Institute of Oceanology, Chinese Academy of Sciences
Article Information
- WANG Jinyan, LI Bin, WANG Yingeng, LIAO Meijie, RONG Xiaojun, ZHANG Zheng
- Influences of immersion bathing in Bacillus velezensis DY-6 on growth performance, non-specific immune enzyme activities and gut microbiota of Apostichopus japonicus
- Journal of Oceanology and Limnology, 37(4): 1449-1459
- http://dx.doi.org/10.1007/s00343-019-8119-8
Article History
- Received May. 8, 2018
- accepted in principle Jul. 3, 2018
- accepted for publication Sep. 6, 2018
2 Shanghai Ocean University, Shanghai 201306, China
Sea cucumber (Apostichopus japonicus) is consumed widely due to its high nutritional value and functions of promoting health and preventing cancers (Purcella et al., 2012), and its farming has been increasing year by year accordingly. The scale of sea cucumber farming reached 218 000 hectares in 2016, with a yield of 200 000 tons in China (Fisheries and Fisheries Administration Bureau of the Ministry of Agriculture, 2017). However, a skin ulceration syndrome (SUS) caused by Vibrio splendidus and other bacterial pathogens has been leading to massive losses of A. japonicus farming (Becker et al., 2004; Deng et al., 2009; Zhao et al., 2012). In addition, the abuses of broad-spectrum antibiotics and chemicals caused heavy pollution of the water and the environment nearby, which may also lead to drug residues in sea cucumber and rise drug-resistant bacteria (Moriarty, 1997; Van, 1997; Luzhetskyy et al., 2007; Keen and Montforts, 2011; Pflumm, 2011). Fortunately, probiotics as effective substitutes for antibiotics and chemicals promised to be applicable for the prevention of diseases and promotion of the growth of aquatic livestock. The mechanisms underlining the performance of probiotics include competition with pathogens for nutrition and attachment (Miranda and Zemelman, 2001; Zhou et al., 2007), secretion of various enzymes and antibacterial peptides and promotion to immune response (Shiri et al., 1997; Irianto and Austin, 2002; Tuohy et al., 2003; Aguilar-Macías et al., 2010; Hai, 2015).
Probiotics have been applied in aquaculture since 1986 (Kozasa, 1986), and experienced a development of more than 30 years. Their application has been increasing, and their study has evolved as one of the hot topics. Several Bacillus strains now are broadly used in aquaculture, especially in the farming of fish, shrimp, and shellfish. Probiotics now play a significant role in aquatic farming, water quality improvement, bacterial pathogen inhibition, immune promotion and gut microbiota optimization (Salinas et al., 2005; Ma et al., 2014; Fang et al., 2015; Li et al., 2016; Jiang et al., 2018). Gullian et al. (2004) showed that Bacillus sp. addition in Fenneropenaeus chinensis for 28 days reduced the death rate of shrimp infected by Vibrio harveyi for 24 h from 51.75% to 18.5%. Balcázar and Rojas-Luna (2007) demonstrated that the average weight of Litopenaeus vannamei with the addition of Bacillus sp. P64 was significantly higher than that of control. Zhao et al. (2012) found that B. subtilis could improve the non-specific immune enzyme activities of A. japonicus. Sun et al. (2008) fed Epinephelus coioides with B. pumilus and B. clausii, and found that the activity of superoxide dismutase (SOD) increased by 11.4% and 17.4%, respectively. Karim et al. (2016) proved that the death rate of Crassostrea talienwhanensis affected by R. crassostreae and V. tubiashii was significantly lower than that of control when Phaeobacter sp. S4 or B. pumilus RI06-95 was used. However, probiotics application in A. japonicus farming is blindly to some extension due to the insufficiency of research. Wang et al. (2000) illustrated that the unreasonable use of probiotics would threat the farmed animals. Therefore, it is very important to analyze the influence of probiotics on A. japonicus, and to decipher the mechanisms underlining such influence.
In our previous studies, a local probiotic strain has been screened out from the sediment of A. japonicus farming ponds and identified as Bacillus velezensis DY-6 (Wang et al., 2018). In this study, this strain was used to study the effect of different concentrations of B. velezensis DY-6 on the growth of sea cucumbers and the non-specific immune enzyme activities of its coelom fluid in order to optimize the application optimum concentration of B. velezensis DY-6. Simultaneously, the effect of Bacillus velezensis DY-6 at the optimum concentration on the gut microbiota of sea cucumber was also determined. Our findings would lay a foundation for the rational use of probiotics in A. japonicus farming.
2 MATERIAL AND METHOD 2.1 Experimental Materials and experimental groupingBacillus velezensis DY-6 used in the experiment was screened out from A. japonicus farming ponds ourselves. Previous studies have revealed that DY-6 inhibit obviously the growth of several pathogens such asP.nigrifaciens, V.splendidus, V.parahaemolyticus and V. alginolyticus. It also has wide temperature and salinity adaptability (Wang et al., 2018). Healthy sea cucumber juveniles from a culture corporation in Qingdao with an average weight of 10.3±2.1 g were randomly grouped and acclimatized in four plastic tanks (83 cm×64 cm×60 cm) contain filtered and ozone sterilized seawater for 3 days. The sea cucumber individuals were randomly divided into 21 tanks containing 35 L seawater, 50 individuals each.
