Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LI Wenjie, NIU Cuijuan, LI Xiaoxuan
- Offspring of aged mothers of rotifer Brachionus calyciflorus shows lower sexual propensity than their elder siblings under crowded conditions
- Journal of Oceanology and Limnology, 37(5): 1604-1610
- http://dx.doi.org/10.1007/s00343-019-8203-0
Article History
- Received Jul. 30, 2018
- accepted in principle Oct. 11, 2018
- accepted for publication Jan. 12, 2019
Maternal effects play an important role in evolution by manipulating the phenotypes of the offspring that related to fitness (Mousseau and Fox, 1998). Studies have shown that mothers can transmit their environmental cues such as presence of predator (Tollrian, 1995; Storm and Lima, 2010), food conditions (Alekseev and Lampert, 2004; Raveh et al., 2016), and population density (JoséCarmona et al., 1994; Sun and Niu, 2012; Dantzer et al., 2013). When the environment changes temporally and/or spatially on a scale in which the environmental conditions of offspring growth are predictable from parental environmental conditions, maternal effects can increase reasonably the fitness of offspring via preconditioning (Uller et al., 2013; Kuijper and Hoyle, 2015).
In addition to the maternal environment, maternal age (birth order) is another factor that can influence the life-history traits of offspring (Hercus and Hoffmann, 2000; Gilbert and McPeek, 2013; Gillespie et al., 2013). Since Lansing (1947) found that the offspring from old mothers had a shorter lifespan compared with those from young mothers, literatures concerning the effects of maternal age on life history traits of the offspring includes body size (Fox, 1993; Bernardo, 1996), viability (Hercus and Hoffmann, 2000; Kern et al., 2001; Berkeley et al., 2004) and lifespan (Plaistow et al., 2015). In an environment that fluctuates on an annual basis, later-born offspring may face higher population density or harsher environmental conditions. Mcintyre and Gooding (2000) reported that old female houseflies (Musca domestica) could produce larvae that are more competitive in a high density, than larvae from young females. If environmental conditions change during the mother's life span, offspring born from different ages of the mother may face different environmental conditions; thus, the phenotype and fitness of offspring depend on birth order through preconditioning maternal effect.
The monogonont rotifer Brachionus calyciflourus has a heterogonic life cycle alternating between asexual and sexual reproduction (Gilbert, 1974). Asexual reproduction via amictic female parthenogenesis allows rapid population growth (a short-term fitness for the population). On the other hand, sexual reproduction (mixis) leads to the production of resting eggs, which remain dormant and can survive in unfavorable conditions (a long term fitness for the population), but do not contribute to the current population growth (Stelzer, 2011). Thus, there is an interesting trade-off between asexual and sexual reproduction for a monogonont rotifer population (Serra et al., 2004; Schröder, 2005). Crowding has been recognized as an important trigger to initiate sexual reproduction in monogonant rotifers (Snell and Boyer, 1988; Gilbert, 2003). Furthermore, a protein signal that originates from crowding individuals was proved a type of trigger in rotifer Brachionus plicatilis (Snell, 1998; Snell et al., 2006). Exposure to crowded conditions significantly increases the amictic B. calyciflourus female's propensity to produce mictic daughters (Fussmann et al., 2003), and this effect is transgenerational. Sun and Niu (2012) found that amictic offspring of mothers from high density population produced a high proportion of mictic daughters even when they are at low density. Besides maternal population density, maternal age also affects the propensity to produce mictic daughters in B. calyciflorus, but in various patterns (Rougier and Pourriot, 1977; Fussmann et al., 2007; Gilbert and Schröder, 2007).
In spite of lots of reports documenting the effects of the maternal environment and maternal age on the sexual propensity of offspring, few studies examined the effects of interaction between maternal environments and maternal age in B. calyciflorus. Considering the deterioration of survival and reproductive ability with aging (Hercus and Hoffmann, 2000; Gillespie et al., 2013), and the differential contribution to population fitness between asexual and sexual offspring, mothers might influence the sexual propensity of offspring in different birth orders according to the current population density via transmission of epigenetic marks (Uller et al., 2015) or hormone (Gilbert, 2007; Yin et al., 2015).
The present study aimed to explore weather maternal age effects can be affected by maternal environment effects in the rotifer B. calyciflorus. We hypothesized that maternal age effects on the propensity of amictic females to produce micitic daughters might be influenced by maternal population density. This work may help to understand the variations in maternal effects as described by Gilbert and Schröder (2007) and Gilbert and McPeek (2013).
