Institute of Oceanology, Chinese Academy of Sciences
Article Information
- HU Guanyu, YU Wei, LI Bai, HAN Dongyan, CHEN Xinjun, CHEN Yong, LI Jianhua
- Impacts of El Niño on the somatic condition of Humboldt squid based on the beak morphology
- Journal of Oceanology and Limnology, 37(4): 1440-1448
- http://dx.doi.org/10.1007/s00343-019-8175-0
Article History
- Received Jun. 16, 2018
- accepted in principle Jul. 22, 2018
- accepted for publication Nov. 1, 2018
2 National Distant-water Fisheries Engineering Research Center, Shanghai Ocean University, Shanghai 201306, China;
3 Key Laboratory of Sustainable Exploitation of Oceanic Fisheries Resources, Ministry of Education, Shanghai Ocean University, Shanghai 201306, China;
4 School of Marine Sciences, University of Maine, Orono, Maine 04469, USA;
5 Collaborative Innovation Center for National Distant-water Fisheries, Shanghai 201306, China;
6 College of Fisheries, Ocean University of China, Qingdao 266003, China
Dosidicus gigas is one of the most important target commercial species in the East Pacific (Nigmatullin et al., 2001; Arkhipkin et al., 2015b). As a predator and prey, D. gigas plays an important role in marine ecosystems (Lu and Ickeringill, 2002; Field et al., 2007).
It is the largest and one of the most abundant ommastrephid squid and widely distributed in the East Pacific from the Gulf of Alaska to southern Chile (Taipe et al., 2001; Field et al., 2007; Zeidberg and Robison, 2007). Its distribution may expand during warm and cold periods combined with the decline of predators throughout the Pacific (Zeidberg and Robison, 2007; Keyl et al., 2008). In addition to the distribution, the adult size and life span of D. gigas can be influenced by the sea temperature (Hoving et al., 2013). The life span of D. gigas is about 1 year, but some huge specimens of the large size group can live up to 1.5 to 2 years (Nigmatullin et al., 2001; Arkhipkin et al., 2015a). Significant negative effect of sea surface temperatures on the life span could lead to a shorter life cycle of squid with a smaller size (higher ambient temperature) or a longer life cycle with a larger size (lower ambient temperature) (Arkhipkin et al., 2015a). The population structure of D. gigas tends to be complex, with three intraspecific groups that can be distinguished based on the adult sizes (Nigmatullin et al., 2001). In addition, the population of D. gigas can be divided into southern and northern groups based on an analysis of randomly amplified polymorphic DNA (RAPD) and mitochondrial DNA (Sandoval-Castellanos et al., 2007, 2010).
Beaks, mainly composed of proteins and chitin fibers, are the important feeding organ of squids (Clarke, 1962; Wolff, 1984; Miserez et al., 2007). They are hard tissues with constant shape and high stiffness and can record information on life history (Guerra et al., 2010; Ikica et al., 2014). The pigmented part of the beak was harder than the transparent part based on tensile and nanoindentation tests, which might result from different contents of catechols and water in the beak (Miserez et al., 2008). In previous studies, pigmentation stages were defined based on the development of pigmentation of beaks (HernańdezGarcía et al., 1998; García, 2003; Fang et al., 2016). The darkening process was suggested to make beaks harder and stronger, which can affect feeding habits and behavior of squid (Castro and Hernández-García, 1995; García, 2003; Franco-Santos and Vidal, 2014). Regular growth lines were found in the rostrum sagittal section and inner surface of the lateral wall, and growth increment was found to be deposited daily, providing an opportunity to estimate the age of squid using beaks (Rodríguez-Domínguez et al., 2013; Villegas Bárcenas et al., 2014; Hu et al., 2016b). Furthermore, the morphology of beaks was widely used for interspecies and intraspecies identification due to their stable configuration (Martínez et al., 2002; Liu et al., 2015; Fang et al., 2017), and body size of squids can be estimated by beaks remains in the stomachs of predators (Wolff, 1984). In previous studies, the beak growth was analyzed for many species, suggesting that the development of beaks might reflect the shifts of the size and types of prey (Jackson and McKinnon, 1996; Lefkaditou and Bekas, 2004; Hu et al., 2016a).