The experimental groups were set to different bathing concentrations of B. velezensis DY-6 (1×102, 1×103, 1×104, 1×105, 1×106 and 1×107 CFU/mL), three tanks each group. Simultaneously, three tanks (no B. velezensis DY-6) addition were used as control. The experiment lasted for 49 d, during which the temperature was maintained at 17±1.5℃, and 1/5 volume of seawater was replaced every day. The bacteria were added upon seawater was replaced. The sea cucumbers were fed a formula at 1% of the total weight of sea cucumber.
2.2 Determination of body weight gain rate of A.japonicusAhead and at the end of experiment, sea cucumber was counted, and the average weight, W0 and Wt, were measured, respectively. The body weight gain rate (WGR) was calculated as follows:
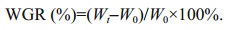
During the experiment, three individuals each tank were randomly every 7 days to collect coelom fluid and intestinal material. The coelom fluid was extracted with a disposable syringe and centrifuged at 4 000 r/min and 4℃ for 10 min with the supernatant transferred into sterilized centrifuge tubes for determining the nonspecific immune enzyme activities.
Acid phosphatase (ACP), alkaline phosphatase (AKP), lysozyme (LZM) and superoxide dismutase (SOD) were selected to represent the non-specific immune enzymes. Enzyme activities were determined using enzyme activity assay kits (Nanjing Jiancheng Bioengineering Institute) following the manufacturer's instructions.
2.4 Structure analysis of gut microbiota of A. japonicusThe optimum B. velezensis DY-6 concentration was selected based on the body weight gain rate of A. japonicus and non-specific immune enzyme activities. The microbiota community structure at this concentration was analyzed through 16S ribosomal RNA (rDNA) sequencing.
The sea cucumber was dissected with the intestine contents were removed and the intestine was washed three times with 1.5% NaCl. Each intestine was then transferred into a separate sterile centrifuge tube and stored at -80℃.
Microbial DNA was extracted using the E.Z.N.A. soil DNA Kit (Omega Biotek, Norcross, GA, U.S.) following the manufacturer's protocol. The V3–V4 region of the prokaryotic ribosomal RNA gene (16S rDNA) was amplified though PCR by denaturing at 95℃ for 2 min which was followed by 27 cycles of denaturing at 98℃ for 10 s, annealing at 62℃ for 30 s, and extending at 68℃ for 30 s and a final extension at 68℃ for 10 min. The primers were 341F (5′-CCTACGGGNGGCWGCAG-3′) and 806R (5′-GGACTACHVGGGTATCTAAT-3′) in which an eight-base barcode was inserted, respectively. Amplicatio product was separated in 2% agarose gels, purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, U.S.) following the manufacturer's instructions, and quantified on QuantiFluor-ST (Promega, U.S.). Purified product was pooled in equimolar and pairedend sequenced (2×250) on an Illumina Hiseq2500 PE250 platform.
2.5 Bioinformatics analysisTwo short Illumina reads were assembled into a complete V3–V4 region, and analyzed with the standard methods. Sequences that were shorter than 55 bp, contained primer mismatches, ambiguous bases or uncorrectable barcodes, were removed. The sequences were clustered into operational taxonomic units (OTUs) at a similarity threshold of ≥ 97% using a UPARSE pipeline (v9.2.64). Chao1, Ace and Shannon indices were calculated using the QIIME (V1.9.1) and principal coordinate analysis (PCoA) was carried out with R (https://www.r-project.org/).
2.6 Correlation between OTU abundance and nonspecific immune enzyme activitiesThe correlation coefficient between the abundance of OTU and non-specific immune enzyme activities was calculated with R (https://www.r-project.org/). OTUs with the Pearson correlation value > 0.7 or < -0.7 and P < 0.05 were selected out and annotated to the phyla based on Greengenes Database (https://www.arb-silva.de/), then the relationship between the OTU abundance and the non-specific immune enzyme activities was deduced.
2.7 Statistical analysisData were expressed as mean±standard deviations (SD). One-way analysis of variance (ANOVA) was performed to determine the significance of difference among groups. Duncan's multiple range test was used to compare the significance of difference among treatments. Statistical analyses were performed using SPSS version 17.0.
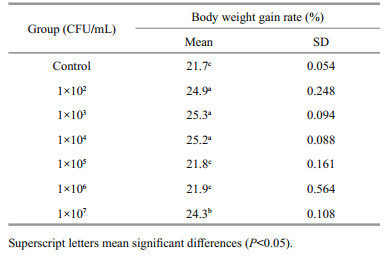
3 RESULT 3.1 Growth performanceThe body weight gain rate of A. japonicus in all the experimental groups is listed in Table 1. The result showed that WGR of all experimental groups was higher than or equal to that of control. The WGR of 1×102 CFU/mL, 1×103 CFU/mL and 1×104 CFU/mL groups was significantly higher than that of other groups (P < 0.05). The growth rate of the 1×103 CFU/mL group achieved the highest WGR (25.3%). The body weight gain rate of the 1×105 CFU/mL and 1×106 CFU/mL groups was not significantly different from that of control (P > 0.05), which was 21.8% and 21.9%, respectively. From our results, it can be seen that B. velezensis DY-6 improved the growth of A. japonicus and that 1×103CFU/mL was the optimum concentration.