2 MATERIAL AND METHOD 2.1 Clone culture and experimental conditionsClonal cultures of B. calyciflourus were derived from one resting egg clone collected in September from Xihai Lake in Beijing, China (39°57ʹN, 116°21ʹE). We deposited the COI sequence of the studied clone in GenBank, accession number: MK344674. Using animals from one clone ancestor is to assure genetic similarities of the animals. Before the experiment, rotifers were reared at the population density of 0.025 female/mL in COMBO medium (Kilham et al., 1998) with Chlorella pyrenoidosa food (FACHB (Freshwater Algae Culture Collection of the Institute of Hydrobiology), 2016) (1×106 cells/mL) at 20℃ (light: dark 16 h:8 h) for 1 month. The medium was changed every two days. To exclude maternal effects, the first one-amictic offspring of the parent generation was repeatedly transferred to the new medium to propagate the next generation. The C. pyrenoidosa was cultured in SE medium, at a constant photoperiod (3 000 Lux) and 23℃.
2.2 Experimental design and procedureAccording to the procedures described by Gilbert (2002), low and high population densities were obtained by culturing single newly hatched amictic females (age < 2 h) in a daily renewed medium in small and large volumes, respectively. The low crowded environment (LD) consisted of 1 female per 40 mL medium and the high crowded (HD) of 1female per 1.5 mL medium. Two experiments (low maternal density experiment: F0 in LD, and high maternal density experiment: F0 in HD) were run parallelly. Newly hatched amictic females of B. calyciflourus (F0) were cultured individually in containers with either 40 mL fresh medium (LD) or 1.5 mL fresh medium (HD) and renewed daily. Any produced offspring (F1) were obtained in birth order and placed into 1.5 mL fresh medium (crowded environment) in 24-cell plastic dishes (Corning costar®) to be reared continually. Mictic F1 females were counted and picked out from each experiment. The medium was renewed daily and newly hatched daughters (F2) from each amictic F1 were removed and cultured individually in 24-cell plastic dishes until they could be categorized as amictic or mictic females. The proportion of mictic daughter (mixis ratio) produced by both F0 and F1 mothers were calculated, separately.
The number of replicate F0 females was 22 in LD treatment and 16 in HD treatment. Daughters (F1) born in the whole reproductive period of F0(5 days) were divided into two groups by the birth order. In LD, the F0mothers produced 2–7 offspring per day for 5 days, and in HD the F0 mothers produced 2–3 offspring per day for 5 days. As F0 mothers produced less offspring over the first day reproduction, those born in the first three days were named as the young mother group (YF0), while those born in the last two days were named as the old mother group (OF0). Thus, we obtained four treatment groups according to the F0 mother's population density and age, named as LD-YF0, LD-OF0, HD-YF0, and HD-OF0 respectively. In LD treatment, we obtained 152 and 135 F1 amictic females from young and old mothers, respectively. In HD treatment, there were 50 F1 amictic females from young mothers and 49 from old mothers.
2.3 Data analysisAll data were calculated as mean±standard error (SE). Mixis ratio of F1 and F2generations were arcsine transformed to fit with the homogeneity of variance (Levene test, P>0.05). We used two-way ANOVA to examine the effects of maternal (F0) age (young or old), maternal (F0) population density (LD or HD), and their interaction on the proportion of mictic females of F1 and F2. Post hoc student t-test was used to compare means. The significant level was set at P < 0.05. All statistical analyses were carried out using the statistical package SPSS version 19.0. Figures are drawn by Origin Pro version 9.0.
3 RESULTMothers (F0) in HD environment produced fewer daughters than those in LD (F1, 36=36.570, P < 0.001, Table 1). However, the former produced significantly more mictic daughters than the latter (F1, 36=43.490, P < 0.001, Table 1). In F0 generation, both age (F1, 72=9.480, P=0.003) and population density (F1, 72=61.600, P < 0.001) significantly affected the mixis ratio of F1. There was no significant interaction between age and population density (F1, 72=3.380, P=0.070). The mixis ratio of F1 from LD-YF0 did not differ from that of LD-OF0(F1, 42=1.340, P=0.260, Fig. 1), and the mixis ratio of F1 from HD-YF0 was muchhigherthan from HD-OF0(F1, 30=7.040, P=0.013, Fig. 1).