Dosidicus gigas is a short-lived species with its abundance and distribution being easily influenced by the environmental variability (Waluda and Rodhouse, 2006; Yu et al., 2017). Waluda and Rodhouse (2006) suggested that the abundance of D. gigas was strongly influenced by mesoscale variability associated with the ENSO, and the low catches were observed during strong El Niño events of 1997 to 1998 off Peru. Robinson et al. (2013) found that the catches were high in a La Niña year (1999) associated with cold sea surface temperature (SST) and high chlorophyll a (Chl a) concentrations. Yu et al. (2016) studied the effects of the climate variability on the habitat suitability of D. gigas and found that an El Niño event weakened upwelling coupled with warm and nutrient-depleted waters, which was unfavorable for squid habitat, leading to reduced catches. However, a La Niña event strengthened upwelling coupled with cool and nutrient-enhanced waters, which was favorable for squid habitat, yielding high catches. In previous studies, the effects of environment variables on the growth of squids were studied (Pecl et al., 2004). Argüelles and Tafur (2010) analyzed the somatic and reproductive investment of D. gigas in Peruvian waters and found that the somatic and reproductive investment varied both among different years and between genders. In the Gulf of California and Mexican waters, the somatic condition of D. gigas among different years was studied using the relative weight (Wr) and Fulton's condition coefficient (K), and the K value was found more sensitive and effective in the estimation of the somatic condition of D. gigas (Bazzino Ferreri, 2014). The poor somatic condition was observed during 1998 and 1999 with unfavorable environmental conditions caused by El Niño event during 1997–1998, and the condition factors differed between genders. The sexual differences in growth for squids were commonly observed in previous studies (Markaida et al., 2004; Liu et al., 2013; Fang et al., 2017). Compared with males, females were found to have a longer life span with larger body size (Mejia-Rebollo et al., 2008).
The objectives of this study were 1) to analyze the inter-annual variability of beak morphology and the link with climate variability on the fishing ground, and 2) to explore the response mechanism of the somatic condition and abundance of D. gigas to an El Niño event.
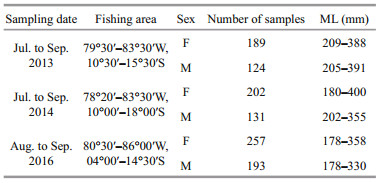
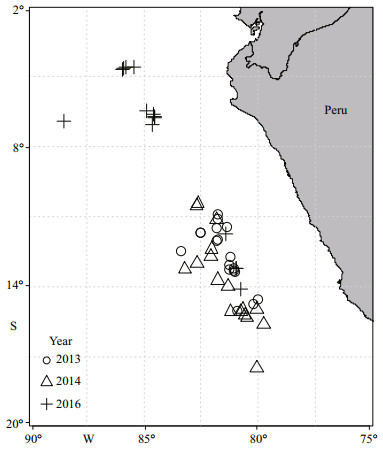
2 MATERIAL AND METHOD 2.1 SamplingA total of 1 096 Jumbo squid were randomly sampled from the catch of Chinese commercial jigging vessels off the Peruvian Exclusive Economic Zone (EEZ) in 2013, 2014, and 2016 (Table 1, Fig. 1). The sampled squids were immediately frozen aboard the vessels. After defrosting the samples in the laboratory, the mantle length (ML) and body weight (BW) were measured to the nearest 1 mm and 1 g, respectively. Meanwhile, sex was identified based on the approach defined by Lipiński and Underhill (1995).
|
| Fig.1 Map of the fishing and sampling locations off the Peruvian coast |
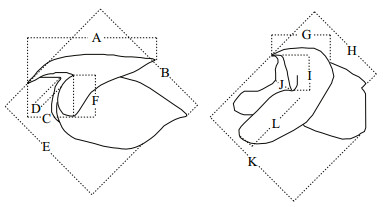
Both the upper and lower beaks were extracted from the buccal mass and stored in 70% alcohol. Twelve morphometric measurements were taken for each beak using digital calipers (Fig. 2), and six beak variables (UHL, UCL, ULWL, LCL, LRL, and LLWL, as seen in Fig. 2) were chosen in this study because they can be measured precisely using calipers (Fang et al., 2015).
|
| Fig.2 Schematic diagram of beak morphometric variables (Fang et al., 2014) A: upper hood length (UHL); B: upper crest length (UCL); C: upper rostrum length (URL); D: upper rostrum width (URW); E: upper lateral wall length (ULWL); F: upper wing length (UWL); G: lower hood length (LHL); H: lower crest length (LCL); Ⅰ: lower rostrum length (LRL); J: lower rostrum width (LRW); K: lower lateral wall length (LLWL); L: lower wing length (LWL). |
The linear, power, exponential, and logarithmic function were used to develop the relationships between ML and beak variables for D. gigas, and the linear regression model was selected as the best model based on the value of Akaike's information criterion (AIC) (Hu et al., 2017). In this study, the relationships between ML and beak variables for squids in three years were performed using linear regression models (Hu et al., 2017):
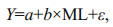
where Y is one of the beak variables, ML is the mantle length of squid. a and b are the parameters to be estimated representing intercept and slope, respectively. ε is the error of the linear model. After simulating the linear models, an analysis of covariance (ANCOVA) was used to test the differences of slopes for different genders and years.
