network, to explore the potential changes in population structure with resource recovery (Wang et al., 2013; Zhang et al., 2014, 2015). These works redefined the spatial scales of the subpopulations, and provide evidence for metapopulation hypotheses in small yellow croaker. The metapopulation theory emphasizes variables but moderate exchanges between subpopulations (Levins, 1970; Hanski and Simberloff, 1997), the exchange requires a spatial and temporal overlap during spawning (namely interbreeding) which could be realized through drifting eggs and larvae and straying adults. For small yellow croaker, a single spawning stock could consist of individuals from different wintering grounds (Liu, 1962; Zhang et al., 2014), but no clear evidences have been obtained to support this assumption.
To clarify the source of a spawning stock, in this study we focus on a local spawning stock in the Haizhou Bay (HZB) that is the boundary area between adjacent subpopulations. HZB is an open bay on the western margin of the middle Yellow Sea, serving as a critical spawning and nursery habitat for the small yellow croaker. A newly observed temporal interstructure of morphological analysis using truss network provides evidence for the two components hypothesis of HZB spawning stock (Zhang et al., 2015). Assuming the two components exist, the different migratory pattern and experienced environmental conditions could result in different reproduction strategies such as egg size variation and life history characteristics such as changes in growth. The aim of this work is to further investigate the reproduction characteristics and structure of the HZB spawning stock through egg distribution and size variation, otolith shape and growth in adult fishes. Our results provide new evidence supporting the two components within a same spawning stock, and we also discuss how this result can influence the sustainable management of this population.
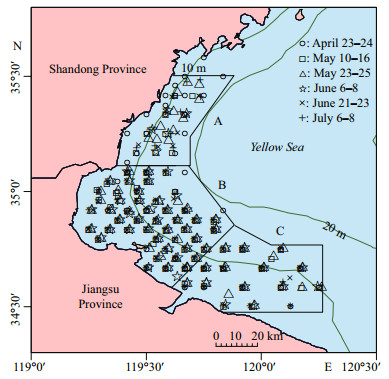
2 MATERIAL AND METHOD 2.1 Study area and sample collectionSix ichthyoplankton surveys were carried out at an interval of two weeks covering spawning season of small yellow croaker during April to July in 2013, in the shallow water of the HZB from 34.5°N to 35.5°N (Fig. 1). The survey grids were comprised of around 70 stations based on a stratified and systematic design.
|
| Fig.1 Sampling stations from six ichthyoplankton surveys in 2013 and sampling strata (A–C) |
Ichthyoplankton samples were collected during daylight using oblique tows of plankton nets (0.505 mm mesh size, 50 cm in diameter and 145 cm in length), with a constant speed of 2–3 knots for about 10 min. The plankton samples were immediately preserved in a 5% buffered formaldehyde seawater solution. A single general oceanic flowmeter was fitted in the mouth of nets to measure volume of waterfi ltered. Temperature (℃), salinity and depth (m) were measured using a Seabird XR-420 CTD.
Adult individuals were randomly sampled from commercial and survey captures with bottom trawlers (mesh size 20 mm×20 mm) in natural waters surrounding the Huangwo, Lianyungang simultaneously with ichthyoplankton samples (Fig. 1). The adults were ice-frozen onboard, stored frozen in the laboratory and thawed in room temperature prior to biological measurement. Standard length (mm), body weight and gonad weight were measured for each individual. Sex and maturity stages of the samples were visually determined according to Yin (1995). Only individuals at pre-spawn, spawning and spawned stages were retained for further analysis. Otoliths were removed from each fish, cleaned in fresh water, air-dried, and stored in plastic tubes.
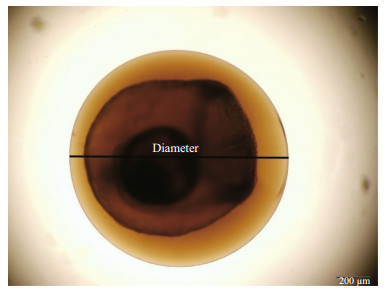
2.2 Egg identification and imagingEggs of small yellow croaker were included in the sorted fractions, and then were selected and allocated to an embryonic development stage under a binocular microscope following morphology criteria (Thompson and Riley, 1981; Zhang et al., 1985). The accuracy of morphology identification has been further confirmed by molecular marker of mitochondrial COI gene (Fan, 2017). Then, the images of the selected eggs were captured using Nikon SMZ1000 video camera. As the proxy for egg size, the egg diameter (nearest to 0.01 mm) was measured using Image-Pro Plus (Version 6.0) (Fig. 2).