|
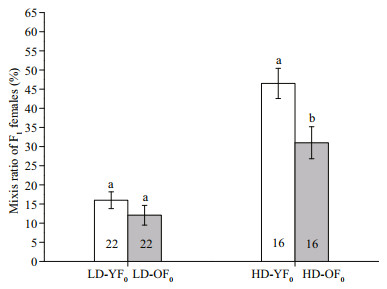
|
| Fig.1 Mixis ratio of F1produced by young or old F0 mothers at low population and high population density in Brachionus calyciflorus LD-YF0 and LD-OF0: young and old mothers F0 cultured in low population density treatment (1 ind. per 40 mL); HD-YF0 and HDOF0: young and old mothers F0 cultured in high population density treatment (1 ind. per 1.5 mL). Number below the bar is the sample size in the treatment. Error bars show the mean±SE. Different letters show a significant difference, significance level P < 0.05. |
In F1 generation, the mixis ratio of daughters was significantly affected by maternal age (F1, 72=11.560, P=0.001), maternal population density (F1, 72=5.720, P=0.019) and their interaction (F1, 72=4.750, P=0.032). Amictic F1 females from HD-YF0 produced more mictic F2 daughters than those from HD-OF0 (F1, 30=9.320, P=0.005, Fig. 2). There was no significant difference in daughters' mixis ratio between F1 females from LD-YF0 and LD-OF0 (F1, 42=1.290, P=0.260, Fig. 2).
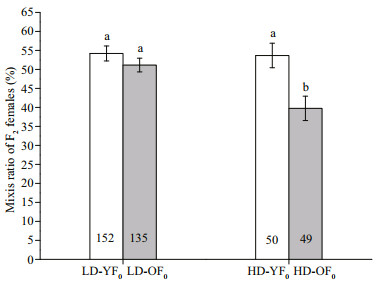
|
| Fig.2 Mixis ratio of F2 produced by amictic F1 which were obtained from young or old F0 mothers at low population and high population density in Brachionus calyciflorus LD-YF0 and LD-OF0: young and old mothers F0 cultured in low population density treatment (1 ind. per 40 mL); HD-YF0 and HDOF0: young and old mothers F0 cultured in high population density treatment (1 ind. per 1.5 mL). Number below the bar is the sample size in the treatment. Error bars show the mean±SE. Different letters show a significant difference, significance level P < 0.05. |
In F1 generation, maternal age (F1, 72=0.268, P=0.606), maternal population density (F1, 72=0.469, P=0.496) and their interaction (F1, 72=0.667, P=0.417) did not significantly affect the total offspring number. The total offspring number of F1 from YF0 was similar with that from OF0 both in HD (F1, 42=0.645, P=0.428, Fig. 3) and LD (F1, 42=0.062, P=0.805, Fig. 3) environments.

|
| Fig.3 Numbers of offspring of Brachionus calyciflorus produced by F1 that were obtained from young or old F0 mothers at low population and high population density in Brachionus calyciflorus LD-YF0 and LD-OF0: young and old mothers F0 cultured in low population density treatment (1 ind. per 40 mL); HD-YF0 and HDOF0: young and old mothers F0 cultured in high population density treatment (1 ind. per 1.5 mL). Number below the bar is the sample size in the treatment. Error bars show the mean±SE. Different letters show a significant difference, significance level P < 0.05. |
Overall, mothers of B. calyciflorus in HD environment produced more mictic daughters than those in LD environment. This result was congruent with those of several previous studies (Carmona et al., 1993; Stelzer and Snell, 2003; Gilbert, 2004, 2007; Sun and Niu, 2012), and confirmed the role of maternal age and crowded condition in mixis in B. calyciflorus.