2.3 Somatic conditionThe Fulton's condition coefficient (K) was conducted to evaluate the somatic condition of D. gigas. The K was determined from the following formula (Froese, 2006):
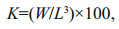
where K is Fulton's condition coefficient, W is body weight (g), and L is mantle length (cm). The differences of the somatic condition of D. gigas for different genders and years were analyzed using t-test (two-tailed test).
2.4 Fishery dataThe D. gigas fishery data from the fishing area were obtained from the Chinese Squid-Jigging Technology Group of Shanghai Ocean University. Data included a catch (tons), fishing effort (in fishing days), and fishing location (latitude and longitude). The nominal catch per unit effort (CPUE) was calculated using the following equation (Chen et al., 2010):

where CPUEyij is the yearly nominal CPUE (tons (t) / days (d)) at longitude i, latitude j in year y; ΣCatchyij the total catch for all the fishing vessels within a fishing grid at longitude i, latitude j in year y; ΣEffortyij is the sum of fishing days for all the fishing vessels within a fishing grid at longitude i, latitude j in year y.
2.5 Climate index and Chl a dataThe definition for an El Niño event was based on the 3-month running mean of SST anomalies in the Niño 3.4 region (5°N–5°S, 120°–170°W) (Yu et al., 2016). An El Niño event was determined by the SSTs above a threshold of +0.5 over at least 5 consecutive months. The Oceanic Niño 3.4 indices from 2013 to 2016 were achieved from the NOAA Climate Prediction Center (http://origin.cpc.ncep.noaa.gov/products/analysis_monitoring/ensostuff/ONI_v5.php). The monthly average Chl a data on the fishing area from 2013 to 2016 were derived from the National Oceanic and Atmospheric Administration (NOAA) Ocean Watch (http://oceanwatch.pifsc.noaa.gov/). The spatial resolution was 0.05°×0.05°.
3 RESULT 3.1 Slopes of beak variables in relation to MLThe parameters in the linear regression models between ML and beak variables were estimated for females and males sampled during 2013, 2014, and 2016 (Table 2), and the slopes for such relationships were compared between years using an analysis of covariance (ANCOVA) (Fig. 3).

|
| Fig.3 The slope of the relationship between ML and beak variable for female (a) and male (b) of D. gigas The error bars are standard errors of slopes. |
In this study, the variation of slopes for beak variables in relation to ML among years was observed, while there was no significant difference (Fig. 3, ANCOVA, P > 0.05). In 2013, slopes of UCL and LRL for females were significantly greater than those of males (ANCOVA, P < 0.05), other beak variables had no significant difference between females and males (ANCOVA, P > 0.05). In 2014 and 2016, all beak variables' slopes for females were greater than those of males, while no significant difference was found (ANCOVA, P > 0.05).
3.2 The variability in somatic conditionThe average K values of females and males from 2013 were 2.80±0.24 and 2.69±0.18, respectively (Table 3). The average K values of females and males caught during 2014 were 2.79±0.31 and 2.74±0.32, respectively (Table 3). The average K values of females and males sampled during 2016 were 2.57±0.23 and 2.55±0.25, respectively (Table 3).

|
K values of females and males during 2013 and 2014 were significantly greater than those of 2016, respectively (t-test, P < 0.01) (Fig. 4), there was no significant difference between 2013 and 2014 (t-test, P >0.05) (Fig. 4). In 2013, K values of females were significantly greater than those of males (ANCOVA, P < 0.05). In 2014 and 2016, K values of females were greater than those of males, while no significant difference was found between females and males (ANCOVA, P > 0.05).

|
| Fig.4 Average values and SDs of Fulton's coefficient (K) for females and males of D. gigas in different years Bar with different letters is significantly different from one another. |
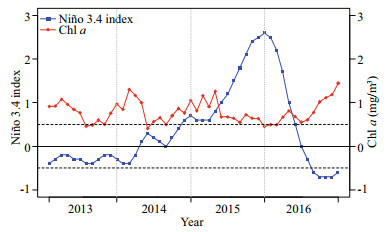
In the normal years (i.e., 2013 and 2014), monthly average Chl a concentration showed an N-shaped variability from January to December on the fishing area (Fig. 5), increasing in the first three months followed with a decrease from March to July and an increase from July to December (Fig. 5).
|
| Fig.5 The monthly Niño 3.4 index and monthly average Chl a concentration in the fishing area from 2013 to 2016 |
In the El Niño years (i.e., 2015 and 2016), the Niño 3.4 indices were slightly greater than 0.5 in the first three months of 2015, and the Chl a concentration fluctuated (Fig. 5). Then the Niño 3.4 index increased rapidly reaching high values from April 2015 to May 2016, and the Chl a concentration decreased and maintained at a lower level from May 2015 to July 2016 (Fig. 5).