|
| Fig.2 Image of Ib stage egg of small yellow croaker illustrating diameter measurement |
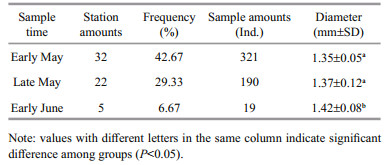
Right otoliths of adults were embedded in translucent epoxy resin, and 0.8 mm-thick transverse sections were cut along the dorsal-ventral axis using IsoMet low speed saw (Buehler). Age was determined by two readers without specimen information, and the conflicting results were determined by a wellexperienced third reader with specimen information. The width of otolith increment was used as the proxy for growth estimates and environmental conditions. The increment was measured from the core of the otolith to the outer margin of each opaque zone along the dorsal tip of sulcus using software ImageJ (Zhang et al., 2014). Due to rapid ontogenetic changes in growth rates, the innermost first increment of each otolith was not used. To exclude the year-class growth differences, only age 2 individuals were selected (Table 1). Fish size distribution varied in a small range, and the length effect on otolith shape could be negligible.
The light stereoscope with a video camera (Nikon SMZ800) was used to capture left otolith images under reflected light on the black background. The otoliths were placed with the distal side upwards and the anterior to the left in horizontal line. The video images were subjected to the shape using R packages of software R. 3.03, and 20 elliptical Fourier harmonics were obtained (Libungan and Pálsson, 2015). The first 12 Fourier harmonics explained 98.5% of the total variance, of which 45 coefficients were determined for shape analysis because the first three coefficients were degenerated and constant for all outlines.
2.4 Data analysisNo significant differences in egg diameter were detected among different embryonic development stages. Therefore, eggs of all different embryonic development stages were pooled together. One-way analysis of variance test (ANOVA) and KruskalWallis test were performed to compare the temporal and spatial variations in egg size with SPSS 20.0.
Random forest (RF) was used for cluster and classification because of its no requirement of distributional assumption, independence of the predictor variables and unbiased out-of-bag (OOB) estimate (Breiman, 2001). The potential groups of otolith morphotype were determined using the partitioning around medoids (PAM) method (Kaufman and Rousseeuw, 1990). RF cluster (an unsupervised way) was applied to partition the data into the identified groups and assign individual to its respective group (Shi and Horvath, 2006). For a finer visual comparison, the average outline of otolith morphotype was reconstructed using shapeR package. RF classification was then applied to test the cluster result.
The fpc, random Forest and Functions RF clustering packages of statistical software R. 3.03 were used for model development.
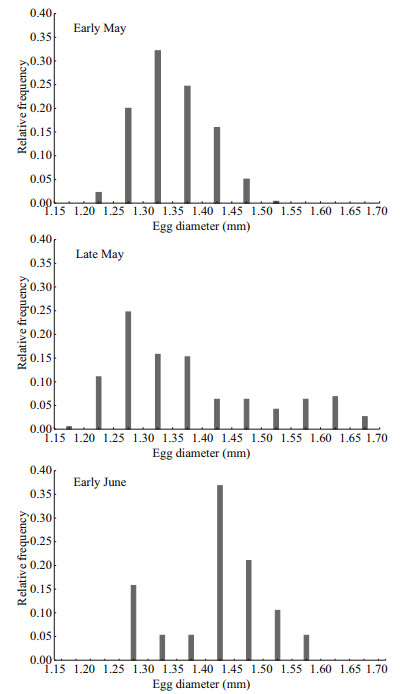
3 RESULTIn total, 530 eggs were collected from three of the six surveys, mainly concentrating in strata B (Fig. 3, Table 2). Significant temporal and spatial differences in egg size were detected among sample stations and times (P < 0.05). Especially in late May, egg distributed as two assemblages: the larger eggs occurred in the north and the smaller ones occurred in the south. The egg size ranged from 1.11 to 1.79 mm (mainly between 1.25 to 1.40 mm), and the average egg size increased 0.05 mm as spawning season progressed (Fig. 4, Table 2).