Our results confirmed our hypothesis on the effect of maternal age and population density on the propensity of amictic females to produce mictic daughters. When mothers were in high population density offspring from younger mothers were more likely to be mictic females than those from older ones. Maternal age effects on the propensity of females to produce mictic daughters have been diverse in Brachionus in the literature (Fussmann et al., 2007; Gilbert and Schrӧder, 2007). Some studies showed that this propensity gradually decreased along with increasing age (Rougier and Pourriot, 1977). Fussmann et al. (2007) found that there was a maximal propensity in the middle of the reproductive period of the mother for B. calyciflorus. Gilbert and Schrӧder (2007) found five of six clones that did not show aging effects on the propensity of females to produce mictic daughters, and for the other clone, the propensity increased with maternal age. Gilbert and Schrӧder (2007) suggested a heritable genetic factor responsible for the variation in the above propensity. According to our results, maternal population density may also be another important factor for the variations in maternal age effects on the propensity to produce mictic daughters. Moreover, the influence of maternal crowding effects on maternal age trait was transgenerational. Even though all mothers were grown in an HD environment, amictic daughters produced by young mothers were more likely to produce mictic daughters than those produced by old mothers. Mixis ratios of the F2 generation were also similar among LD-YF0 and LDOF0 groups, indicating that the mixis ratio was mainly affected by the environment of F0. Transgenerational effects in B. calyciflourus have been documented in predator-induced defensive morphology (Yin et al., 2015), and crowding-induced mixis (Sun and Niu, 2012). It is considered adaptive when maternal environmental conditions accurately reflect the conditions of future generations and mothers modify the traits of offspring to best match the environmental conditions (Uller, 2008).
The timing and extent of sexual reproduction reflect the trade-off between short-and long-term fitness in cyclically parthenogenetic rotifers. Sexual reproduction is necessary for resting egg production, which can survive adverse conditions, but too early and too much might prevent population growth when the environment is still favorable (Serra et al., 2004). Population density works as a predictable environmental cue to the timing of mixis in Brachionus. When females were in LD environment, competition probability is relatively loose and the chances of encounters between fertilizable mictic females and males are low, and thus it might be advantageous for both young and old mothers to enlarge predominantly the population via asexual reproduction. When females were in HD environment, according to the male-female encounter hypothesis (Serra et al., 2004), the chances of encounters between males and fertilizable mictic females are high, and the production of resting eggs will be high, thus sexual reproduction is preferred. Considering the deterioration of survival and reproductive ability with increasing age, it is advantageous for the amictic females to firstly produce mictic daughters. The decreased propensity of mictic daughters in late reproductive period of the mother may be a bethedging strategy to maintain the population growth and offset the reduced population growth due to sexual reproduction as reported by Stelzer (2011). Age-mediated variance caused by maternal physiological aging has been found in the offspring's survival (Lansing, 1947; Berkeley et al., 2004; Opit and Throne, 2007) and fecundity (Lints and Hoste, 1977; Hercus and Hoffmann, 2000). Interestingly, in the present work, the number of F2 was similar between the early-and late-born F1 in both treatments, indicating that maternal age and environment effects influence mainly the mixis response of offspring rather the productive ability. As LD environment of the F0 mother is essential for the offspring to keep fast parthenogenetic population growth, we suggest low population density in rearing 'original clones' for mass rotifer culture in aquaculture.
This is the first study that investigated the interaction of maternal age and population density on the offspring's mixis response. Our study demonstrated that maternal age effects on the sexual propensity of offspring might be affected by maternal population density. This study might provide another explanation for the various patterns of the aging effects on the propensity of females to produce mictic daughters (Fussmann et al., 2007; Gilbert and Schrӧder, 2007), and be applied for mass rotifer culture for freshwater aquaculture.
5 CONCLUSIONMaternal age affected not only the propensity to produce mictic daughters, but also her offspring's sexual propensity in response to crowding. In addition, this maternal age effects can be influenced by the population density a mother is experiencing. In the LD environment, both the young and aged mothers are less likely to produce mictic daughters, and their amictic daughters have a low propensity to produce mictic females. When mothers are experiencing high population density they firstly produce mictic daughters, and amictic daughters from younger mothers have higher propensity to produce mictic females than those from aged mothers. The lower mixis response of females from aged mothers might result from the compensation of the reduced population size due to the sexual reproduction.
6 DATA AVAILABILITY STATEMENTThe data that support the findings of this study are available on request from the corresponding author (email: cjniu@bnu.edu.cn).
Alekseev V, Lampert W. 2004. Maternal effects of photoperiod and food level on life history characteristics of the Cladoceran Daphnia Pulicaria Forbes. Hydrobilogia, 526(1): 225-230.
DOI:10.1023/B:HYDR.0000041600.16226.12 |
Berkeley S A, Chapman C, Sogard S M. 2004. Maternal age as a determinant of larval growth and survival in a marine fish, Sebastes melanops. Ecology, 85(5): 1258-1264.
DOI:10.1890/03-0706 |
Bernardo J. 1996. The particular maternal effect of propagule size, especially egg size:patterns, models, quality of evidence and interpretations. Integr. Comp. Biol., 36(2): 216-236.