4 DISCUSSIONSlopes of all beak variables in relation to ML for females were greater than those of males during 2013, 2014, and 2016, and slopes of UCL and LRL significantly differed between females and males in 2013 (P < 0.05). Meanwhile, K values of females were greater than those of males during 2013, 2014, and 2016, and K values significantly differed between females and males in 2013. Beaks are the important feeding organ of squids. The upper beak is a positive moving body, and the lower beak is the passive one to slice food coupled with upper beak while squid biting and then provide support for chewing (Raya and Hernández-González, 1998; Uyeno and Kier, 2007). The behavior and foraging habits of squids might be shifted with the morphological changes of beaks (Franco-Santos et al., 2014; Fang et al., 2017). As the beaks become more stiff and robust with the growth of beaks, the squids can forage on larger prey items (Castro and Hernández-García, 1995; Franco-Santos and Vidal, 2014; Hu et al., 2016a). Therefore, the somatic condition of squids is likely influenced by the beak shape, the somatic condition of females with greater slopes of beak variables was better than that of males.
We found that the somatic condition of females and males during the normal years (2013 and 2014) were significantly better than those from the El Niño year (2016), respectively (t-test, P < 0.01), while there was no significant difference between normal years (2013 and 2014) (Fig. 4). However, no significant difference was found for slopes of beak variables among years in this study. Therefore, the annual variability of somatic condition was not influenced by beak shape. In previous studies, the poor nutritional condition of D. gigas from the Gulf of California and Mexican waters during 1998 and 1999 was suggested to be caused by El Niño event 1997-1998 (Bazzino Ferreri, 2014). Pecl et al. (2004) suggested that females from cooler years had a better somatic condition and tended to have a higher level of reproductive investment. In this study, we found that the Chl a concentration in El Niño year was lower than that in normal years, which was also observed in previous studies (Barber et al., 1996; Radenac et al., 2012; Espinoza-Morriberón et al., 2017). Therefore, the common prey items of D. gigas were likely deficient, which was caused by the low primary production (Schwing, 2000; Robinson et al., 2013). Yu et al. (2016) studied the effect of the climate change on the habitat suitability of D. gigas and found that El Niño event weakened upwelling coupled with warm and nutrient-depleted waters, which were unfavorable for squid habitat and reduced catches. In this study, we suggested that the bad somatic condition of D. gigas during the El Niño year probably resulted from the low Chl a concentration in the seawater. It is adverse for D. gigas to grow and survive in the ambient environment in the El Niño year, and the abundance of D. gigas might decrease due to the unfavorable environment and the lack of prey items.
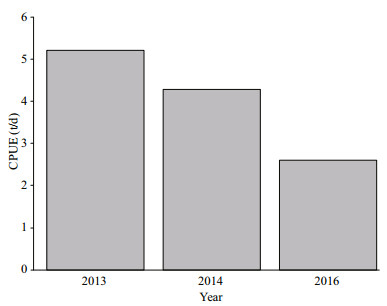
We figured out the CPUE during 2013, 2014, and 2016, and found that the CPUE of D. gigas in a normal year (5.22 t/d for 2013 and 4.29 t/d for 2014) was much greater than the El Niño year (2.61 t/d for 2016) (Fig. 6). In previous studies, the abundance of D. gigas in warm periods was also found less than that in normal years (Waluda and Rodhouse, 2006; Waluda et al., 2006; Yu et al., 2016).
|
| Fig.6 CPUE of D. gigas during 2013, 2014 and 2016 off Peruvian EEZ |
In this study, the relationships between ML and beak variables were developed (Table 2). The squid beaks are hard to digest and usually found in the stomachs of marine predators (Lu and Ickeringill, 2002). This durability offers an opportunity to estimate squid size and biomass of their post-predation (Gröger et al., 2000; Lalas, 2009). Therefore, the beaks that remained in predators' stomachs can be used to estimate the body size of D. gigas based on the relationships developed in this study.