|
| Fig.3 Spatial and temporal distributions of the eggs |
|
| Fig.4 Distribution of egg diameter in the three surveys |
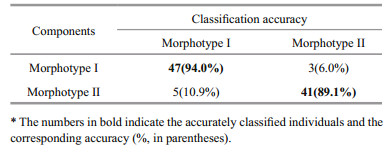
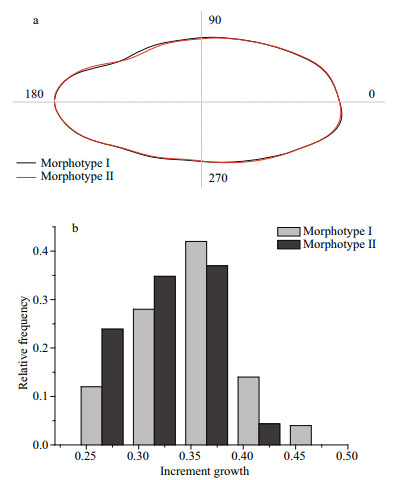
The PAM suggested two morphotypes within the samples, which mainly differed in the ventral margin of posterior (Fig. 5a). Morphotype Ⅰ individuals demonstrated higher gonad development than the morphotype Ⅱ ones in early May and wider increment growth (ANOVA, P < 0.5) (Fig. 5b). RF classification showed overall 91.7% success in assigning individual to its morphotype (Table 3).
|
| Fig.5 The reconstructed average outlines of two otolith morphotypes (a) and distribution of otolith increment of the two morphotypes (b) |
The reproduction characteristics and the spatialtemporal pattern in the distributions of eggs of small yellow croaker in the HZB were investigated in this work. The eggs occurred from early May to early June, lasting almost one month and peaking in the May. The egg distribution concentrated in the B-strata with brackish waters from river influx. Egg size generally shows increasing trend with a decrease of temperature, namely, the 'Thorson-Rass's rule (Mileikovsky, 1971). Meanwhile, more specifically egg size increases with latitude and decreases for a single stock with the progression of the spawning season. This rule has been confirmed in many species, such as anchoveta (Engraulis ringens) (Llanos-Rivera and Castro, 2004) and masu salmon (Oncorhynchus masou) (Morita et al., 2009) including the small yellow croaker (Qiu et al., 1962; Lin et al., 2009). Contrarily, the egg size of the small yellow croaker increased during spawning season in this study, and the size range of eggs in the late May showed large variation (normally 0.3 to 0.4 mm). The spatial distribution pattern also differed with larger eggs mainly occurring in the northern waters. The two egg assemblages with different sizes suggest two potential types of spawning strategy for the small yellow croaker in this area. However, eggs collected in the HZB may source multiple spawning sites and were transported from north to south during the spawning season due to oceanographic transport (Yuan et al., 2008). Spatial difference in the egg size might be caused by the southern shift in egg distribution from May to June. Therefore, this conclusion is merely a conjecture that needs further confirmation through other method (e.g. molecular techniques) in the future.
Increment growth and shape of fish otolith have been proven effective tools in population study for small yellow croaker (Zhang et al., 2014). The otolith shape analysis of small yellow croaker revealed two distinct morphotypes within the spawning stocks. In addition, significant difference in otolith increment growth was detected between the two morphotypes. The experienced differences in depth, temperature and salinity contribute to the differences in otolith increment growth and shape (Wright, 1991; Begg and Brown, 2000; Cardinale et al., 2004; Petursdottir et al., 2006). Generally, the higher temperature becomes, the larger otolith increment grows (Lombarte et al., 2003). This has also been proven in small yellow croaker (Zhang et al., 2014). That suggested that fish of morphotype Ⅰ with larger increment growth might experience higher water temperature than individuals of morphotype Ⅱ.
The echoing variation in distribution and size of eggs, otolith growth and shape of adults suggest two groups of different life histories and migration routes within the HZB spawning stocks. Morphotype Ⅰ fishes from the southern over-wintering ground spawned smaller eggs firstly in early May, and then both morphotypes spawned in late May, which resulted in the two assemblages of eggs. In June, only fish of morphotype Ⅱ from the northern over-wintering ground spawned few large eggs.
These findings provided further evidences to support the hypothesis that two components co-exist within the same HZB spawning stocks. This hypothesis is also supported by spatial distribution, migration route and body morphometrics analysis (Xu and Chen, 2009; Zhong et al., 2011; Zhang et al., 2015). The co-existence of the two components indicates a limited interbreeding between adjacent subpopulations, which provides an opportunity to quantify the genetic connections and puts insight to the metapopulation structure for small yellow croaker. However, an investigation of one year could be merely a 'snapshot', further studies are required to confirm the structure of the two components using multi-disciplines methods such as otolith microchemistry for wintering period. Protecting the HZB stock contributes to maintain connective between adjacent subpopulations and genetic diversity of the population.