|
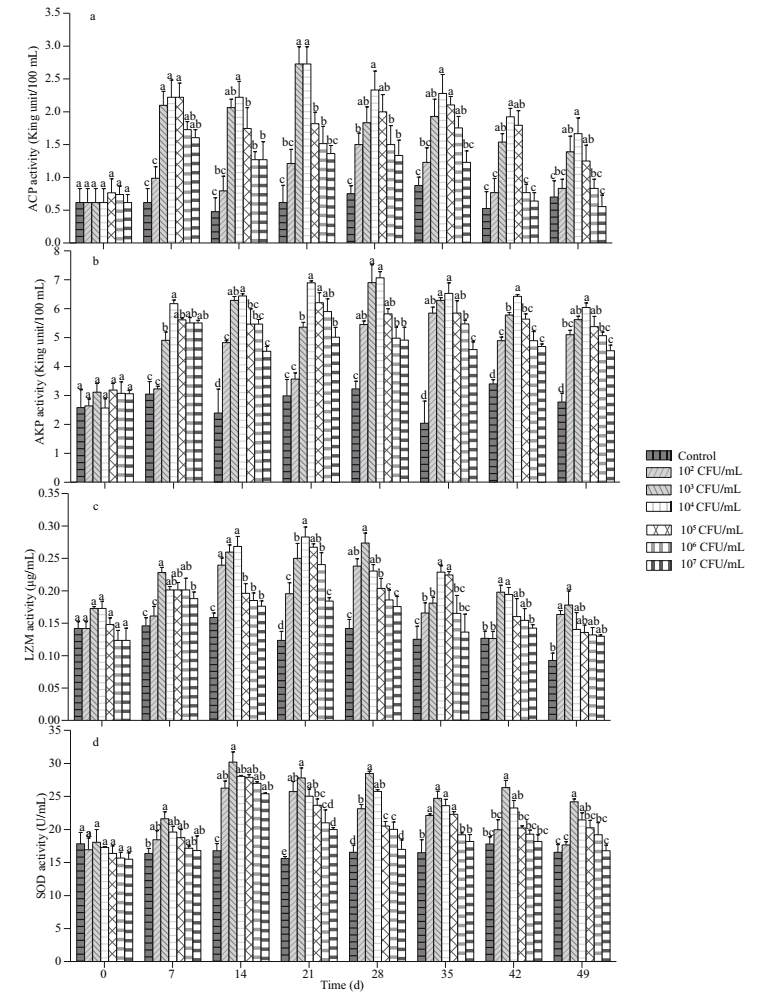
The effects of immersion bathing in different concentrations of B. velezensis DY-6 on the nonspecific immune enzyme activities of A. japonicus are shown in Fig. 1a–d. At the 0 d of the experiment, each non-specific immune enzyme activities had no significant difference among groups. Compared with the control, the non-specific immune enzyme activities fluctuated (increasing at early stages and decreasing at later stages) among experimental groups along observing dates. The ACP activity of 1×103 and 1×104 CFU/mL groups peaked on day 21, which was significantly higher than that of other two groups (P < 0.05). The AKP activity of all experimental groups was significantly higher than that of control (P < 0.05) after day 14, and reached the highest on day 28 in 1×104 CFU/mL group. As to the activity of LZM, that of 1×103 and 1×104 CFU/mL groups was significantly higher than that of control (P < 0.05) in the first 42 days, consistently peaked from day 14 to day 28, and then decreased to a value similar to that of control on day 49 in 1×104 CFU/mL group (P > 0.05). The SOD activity of A. japonicus was significantly higher than that of control (P < 0.05) in 1×103 CFU/mL group and reached its peak from day 14 to day 28. These findings indicated that 1×103 and 1×104 CFU/mL groups showed the best effect.
|
| Fig.1 The effect of different concentrations of B. velezensis on the activity of ACP (a), AKP (b), LZM (c) and SOD (d) of A.japonicus (mean±SD, n=3) |
According to the body weight gain rate and economic cost, the concentration of B. velezensis DY- 6, 1×103 CFU/mL, was accepted as the optimum. At 1×103 CFU/mL, the influence of B. velezensis DY-6 on the gut microbiota of A. japonicus during the experimental period (from day 0 to day 49) was evaluated through 16S rDNA sequencing analysis.
The top 10 OTUs of each sample were selected, and the stack map of species distribution was constructed at phylum level using all selected OTUs (Fig. 2). At the beginning (day 0), the relative abundances of the bacterial phyla Proteobacteria, Bacteroidetes, Verrucomicrobia and Firmicutes were relatively higher, which was 47.5%, 36.2%, 9.16% and 5.51%, respectively. On day 7, the species distribution did not change significantly. The relative abundance of Planctomycetes increased significantly on day 14 (0.045 4% vs. 0.513 2%, P < 0.05). On day 21, the relative abundance of Planctomycetes increased significantly in comparison with that of day 0 (0.045 4% vs. 0.874 8%, P < 0.01). On day 28, the relative abundance of Proteobacteria significantly increased (47.506 3% vs. 85.003 3%, P < 0.01) and the relative abundance of Bacteroidetes decreased significantly (36.170 8% vs. 4.603 1%, P < 0.05) compared with that on day 0. The species distribution did not change significantly on day 35 compared with that on day 0, but there was a significant difference from that on day 28. On day 42, the relative abundance of Cyanobacteria was significantly decreased (0.947 6% vs. 0.095 4%, P < 0.05). On day 49, the relative abundances of Planctomycetes and Verrucomicrobia were significantly increased (0.045 4% vs. 0.233 7%, P < 0.05; 9.162% vs. 32.149 2%, P < 0.01, respectively), and the relative abundance of Firmicutes decreased significantly (5.507 7% vs. 0.244%, P < 0.05). The addition of B. velezensis DY-6 induced gut microbiota of sea cucumbers into a dynamic process, the relative abundance of Planctomycetes, Proteobacteria, Bacteroidetes, Cyanobacteria, Verrucomicrobia and Firmicutes changed significantly during the experiment period.