5 CONCLUSIONHaving analyzed the D. gigas samples collected during 2013, 2014, and 2016, we revealed the variance of the somatic condition of D. gigas between normal years and the El Niño year. We believe that the relatively poor somatic condition of D. gigas during the El Niño year is resulted from the low Chl a concentration in the water. To make the Humboldt squid fishery sustainable and reduce the negative impacts of El Niño, more scientific management measures should be implemented in the year to come. The conclusion derived may need to be evaluated with more years of data in the future.
6 DATA AVAILABILITY STATEMENTThe data that support the findings of this study are available from the corresponding author upon reasonable request.
7 ACKNOWLEDGMENTThe data analysis and paper writing were done mainly when the senior author studied in Dr. CHEN Yong's lab in the School of Marine Sciences, the University of Maine, which is supported by Shanghai Ocean University and the University of Maine. We thank XING Lei (Ocean University of China) for statistical consultancy and advice on the figures.
Argüelles J, Tafur R. 2010. New insights on the biology of the jumbo squid Dosidicus gigas in the Northern Humboldt Current System:size at maturity, somatic and reproductive investment. Fisheries Research, 106(2): 185-192.
DOI:10.1016/j.fishres.2010.06.005 |
Arkhipkin A I, Rodhouse P G K, Pierce G J, Sauer W, Sakai M, Allcock L, Arguelles J, Bower J R, Castillo G, Ceriola L, Chen C S, Chen X J, Diaz-Santana M, Downey N, González A F, Amores J G, Green C P, Guerra A, Hendrickson L C, Ibáñez C, Ito K, Jereb P, Kato Y, Katugin O N, Kawano M, Kidokoro H, Kulik V V, Laptikhovsky V V, Lipinski M R, Liu B L, Mariátegui L, Marin W, Medina A, Miki K, Miyahara K, Moltschaniwskyj N, Moustahfid H, Nabhitabhata J, Nanjo N, Nigmatullin C M, Ohtani T, Pecl G, Perez J A A, Piatkowski U, Saikliang P, Salinas-Zavala C A, Steer M, Tian Y J, Ueta Y, Vijai D, Wakabayashi T, Yamaguchi T, Yamashiro C, Yamashita N, Zeidberg L D. 2015b. World squid fisheries. Reviews in Fisheries Science & Aquaculture, 23(2): 92-252.
|
Arkhipkin A, Argüelles J, Shcherbich Z, Yamashiro C. 2015a. Ambient temperature influences adult size and life span in jumbo squid (Dosidicus gigas). Canadian Journal of Fisheries and Aquatic Sciences, 72(3): 400-409.
DOI:10.1139/cjfas-2014-0386 |
Barber R T, Sanderson M P, Lindley S T, Chai F, Newton J, Trees C C, Foley D G, Chavez F P. 1996. Primary productivity and its regulation in the equatorial Pacific during and following the 1991-1992 El Niño. Deep Sea Research Part Ⅱ:Topical Studies in Oceanography, 43(4-6): 933-969.
DOI:10.1016/0967-0645(96)00035-5 |
Bazzino Ferreri G A. 2014. Length-weight relationships and condition factors of the humboldt squid (Dosidicus gigas) from the gulf of california and the pacific ocean. Journal of Shellfish Research, 33(3): 769-780.
DOI:10.2983/035.033.0311 |
Castro J J, Hernández-García V. 1995. Ontogenetic changes in mouth structures, foraging behaviour and habitat use of Scomber japonicus and Illex coindetii. Scientia Marina, 59(3-4): 347-355.
|
Chen X J, Tian S Q, Chen Y, Liu B L. 2010. A modeling approach to identify optimal habitat and suitable fishing grounds for neon flying squid (Ommastrephes bartramii) in the Northwest Pacific Ocean. Fishery Bulletin, 108(1): 1-14.
|
Clarke M R. 1962. The identification of cephalopod " beaks" and the relationship between break size and total body weight. Bulletin of the British Museum (Natural History) (Zoology), 8: 419-480.
|
Espinoza-Morriberón D, Echevin V, Colas F, Tam J, Ledesma J, Vásquez L, Graco M. 2017. Impacts of El Niño events on the Peruvian upwelling system productivity. Journal of Geophysical Research:Oceans, 122(7): 5423-5444.
DOI:10.1002/jgrc.v122.7 |
Fang Z, Chen X J, Su H, Thompson K, Chen Y. 2017. Evaluation of stock variation and sexual dimorphism of beak shape of neon flying squid, Ommastrephes bartramii, based on geometric morphometrics. Hydrobiologia, 784(1): 367-380.