5 DATA AVAILABILITY STATEMENTAll data of this study are available from the corresponding author upon reasonable request.
6 ACKNOWLEDGEMENTWe also thank all of the undergraduates and fishermen involving in this work for their assistance in sample collection.
Begg G A, Brown R W. 2000. Stock identification of haddock Melanogrammus aeglefinus on Georges Bank based on otolith shape analysis. Transactions of the American Fisheries Society, 129(4): 935-945.
DOI:10.1577/1548-8659(2000)129<0935:SIOHMA>2.3.CO;2 |
Breiman L. 2001. Random forests. Machine Learning, 45(1): 5-32.
DOI:10.1023/A:1010933404324 |
Cardinale M, Doering-Arjes P, Kastowsky M, Mosegaard H. 2004. Effects of sex, stock, and environment on the shape of known-age Atlantic cod(Gadus morhua) otoliths. Canadian Journal of Fisheries and Aquatic Sciences, 61(2): 158-167.
DOI:10.1139/f03-151 |
Fan Y N, Ye Z J, Jiang Y Q, Wan R, Ren Y P, Yu H L. 2016. Morphological and Cenetic Identification of Larimichthys polyactis Eggs. Journal of Zhejiang Ocean University (Natural Science), 35(5): 366-371+424.
(in Chinese with English abstract) |
Hanski I, Simberloff D. 1997. The metapopulation approach, its history, conceptual domain, and application to conservation. In: Hanski I A, Gilpin M E eds. Metapopulation Biology: Ecology, Genetics, and Evolution. Academic Press, San Diego, p.5-26. https://www.sciencedirect.com/science/article/pii/B9780123234452500031
|
Kaufman L, Rousseeuw P J. 1990. Partitioning around medoids (program PAM). In: Kaufman L, Rousseeuw P J eds. Finding Groups in Data: An Introduction to Cluster Analysis. Wiley Series in Probability and Statistics, Hoboken, NJ, USA, p.68-125, https://doi.org/10.1002/9780470316801.ch1.
|
Kim J K, Kim Y H, Kim M J, Park J Y. 2010. Genetic diversity, relationships and demographic history of the small yellow croaker, Larimichthys polyactis (Pisces:Sciaenidae) from Korea and China inferred from mitochondrial control region sequence data. Animal Cells and Systems, 14(1): 45-51.
DOI:10.1080/19768351003764973 |
Levins R. 1970. Extinction. In: Gerstenhaber M ed. Some Mathematical Problems in Biology. American Mathematical Society, Providence, RI. p.77-107.
|
Libungan L A, Pálsson S. 2015. ShapeR:an R package to study otolith shape variation among fish populations. PLoS One, 10(3): e0121102.
DOI:10.1371/journal.pone.0121102 |
Lin L S, Jiang Y Z, Yan L P, Gao T X, Wang J H. 2009. Study on the distribution characteristics and fecundity of spawning stock of Larimichthys polyactis in the southern Yellow Sea and the East China Sea. Journal of Shanghai Ocean University, 18(4): 453-459.
(in Chinese with English abstract) |
Lin X Z, Deng S M, Huang Z Y, Wang Q Z. 1964. Study of population on biometrics of small yellow croaker (Pseudosciaena Ployactis Bleeker). In: Zhu Y D, Zhu S P eds. Collections of Marine Fishery Resource. China Agriculture Press, Beijing. p.84-108. (in Chinese with English abstract)
|
Liu X S. 1962. Studies on the local groups and the maturity of the yellow croaker, Pseudosciaena polyactis Bleeker. In: C.C.O.o Fisheries Research Committee of Western Pacific Ocean. Collection of the Papers at 7th Plenary Session of Fisheries Research Committee of Western Pacific Ocean. Science Press, Beijing. p.35-70. (in Chinese with English abstract)
|
Llanos-Rivera A, Castro L R. 2004. Latitudinal and seasonal egg-size variation of the anchoveta (Engraulis ringens) off the Chilean coast. Fishery Bulletin, 102: 207-212.
|
Lombarte A, Torres G J, Morales-Nin B. 2003. Specific Merluccius otolith growth patterns related to phylogenetics and environmental factors. Journal of the Marine Biological Association of the United Kingdom, 83(2): 277-281.