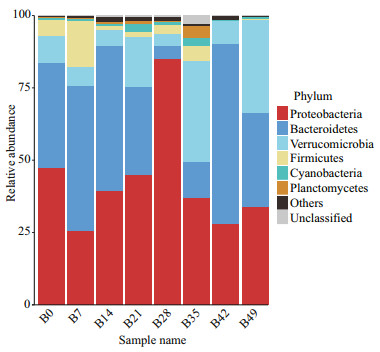
|
| Fig.2 Relative abundances of the gut microbiota at the phylum level in sea cucumber in 1×103 CFU/mL group The stack column diagram indicates the bacterial phyla composition at different times, and different colors represent different kinds of bacterial phyla. The characters B0, B7, B14, B21, B28, B35, B42 and B49, respectively, represent 0 d, 7 d, 14 d, 21 d, 28 d, 35 d, 42 d, and 49 d (n=3). |
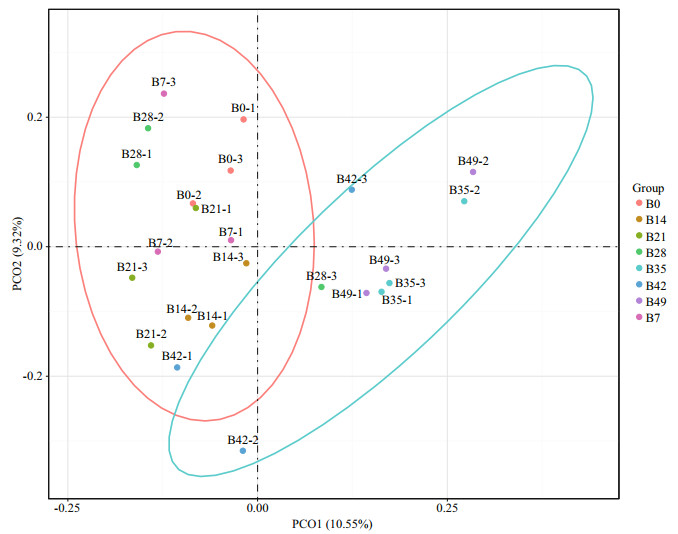
|
| Fig.3 Principal coordinates analysis (PCoA) of the gut microbiota in sea cucumber PCoA was plotted to summarize the microbial compositional differences between samples of different groups. Points that are closer each other represent microbial communities that are more similar. The characters B0, B7, B14, B21, B28, B35, B42 and B49, represent 0 d, 7 d, 14 d, 21 d, 28 d, 35 d, 42 d and 49 d, respectively, n=3. |
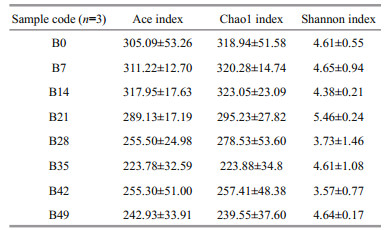
The Ace and Chao1 index reached their highest on day 14, which indicated that the intestine of A. japonicus on day 14 had the highest bacterial richness. The Shannon index reached the highest on day 21, which indicated immersion bathing in B. velezensis DY-6, the biodiversity of the intestine reached the peak on day 21.
Principal coordinates analysis (PCoA) shows the similarity between samples in difference distance. This analysis divided the samples into two clusters based on a 95% confidence interval, B0, B7, B14, B21, and B28 as cluster one and B35, B42 and B49 as cluster 2, indicating that a dramatic change of intestinal microbiota community structure has taken place on day 28.
3.4 Correlation analysis between enzyme activities and the abundance of OTUsThe correlation efficiency between the abundance of OTUs and the changing trend of non-specific immune enzyme activities of coelom fluid is listed in Table 3 and illustrated in Fig. 4a–d. The OTU which had significant correlationship with each enzyme activities and the highest Pearson correlation absolute value was screened out and annotated. Totally, four OTUs (OTU164, OTU304, OTU534, and OTU122) were selected. There is a positive correlation between OTUs and non-specific immune enzyme activity if the Pearson correlation is positive, while a negative value indicates a negative correlation. OTU164 was negatively correlated with ACP activity (P < 0.05) with a Pearson correlation coefficient value of -0.83. OTU304 was negatively correlated with AKP activity (P < 0.01) and the Pearson correlation coefficient value was -0.94. OTU534 was negatively correlated with LZM activity (P < 0.01) and the Pearson correlation coefficient value was -0.89. OTU122 was negatively correlated with SOD activity (P < 0.01) and Pearson's correlation coefficient value was -0.86. OTU164, OTU304, OTU534, and OTU122 were annotated as Firmicutes, Bacteroidetes, Proteobacteria, and Firmicutes, respectively.