DOI:10.1007/s10750-016-2898-0 |
Fang Z, Liu B L, Chen X J, Jin Y, Li J H, Chen Y. 2016. Sexual asynchrony in the development of beak pigmentation for the neon flying squid Ommastrephes bartramii in the North Pacific Ocean. Fisheries Science, 82(5): 737-746.
DOI:10.1007/s12562-016-1011-y |
Fang Z, Liu B L, Li J H, Su H, Chen X J. 2014. Stock identification of neon flying squid (Ommastrephes bartramii) in the North Pacific Ocean on the basis of beak and statolith morphology. Scientia Marina, 78(2): 239-248.
DOI:10.3989/scimar.2014.78n2 |
Fang Z, Xu L L, Chen X J, Liu B L, Li J H, Chen Y. 2015. Beak growth pattern of purpleback flying squid Sthenoteuthis oualaniensis in the eastern tropical Pacific equatorial waters. Fisheries Science, 81(3): 443-452.
DOI:10.1007/s12562-015-0857-8 |
Field J C, Baltz K, Phillips A J, Walker W A. 2007. Range expansion and trophic interactions of the jumbo squid, Dosidicus Gigas, in the California Current. California Cooperative Oceanic Fisheries Investigations Report, 48: 131-146.
|
Franco-Santos R M, Iglesias J, Domingues P M, Vidal E A G. 2014. Early beak development in Argonauta nodosa and Octopus vulgaris (Cephalopoda:incirrata) paralarvae suggests adaptation to different feeding mechanisms. Hydrobiologia, 725(1): 69-83.
DOI:10.1007/s10750-013-1721-4 |
Franco-Santos R M, Vidal E A G. 2014. Beak development of early squid paralarvae (Cephalopoda:teuthoidea) may reflect an adaptation to a specialized feeding mode. Hydrobiologia, 725(1): 85-103.
DOI:10.1007/s10750-013-1715-2 |
Froese R. 2006. Cube law, condition factor and weight-length relationships:history, meta-analysis and recommendations. Journal of Applied Ichthyology, 22(4): 241-253.
DOI:10.1111/jai.2006.22.issue-4 |
García V H. 2003. Growth and pigmentation process of the beaks of Todaropsis eblanae (Cephalopoda: ommastrephidae). Berliner Paläobiologische Abhandlungen, Berlin, 3: 131-140.
|
Gröger J, Piatkowski U, Heinemann H. 2000. Beak length analysis of the Southern Ocean squid Psychroteuthis glacialis (Cephalopoda:psychroteuthidae) and its use for size and biomass estimation. Polar Biology, 23(1): 70-74.
DOI:10.1007/s003000050009 |
Guerra Á, Rodríguez-Navarro A B, González Á F, Romanek C S, Álvarez-Lloret P, Pierce G J. 2010. Life-history traits of the giant squid Architeuthis dux revealed from stable isotope signatures recorded in beaks. ICES Journal of Marine Science:Journal du Conseil, 67(7): 1425-1431.
|
Hernańdez-García V, Piatkowski U, Clarke M R. 1998. Development of the darkening of Todarodes sagittatus beaks and its relation to growth and reproduction. South African Journal of Marine Science, 20(1): 363-373.
DOI:10.2989/025776198784126485 |
Hoving H J T, Gilly W F, Markaida U, Benoit-Bird K J, Brown Z W, Daniel P, Field J C, Parassenti L, Liu B L, Campos B. 2013. Extreme plasticity in life-history strategy allows a migratory predator (jumbo squid) to cope with a changing climate. Global Change Biology, 19(7): 2089-2103.
DOI:10.1111/gcb.12198 |
Hu G Y, Chen X J, Fang Z. 2016a. Effect of individual growth on beak morphometry of jumbo flying squid, Dosidicus gigas off the Peruvian Exclusive Economic Zone. Journal of Fisheries of China, 40(1): 36-44.
(in Chinese with English abstract) |
Hu G Y, Fang Z, Liu B L, Yang D, Chen X J, Chen Y. 2016b. Age, growth and population structure of jumbo flying squid Dosidicus gigas off the Peruvian Exclusive Economic Zone based on beak microstructure. Fisheries Science, 82(4): 579-604.
|
Hu G Y, Jin Y, Chen X J. 2017. Beak morphological characteristics of Dosidicus gigas off the Peruvian Exclusive Economic Zone (EEZ) and their relationship with body size and daily age. Marine Fisheries, 39(4): 361-371.