DOI:10.1017/S0025315403007070h |
Mileikovsky S A. 1971. Types of larval development in marine bottom invertebrates, their distribution and ecological significance:a re-evaluation. Marine Biology, 10(3): 193-213.
DOI:10.1007/BF00352809 |
Morita K, Tamate T, Sugimoto Y, Tago Y, Watanabe T, Konaka H, Sato M, Miyauchi Y, Nagasawa T. 2009. Latitudinal variation in egg size and number in anadromous masu salmon Oncorhynchus masou. Journal of Fish Biology, 74(3): 699-705.
DOI:10.1111/j.1095-8649.2008.02150.x |
Petursdottir G, Begg G A, Marteinsdottir G. 2006. Discrimination between Icelandic cod (Gadus morhua L.) populations from adjacent spawning areas based on otolith growth and shape. Fisheries Research, 80(2-3): 182-189.
DOI:10.1016/j.fishres.2006.05.002 |
Qiu W C, Zhu Q Q, Jiang D H. 1962. A Morphology Study on the Egg and Larvae of the Small Yellow Croaker (Larimichthys polyactis). In: Zhu Y D, Zhu S P eds. Collections of Marine Fishery Resource. China Agriculture Press, Beijing. p.28-33. (in Chinese with English abstract)
|
Shi T, Horvath S. 2006. Unsupervised learning with random forest predictors. Journal of Computational and Graphical Statistics, 15(1): 118-138.
|
Thompson B M, Riley J D. 1981. Egg and larval development studies in the North Sea cod (Gadus morhua L.). The early life history of fish:recent studies. Rapports et Procès-Verbaux des Réunions du Conseil International pour l'Exploration de la MerRapports et Procès-Verbaux des Réunions du Conseil International pour l'Exploration de la Mer, 178: 553-559.
|
Wang L, Liu S F, Zhuang Z M, Guo L, Meng Z N, Lin H R. 2013. Population genetic studies revealed local adaptation in a high gene-flow marine fish, the small yellow croaker (Larimichthys polyactis). PLoS One, 8(12): e83493.
DOI:10.1371/journal.pone.0083493 |
Wright P. 1991. The influence of metabolic rate on otolith increment width in Atlantic salmon parr, Salmo salar L. Journal of Fish Biology, 38(6): 929-933.
DOI:10.1111/j.1095-8649.1991.tb03632.x |
Xu Z L, Chen J J. 2009. Analysis on migratory routine of Larimichthy polyactis. Journal of Fishery Sciences of China, 16(6): 931-940.
(in Chinese with English abstract) |
Yin M C. 1995. Fishes Ecology China Agriculture Press, Beijing, p.110-111. (in Chinese with English abstract)
|
Yuan D L, Zhu J R, Li C Y, Hu D X. 2008. Cross-shelf circulation in the Yellow and East China Seas indicated by MODIS satellite observations. Journal of Marine Systems, 70(1-2): 134-149.
DOI:10.1016/j.jmarsys.2007.04.002 |
Zhang C, Ye Z J, Wan R, Ma Q Y, Li Z G. 2014. Investigating the population structure of small yellow croaker (Larimichthys polyactis) using internal and external features of otoliths. Fisheries Research, 153: 41-47.
DOI:10.1016/j.fishres.2013.12.012 |
Zhang C, Jiang Y Q, Ye Z J, Li Z G, Dou S Z. 2015. A morphometric investigation of the small yellow croaker (Larimichthys polyactis Bleeker, 1877):evidence for subpopulations on the Chinese coast. Journal of Applied Ichthyology, 32(1): 67-74.
DOI:10.1111/jai.12923 |
Zhang R Z, Lu S F, Zhao C X, Chen L F, Zang Z, Zhang X. 1985. Fish Eggs and Larvae in Offshore Waters of China. Shanghai Scientific and Technical Publishers, Shanghai.
(in Chinese with English abstract)
|
Zhong X M, Zhang H, Tang J H, Zhong F, Zhong J S, Xiong X, Gao Y S, Ge K K, Yu W W. 2011. Temporal and spatial distribution of Larimichthys polyactis Bleeker resources in offshore areas of Jiangsu Province. Journal of Fisheries of China, 35(2): 238-246.
(in Chinese with English abstract) |
 2019, Vol. 37
2019, Vol. 37