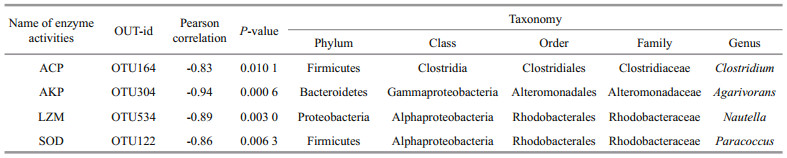
|
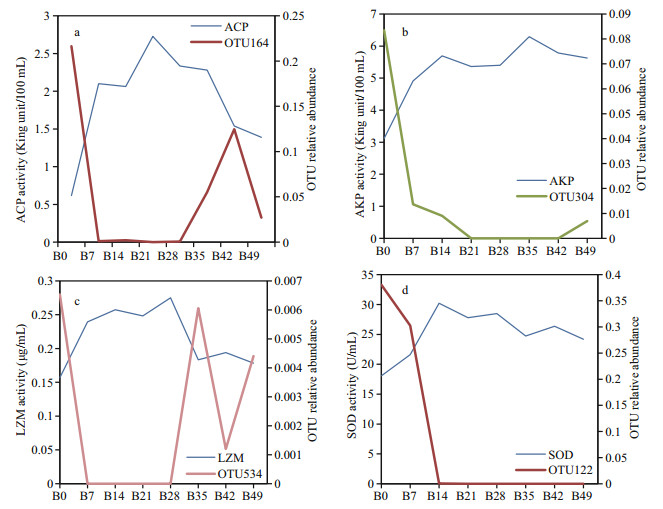
|
| Fig.4 The correlation analysis between the selected OTUs and the change trend of non-specific immune enzyme activities |
Probiotics can stimulate the appetite of cultured organisms, promote nutrient absorption and optimize energy intake through the secretion of extracellular enzymes. They can also regulate the community structure of intestinal microorganisms, accelerate the digestion and absorption of food, and increase the growth rate of cultured organisms (Gómez and Balcázar, 2008; Ringø et al., 2010). Ghosh et al. (2003) revealed that adding Bacillus sp. to the feed of Labeo rohita could significantly increase the growth of Labeo rohita, reduce feed conversion rate, and improve protein efficiency. Ziaei-Nejad et al. (2006) added Bacillus sp. to Fenneropenaeus indicus and discovered that its viability increased by 11%–17% and body weight increased by 8%–22% compared with control. In this study, we found that the B. velezensis DY-6 can improve the WGR of a sea cucumber to some extent, and the added bacteria at concentrations of (1×102)–(1×104) CFU/mL had significantly larger effects than at other concentrations. Liu et al. (2009) showed that adding different concentrations of Bacillus subtilis into the culture water of Oreochromis niloticus could notably increase the body weight by 666.8% and improve water quality when the concentration was 1×104 CFU /mL.
Probiotics can increase the body's humoral immunity and cellular immunity by stimulating the transformation of lymphocytes in the lamina propria of the intestinal mucosa. Regulating the immune system of both humans and animals is one of the most common benefits of probiotics (Panigrahi et al., 2017). Studies have shown that Bacillus sp. could enhance the non-specific immune enzyme activities of aquatic animals (Salinas et al., 2005; Balcázar and Rojas-Luna, 2007). The mechanism involves the bacteria themselves or components of the cell wall stimulating the non-specific immune system of the host, thereby enhancing animal immunity (Rengpipat et al., 2000). This study found that non-specific immune enzyme activities (ACP, AKP, SOD, and LZM) in the coelom fluid of A. japonicus increased at different concentrations of B. velezensis DY-6, which demonstrated that the addition of B. velezensis DY-6 can improve the immunity of A. japonicus. Nair et al. (2011) found that non-specific immune enzyme activities of Litopenaeus vannamei significantly increased after inoculation with Bacillus cereus, and Suzer et al. (2008) reported that Lactobacillus sp. could improve the growth performance and digestive enzyme activities of Sparus aurata larvae. In this study, we found also that the enzyme activities in the coelom fluid of A. japonicus in 1×103 CFU/mL and 1×104 CFU/mL groups were higher than that of control and reached their peaks either on day 21 or day 28. Liu et al. (2011) added Bacillus subtilis to the feed of hybrid sturgeon (Cipenser baerii ♂×Acipenser schrenckii ♀) juveniles and found that the LZM and SOD activities first increased and then decreased. Sui (2003) reported that adding different combinations of probiotic preparations to the aquaculture water of Litopenaeus vannamei could significantly increase activities of different kinds of non-specific immune enzymes and decrease after they maximized at different times. After using probiotics, the trend of enzyme activities was consistent with that of this study, but the peak times of non-specific immune enzyme activities were different, which may be caused by varietal differences in probiotics and cultured organisms.
Gut microbiota plays important roles in the host's nutrition, metabolism, growth, and differentiation of epithelial cells, regulation of immune functions, and protection from outside attacks (Böttcher et al., 2000; Forchielli and Walker, 2005; Vael et al., 2008). This study used the 16S rDNA sequence to analyze the gut microbiota of A. japonicus at the concentration of 1×103 CFU/mL. The result indicated that the intervention of B. velezensis DY-6 induced the structure of the gut microbiota of A. japonicus into a dynamic process. The same phenomena were also investigated in farmed fish and mice: Huys et al. (2001) found that the use of probiotics significantly altered gut microbiota of farmed fish. Zhao et al. (2017) found that using Bacillus subtilis can significantly change the gut microbiota of mice, which is consistent with the results of this study. Zhao et al. (2017) also deduced that using Bacillus subtilis could regulate intestinal flora structure possibly via interacting with intestinal microbial but not via colonizing. As for this study, the mechanism of B. velezensis DY-6 on A. japonicus should be investigated in future.