(in Chinese with English abstract) |
Ikica Z, Vuković V, Đurović M, Joksimović A, Krstuluvić Šifner S. 2014. Analysis of beak morphometry of the horned octopus Eledone cirrhosa, Lamarck 1798 (Cephalopoda:octopoda), in the south-eastern Adriatic Sea. Acta Adriatica, 55(1): 43-56.
|
Jackson G D, McKinnon J F. 1996. Beak length analysis of arrow squid Nototodarus sloanii (Cephalopoda: ommastrephidae) in southern New Zealand waters. Polar biology, 16(3): 227-230.
DOI:10.1007/BF02329211 |
Keyl F, Argüelles J, Mariátegui L, Tafur R, Wolff M, Yamashiro C. 2008. A hypothesis on range expansion and spatiotemporal shifts in size-at-maturity of jumbo squid (Dosidicus gigas) in the Eastern Pacific Ocean. CalCOFI Report, 49: 119-128.
|
Lalas C. 2009. Estimates of size for the large octopus Macroctopus maorum from measures of beaks in prey remains. New Zealand Journal of Marine and Freshwater Research, 43(2): 635-642.
DOI:10.1080/00288330909510029 |
Lefkaditou E, Bekas P. 2004. Analysis of beak morphometry of the horned octopus Eledone cirrhosa (Cephalopoda: octopoda) in the Thracian Sea (NE Mediterranean). Mediterranean Marine Science, 5(1): 143-149.
DOI:10.12681/mms.219 |
Lipiński M R, Underhill L G. 1995. Sexual maturation in squid:quantum or continuum?. South African Journal of Marine Science, 15(1): 207-223.
DOI:10.2989/02577619509504844 |
Liu B L, Chen X J, Chen Y, Tian S Q, Li J H, Fang Z, Yang M Z. 2013. Age, maturation, and population structure of the Humboldt squid Dosidicus gigas off the Peruvian Exclusive Economic Zones. Chinese Journal of Oceanology and Limnology, 31(1): 81-91.
DOI:10.1007/s00343-013-2036-z |
Liu B L, Fang Z, Chen X J, Chen Y. 2015. Spatial variations in beak structure to identify potentially geographic populations of Dosidicus gigas in the Eastern Pacific Ocean. Fisheries Research, 164: 185-192.
DOI:10.1016/j.fishres.2014.12.001 |
Lu C C, Ickeringill R. 2002. Cephalopod beak identification and biomass estimation techniques: tools for dietary studies of southern Australian finfishes. Museum Victoria Science Reports, Victoria, Australia. https://www.researchgate.net/publication/283437777_Cephalopod_beak_identification_and_biomass_estimation_techniques_Tools_for_dietary_studies_of_southern_Australian_finfishes
|
Markaida U, Quiñónez-Velázquez C, Sosa-Nishizaki O. 2004. Age, growth and maturation of jumbo squid Dosidicus gigas (Cephalopoda:ommastrephidae) from the Gulf of California, Mexico. Fisheries Research, 66(1): 31-47.
DOI:10.1016/S0165-7836(03)00184-X |
Martínez P, Sanjuan A, Guerra A. 2002. Identification of Illex coindetii, I.illecebrosus and I. argentinus (Cephalopoda: ommastrephidae) throughout the Atlantic Ocean; by body and beak characters. Marine Biology, 141(1): 131-143.
DOI:10.1007/s00227-002-0796-7 |
Mejia-Rebollo A, Quiñónez-Velázquez C, Salinas-Zavala C A, Markaida U. 2008. Age, growth and maturity of jumbo squid (Dosidicus gigas d'Orbigny, 1835) off the western coast of the Baja California Peninsula. CalCOFI Reports, 49: 256-262.
|
Miserez A, Li Y L, Waite J H, Zok F. 2007. Jumbo squid beaks: inspiration for design of robust organic composites. Acta Biomaterialia, 3(1): 139-149.
DOI:10.1016/j.actbio.2006.09.004 |
Miserez A, Schneberk T, Sun C J, Zok F W, Waite J H. 2008. The transition from stiff to compliant materials in squid beaks. Science, 319(5871): 1816-1819.
DOI:10.1126/science.1154117 |
Nigmatullin C M, Nesis K N, Arkhipkin A. 2001. A review of the biology of the jumbo squid Dosidicus gigas (Cephalopoda:ommastrephidae). Fisheries Research, 54(1): 9-19.
DOI:10.1016/S0165-7836(01)00371-X |
Pecl G T, Moltschaniwskyj N A, Tracey S R, Jordan A R. 2004. Inter-annual plasticity of squid life history and population structure:ecological and management implications. Oecologia, 140(2): 380.
DOI:10.1007/s00442-004-1619-y |
Radenac M H, Léger F, Singh A, Delcroix T. 2012. Sea surface chlorophyll signature in the tropical Pacific during eastern and central Pacific ENSO events. Journal of Geophysical Research:Oceans, 117(C4): C04007.
|
Raya C P, Hernández-González C L. 1998. Growth lines within the beak microstructure of the octopus Octopus vulgaris Cuvier, 1797. South African Journal of Marine Science, 20(1): 135-142.
DOI:10.2989/025776198784126368 |
Robinson C J, Gómez-Gutiérrez J, de León D A S. 2013. Jumbo squid (Dosidicus gigas) landings in the Gulf of California related to remotely sensed SST and concentrations of chlorophyll a (1998-2012). Fisheries Research, 137: 97-103.
DOI:10.1016/j.fishres.2012.09.006 |
Rodríguez-Domínguez A, Rosas C, Méndez-Loeza I, Markaida U. 2013. Validation of growth increments in stylets, beaks and lenses as ageing tools in Octopus maya. Journal of Experimental Marine Biology and Ecology, 449: 194-199.
DOI:10.1016/j.jembe.2013.10.001 |
Sandoval-Castellanos E, Uribe-Alcocer M, Díaz-Jaimes P. 2007. Population genetic structure of jumbo squid (Dosidicus gigas) evaluated by RAPD analysis. Fisheries Research, 83(1): 113-118.
DOI:10.1016/j.fishres.2006.09.007 |
Sandoval-Castellanos E, Uribe-Alcocer M, Díaz-Jaimes P. 2010. Population genetic structure of the Humboldt squid (Dosidicus gigas d'Orbigny, 1835) inferred by mitochondrial DNA analysis. Journal of Experimental Marine Biology and Ecology, 385(1-2): 73-78.
DOI:10.1016/j.jembe.2009.12.015 |
Schwing F B, Moor C S, Ralston S, Sakuma K M. 2000. Record coastal upwelling in the California Current in 1999. CalCOFI Reports, 41: 148-160.
|
Taipe A, Yamashiro C, Mariategui L, Rojas P, Roque C. 2001. Distribution and concentrations of jumbo flying squid (Dosidicus gigas) off the Peruvian coast between 1991 and 1999. Fisheries Research, 54(1): 21-32.
DOI:10.1016/S0165-7836(01)00377-0 |
Uyeno T A, Kier W M. 2007. Electromyography of the buccal musculature of octopus (Octopus bimaculoides):a test of the function of the muscle articulation in support and movement. Journal of Experimental Biology, 210: 118-128.
DOI:10.1242/jeb.02600 |
Villegas Bárcenas G, Perales-Raya C, Bartolomé A, Almansa E, Rosas C. 2014. Age validation in Octopus maya (Voss and Solís, 1966) by counting increments in the beak rostrum sagittal sections of known age individuals. Fisheries Research, 152: 93-97.
DOI:10.1016/j.fishres.2013.08.007 |
Waluda C M, Rodhouse P G. 2006. Remotely sensed mesoscale oceanography of the Central Eastern Pacific and recruitment variability in Dosidicus gigas. Marine Ecology Progress, 310: 25-32.
DOI:10.3354/meps310025 |
Waluda C M, Yamashiro C, Rodhouse P G. 2006. Influence of the ENSO cycle on the light-fishery for Dosidicus gigas in the Peru Current:an analysis of remotely sensed data. Fisheries Research, 79(1-2): 56-63.
DOI:10.1016/j.fishres.2006.02.017 |
Wolff G A. 1984. Identification and estimation of size from the beaks of 18 species of cephalopods from the Pacific Ocean. NOAA Technical Report NMFS 17, US.
|
Yu W, Yi Q, Chen X J, Chen Y. 2016. Modelling the effects of climate variability on habitat suitability of jumbo flying squid, Dosidicus gigas, in the Southeast Pacific Ocean off Peru. ICES Journal of Marine Science, 73(2): 239-249.
DOI:10.1093/icesjms/fsv223 |
Yu W, Yi Q, Chen X J, Chen Y. 2017. Climate-driven latitudinal shift in fishing ground of jumbo flying squid (Dosidicus gigas) in the Southeast Pacific Ocean off Peru. International Journal of Remote Sensing, 38(12): 3531-3550.
DOI:10.1080/01431161.2017.1297547 |
Zeidberg L D, Robinson B H. 2007. Invasive Range Expansion by the Humboldt Squid, Dosidicus gigas, in the Eastern North Pacific. Proceedings of the National Academy of Sciences of the United States of America, 104(31): 12948-12950.
DOI:10.1073/pnas.0702043104 |
 2019, Vol. 37
2019, Vol. 37