Many studies have found that changes in the abundance of certain bacteria in the gut can affect immune processes of the host. Garrett et al. (2010) and Carvalho et al. (2009) found that Proteobacteria contain plenty of gut pathogens and may cause intestinal inflammation. Smith et al. (2013) and Furusawa et al. (2013) found that decreasing the abundance of Firmicutes can reduce the production of short chain fatty acids, whereas short chain fatty acids can increase T-cell tolerance in the intestinal mucosa. Carding et al. (2015) found that gut microbiota could help the host to gain access to essential nutrients and energy to prevent intrusion from outside germs, thus maintaining the integrity of the intestinal barrier and promoting the development of the host's immune system. This study correlated the abundance of OTUs at different time points and the non-specific immune enzyme activities in the body fluid of A. japonicus. The results showed that after an immersion bathing with B. velezensis DY-6, there was a significant correlation between the changes in non-specific immune enzyme activities of A. japonicus and some bacteria in the sea cucumbers gut. However, the specific mechanism of action between the changes in the gut microbiota and non-specific immune enzyme activities after adding probiotics should be studied further.
Through analyzing the influence of different concentrations of B. velezensis DY-6 on non-specific immune enzyme activities in coelom fluid and the body weight gain rate of A. japonicus, this study determined that the best concentrations of B. velezensis DY-6 are 1×103 CFU/mL or 1×104 CFU/mL. Taking the cost into account, 1×103 CFU/mL is the optimum for practical use. The community structure of gut microbiota of A. japonicus was dynamic, and the change inflection point was on day 28 when B. velezensis DY-6 was used at the optimum concentration. Non-specific immune enzyme activities of the coelom fluid of A. japonicus reached their peaks on day 21 or day 28. This finding can provide a theoretical basis for using probiotics rationally in A. japonicus farming.
5 CONCLUSIONIn summary, the addition of B. velezensis DY-6 could significantly improve the growth of and nonspecific immune enzyme activities in sea cucumber, and the optimum concentration is 1×103 CFU/mL. The gut microbiota changed with the artificial involvement of B. velezensis DY-6 and could be obviously divided into two clusters on day 28. The activities changes for ACP, AKP, LZM, and SOD have significant correlations with the abundance of Firmicutes, Bacteroidetes, Proteobacteria, and Firmicutes, respectively.
6 DATA AVAILABILITY STATEMENTThe datasets generated and analyzed during the current study are available from the corresponding author.
Aguilar-Macías O L, Ojeda-Ramírez J J, Campa-Córdova A I, Saucedo P E. 2010. Evaluation of natural and commercial probiotics for improving growth and survival of the pearl oyster, Pinctada mazatlanica, during late hatchery and early field culturing. Journal of the World Aquaculture Society, 41(3): 447-454.
DOI:10.1111/jwas.2010.41.issue-3 |
Balcázar J L, Rojas-Luna T. 2007. Inhibitory activity of probiotic Bacillus subtilis UTM 126 against Vibrio species confers protection against Vibriosis in juvenile shrimp (Litopenaeus vannamei). Current Microbiology, 55(5): 409-412.
DOI:10.1007/s00284-007-9000-0 |
Becker P, Gillan D, Lanterbecq D, Jangoux M, Rasolofonirina R, Rakotovao J, Eeckhaut I. 2004. The skin ulceration disease in cultivated juveniles of Holothuria scabra (Holothuroidea, Echinodermata). Aquaculture, 242(1-4): 13-30.
DOI:10.1016/j.aquaculture.2003.11.018 |
Böttcher M F, Nordin E K, Sandin A, Midtvedt T, Björkstén B. 2000. Microflora-associated characteristics in faeces from allergic and nonallergic infants. Clinical & Experimental Allergy, 30(11): 1591-1596.
|
Carding S, Verbeke K, Vipond D T, Corfe B M, Owen L J. 2015. Dysbiosis of the gut microbiota in disease. Microbial Ecology in Health and Disease, 26(1): 26191.
|
Carvalho F A, Barnich N, Sivignon A. Darcha C, Chan C H, Stanners C P, Darfeuille-Michaud A. 2009. Crohn's disease adherent-invasive Escherichia coli colonize and induce strong gut inflammation in transgenic mice expressing human CEACAM. Journal of Experimental Medicine, 206(10): 2179-2189.
DOI:10.1084/jem.20090741 |
Deng H, He C B, Zhou Z C, Liu C, Tan K F, Wang N B, Jiang B, Gao X G, Liu W D. 2009. Isolation and pathogenicity of pathogens from skin ulceration disease and viscera ejection syndrome of the sea cucumber Apostichopus japonicus. Aquaculture, 287(1-2): 18-27.
DOI:10.1016/j.aquaculture.2008.10.015 |
Fang C, Ma M Y, Ji H, Ren T J, Mims S D. 2015. Alterations of digestive enzyme activities, intestinal morphology and microbiota in juvenile paddlefish, Polyodon spathula, fed dietary probiotics. Fish Physiology and Biochemistry, 41(1): 91-105.
|
Fisheries and Fisheries Administration Bureau of the Ministry of Agriculture. 2017. China Fishery Statistical YearBook. China Agriculture Press, Beijing, China, p.23-50. (in Chinese)
|
Forchielli M L, Walker W A. 2005. The role of gut-associated lymphoid tissues and mucosal defence. British Journal of Nutrition, 93(S1): S41-S48.
DOI:10.1079/BJN20041356 |
Furusawa Y, Obata Y, Fukuda S, Endo T A, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda N N, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke J M, Topping D L, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. 2013. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature, 504(7480): 446-450.
DOI:10.1038/nature12721 |
Garrett W S, Gallini C A, Yatsunenko T, Michaud M, DuBois A, Delaney M L, Punit S, Karlsson M, Bry L, Glickman J N, Gordon J I, Onderdonk A B, Glimcher L H. 2010. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host & Microbe, 8(3): 292-300.
|
Ghosh K, Sen S K, Ray A K. 2003. Supplementation of an isolated fish gut bacterium, Bacillus circulans, in formulated diets for Rohu, Labeo rohita, fingerlings. The Israeli Journal of Aquaculture-Bamidgeh, 55(1): 13-21.
|
Gómez G D, Balcázar J L. 2008. A review on the interactions between gut microbiota and innate immunity of fish. FEMS Immunology & Medical Microbiology, 52(2): 145-154.
|
Gullian M, Thompson F, Rodriguez J. 2004. Selection of probiotic bacteria and study of their immunostimulatory effect in Penaeus vannamei. Aquaculture, 233(1-4): 1-14.
DOI:10.1016/j.aquaculture.2003.09.013 |
Hai N V. 2015. The use of probiotics in aquaculture. Journal of Applied Microbiology, 119(4): 917-935.
DOI:10.1111/jam.12886 |
Huys L, Dhert P, Robles R, Ollevier F, Sorgeloos P, Swings J. 2001. Search for beneficial bacterial strains for turbot (Scophthalmus maximus L.) larviculture. Aquaculture. Aquaculture, 193(1-2): 25-37.
DOI:10.1016/S0044-8486(00)00474-9 |
Irianto A, Austin B. 2002. Use of probiotics to control furunculosis in rainbow trout, Oncorhynchus mykiss (Walbaum). Journal of Fish Diseases, 25(6): 333-342.
DOI:10.1046/j.1365-2761.2002.00375.x |
Jiang Y, Zhang Z, Wang Y G, Jing Y Y, Liao M J, Rong X J, Li B, Chen G P, Zhang H S. 2018. Effects of probiotic on microfloral structure of live feed used in larval breeding of turbot Scophthalmus maximus. Journal of Oceanology and Limnology, 36(3): 1002-1012.
DOI:10.1007/s00343-018-7049-1 |
Karim M, Zhao W J, Rowley D, Nelson D, Gomez-Chiarri M. 2016. Probiotic strains for shellfish aquaculture:protection of eastern oyster, Crassostrea virginica, larvae and juveniles against bacterial challenge. Journal of Shellfish Research, 32(2): 401-408.
|
Keen P L, Montforts M H M M. 2011. Antimicrobial Resistance in the Environment. John Wiley & Sons, Inc., Hoboken, NJ. p.325-335.
|
Kozasa M. 1986. Toyocerin (Bacillus toyoi) as growth promotor for animal feeding. Microbiologie Aliments Nutrition, 4(2): 121-135.
|
Li F H, Gao F, Tan J, Fan C J, Sun H L, Yan J P, Chen S Q, Wang X J. 2016. Characterization and identification of enzyme-producing microflora isolated from the gut of sea cucumber Apostichopus japonicus. Chinese Journal of Oceanology and Limnology, 34(1): 153-162.
DOI:10.1007/s00343-015-4149-z |
Liu H L, Huang X W, Li C L, Li R W, Luo Y P. 2009. Effect of different concentrations of Bacillus substilis on water quality and disease resistence of Tilapia fry. Journal of Aquaculture, 30(10): 5-9.
(in Chinese with English abstract) |
Liu X Y, Zhang Y, Qi Q, Zhao M J, Mai L K, Sun D J. 2011. Effects of Bacillus subtilis on growth, digestive enzyme activity, and non-specific immunity in hybrid sturgeon (Acipenser baeri ♂×Acipenser schrenkii ♀) juveniles. Journal of Fishery Sciences of China, 18(6): 1315-1320.
(in Chinese with English abstract) |
Luzhetskyy A, Pelzer S, Bechthold A. 2007. The future of natural products as a source of new antibiotics. Current Opinion in Investigational Drugs, 8(8): 608-613.
|
Ma Y X, Liu Z M, Yang Z P, Bao P Y, Zhang C Y, Ding J F. 2014. Effects of Hanseniaspora opuntiae C21 on the growth and digestive enzyme activity of juvenile sea cucumber Apostichopus japonicus. Chinese Journal of Oceanology and Limnology, 32(4): 743-748.
DOI:10.1007/s00343-014-3304-2 |
Miranda C D, Zemelman R. 2001. Antibiotic resistant bacteria in fish from the Concepción Bay, Chile. Marine Pollution Bulletin, 42(11): 1096-1102.
DOI:10.1016/S0025-326X(01)00093-5 |
Moriarty D J W. 1997. The role of microorganisms in aquaculture ponds. Aquaculture, 151(1-4): 333-349.
DOI:10.1016/S0044-8486(96)01487-1 |
Nair A G H, Donio M T B S, Viji V R T, Michaelbabu M, Citarasu T. 2011. Isolation from coconut retting effluent of Bacillus cereus TC-2 antagonistic to pathogenic Vibrios. Annals of Microbiology, 61(3): 631-637.
DOI:10.1007/s13213-010-0183-4 |
Panigrahi P, Parida S, Nanda N C, Satpathy R, Pradhan L, Chandel D S, Baccaglini L, Mohapatra A, Mohapatra S S, Misra P R, Chaudhry R, Chen H H, Johnson J A, Morris J G, Paneth N, Gewolb Ira H. 2017. A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature, 548(7668): 407-412.
DOI:10.1038/nature23480 |
Pflumm M. 2011. Persistence may pay off for antibiotics innovators. Nature Medicine, 17(6): 652.
DOI:10.1038/nm0611-652 |
Purcella S W, Hairb C A, Mills D J. 2012. Sea cucumber culture, farming and sea ranching in the tropics:progress, problems and opportunities. Aquaculture, 368-369: 68-81.
DOI:10.1016/j.aquaculture.2012.08.053 |
Rengpipat S, Rukpratanporn S, Piyatiratitivorakul S, Menasaveta P. 2000. Immunity enhancement in black tiger shrimp (Penaeus monodon) by a probiont bacterium (Bacillus S11). Aquaculture, 191(4): 271-288.
DOI:10.1016/S0044-8486(00)00440-3 |
Ringø E, Olsen R E, Gifstad T Ø, Dalmo R A, Amlund H, Hemre G I, Bakke A M. 2010. Prebiotics in aquaculture:a review. Aquaculture Nutrition, 16(2): 117-136.
DOI:10.1111/anu.2010.16.issue-2 |
Salinas I, Cuesta A, Esteban M Á, Meseguer J. 2005. Dietary administration of Lactobacillus delbrüeckii and Bacillus subtilis, single or combined, on gilthead seabream cellular innate immune responses. Fish & Shellfish Immunology, 19(1): 67-77.
|
Shiri Harzevili A R, Van D H, Defoort T, Dhert P, Sorgeloos P, Swings J. 1997. The influence of a selected bacterial strain Vibrio anguillarum TR 27 on the growth rate of rotifers in different culture conditions. Aquaculture International, 5(2): 183-188.
|
Smith P M, Howitt M R, Panikov N, Michaud M, Gallini C A, Bohlooly-Y M, Glickman J N, Garrett W S. 2013. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science, 341(6145): 569-573.
|
Sui D P. 2003. The Research of Effects of Microecologics on the Growth and Non-special Immunity Factors of Penaeus vannamei Boone. Ocean University of China, Qingdao, China. 78p. (in Chinese with English abstract)
|
Sun Y X, Jin L J, Wang T T, Xue G, Li X Y, You J S, Li S Y, Xu Y P. 2008. Polysaccharides from Astragalus membranaceus promote phagocytosis and superoxide anion (O2-) production by coelomocytes from sea cucumber Apostichopus japonicus in vitro. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 147(3): 293-298.
|
Suzer C, Çoban D, Kamaci H O, Saka Ş, Firat K, Otgucuoğlu Ö, Küçüksari H. 2008. Lactobacillus spp. Bacteria as probiotics in gilthead sea bream (Sparus aurata, L.) larvae:effects on growth performance and digestive enzyme activities. Aquaculture, 280(1-4): 140-145.
|
Tuohy K M, Probert H M, Smejkal C W, Gibson G R. 2003. Using probiotics and prebiotics to improve gut health. Drug Discovery Today, 8(15): 692-700.
DOI:10.1016/S1359-6446(03)02746-6 |
Vael C, Nelen V, Verhulst S L, Goossens H, Desager K N. 2008. Early intestinal Bacteroides fragilis colonisation and development of asthma. BMC Pulmonary Medicine, 8(1): 19.
DOI:10.1186/1471-2466-8-19 |
Van D B AE. 1997. Antimicrobial resistance-relation to human and animal exposure to antibiotics. Journal of Antimicrobial Chemotherapy, 40(3): 453-461.
DOI:10.1093/jac/40.3.453 |
Wang J Y, Li B, Wang Y G, Liao M J, Rong X J, Zhang Z, Niu Y Y, Ning L G. 2018. Screening and characteristic analysis of Bacillus velezensis from sea cucumber (Apostichopus japonicus) ponds. Journal of Fishery Sciences of China, 25(3): 567-575.
(in Chinese with English abstract) DOI:10.3724/SP.J.1118.2018.17383 |
Wang Y G, Lee K L, Najiah M, Shariff M, Hassan M D. 2000. A new bacterial white spot syndrome (BWSS) in cultured tiger shrimp Penaeus monodon and its comparison with white spot syndrome (WSS) caused by virus. Diseases of Aquatic Organisms, 41(1): 9-18.
|
Zhao L, Li L X, Chen X F, Zuo X L, Li Y Q. 2017. Effect of Bacillus subtilis gavage on the intestinal microbiota of mice. Journal of Shandong University (Health Science), 55(10): 28-35.
(in Chinese with English abstract) |
Zhao Y C, Zhang W B, Xu W, Mai K S, Zhang Y J, Liufu Z G. 2012. Effects of potential probiotic Bacillus subtilis T13 on growth, immunity and disease resistance against Vibrio splendidus infection in juvenile sea cucumber Apostichopus japonicus. Fish & Shellfish Immunology, 32(5): 750-755.
|
Zhou Z G, Ding Z K, Huiyuan L V. 2007. Effects of dietary short-chain fructooligosaccharides on intestinal microflora, survival, and growth performance of juvenile white shrimp, Litopenaeus vannamei. Journal of the World Aquaculture Society, 38(2): 296-301.
DOI:10.1111/jwas.2007.38.issue-2 |
Ziaei-Nejad S, Rezaei M H, Takami G A, Lovett D L, Mirvaghefi A R, Shakouri M. 2006. The effect of Bacillus spp. bacteria used as probiotics on digestive enzyme activity, survival and growth in the Indian white shrimp Fenneropenaeus indicus. Aquaculture, 252(2-4): 516-524.
|
 2019, Vol. 37
2019, Vol. 37