DOI:10.1093/icb/36.2.216 |
Carmona M J, Serra M, Miracle M R. 1993. Relationships between mixis in Brachionus plicatilis and preconditioning of culture medium by crowding. Hydrobiologia, 255-256(1): 145-152.
DOI:10.1007/BF00025832 |
Dantzer B, Newman A E M, Boonstra R, Palme R, Boutin S, Humphries M M, McAdam A G. 2013. Density triggers maternal hormones that increase adaptive offspring growth in a wild mammal. Science, 340(6137): 1215-1217.
DOI:10.1126/science.1235765 |
FACHB-2016 (Freshwater Algae Culture Collection of the Institute of Hydrobiology). 2016. SE (Brostol's solution)medium. http://algae.ihb.ac.cn/Products/ProductDetail.aspx?product=3.
|
Fox C W. 1993. The influence of maternal age and mating frequency on egg size and offspring performance in Callosobruchus maculatus (Coleoptera:Bruchidae). Oecologia, 96(1): 139-146.
DOI:10.1007/BF00318042 |
Fussmann G F, Ellner S P, Hairston N G. 2003. Evolution as a critical component of plankton dynamics. Proc. Roy. Soc.B Biol. Sci., 270(1519): 1015-1022.
DOI:10.1098/rspb.2003.2335 |
Fussmann G F, Kramer G, Labib M. 2007. Incomplete induction of mixis in Brachionus calyciflorus:patterns of reproduction at the individual level. Hydrobiologia, 593(1): 111-119.
DOI:10.1007/s10750-007-9041-1 |
Gilbert J J, McPeek M A. 2013. Maternal age and spine development in a rotifer:ecological implications and evolution. Ecology, 94(10): 2166-2172.
DOI:10.1890/13-0768.1 |
Gilbert J J, Schröder T. 2007. Intraclonal variation in propensity for mixis in several rotifers:variation among females and with maternal age. Hydrobiologia, 593(1): 121-128.
DOI:10.1007/s10750-007-9040-2 |
Gilbert J J. 1974. Dormancy in rotifers. Trans. Am. Microsc.Soc., 93(4): 490-513.
DOI:10.2307/3225154 |
Gilbert J J. 2002. Endogenous regulation of environmentally induced sexuality in a rotifer:a multigenerational parental effect induced by fertilisation. Freshw. Biol., 47(9): 1633-1641.
DOI:10.1046/j.1365-2427.2002.00900.x |
Gilbert J J. 2003. Environmental and endogenous control of sexuality in a rotifer life cycle:developmental and population biology. Evol. Dev., 5(1): 19-24.
DOI:10.1046/j.1525-142X.2003.03004.x |
Gilbert J J. 2004. Population density, sexual reproduction and diapause in monogonont rotifers:new data for Brachionus and a review. J. Limnol., 63(1S): 32-36.
DOI:10.4081/jlimnol.2004.s1.32 |
Gilbert J J. 2007. Induction of mictic females in the rotifer Brachionus:oocytes of amictic females respond individually to population-density signal only during oogenesis shortly before oviposition. Freshw. Biol., 52(8): 1417-1426.
DOI:10.1111/j.1365-2427.2007.01782.x |
Gillespie D O S, Russell A F, Lummaa V. 2013. The effect of maternal age and reproductive history on offspring survival and lifetime reproduction in preindustrial humans. Evolution, 67(7): 1964-1974.
DOI:10.1111/evo.12078 |
Hercus M J, Hoffmann A A. 2000. Maternal and grandmaternal age influence offspring fitness in Drosophila. Proc. Roy.Soc. B Biol. Sci., 267(1457): 2105-2110.
DOI:10.1098/rspb.2000.1256 |
JoséCarmona M, Serra M, Miracle M R. 1994. Effect of population density and genotype on life-history traits in the rotifer Brachionus plicatilis O.F. Müller. J. Exp. Mar.Biol.Ecol., 182(2): 223-235.
DOI:10.1016/0022-0981(94)90053-1 |
Kern S, Ackermann M, Stearns S C, Kawecki T J. 2001. Decline in offspring viability as a manifestation of aging in Drosophila melanogaster. Evolution, 55(9): 1822-1831.
DOI:10.1111/j.0014-3820.2001.tb00831.x |
Kilham S S, Kreeger D A, Lynn S G, Goulden C E, Herrera L. 1998. COMBO:a defined freshwater culture medium for algae and zooplankton. Hydrobiologia, 377(1-3): 147-159.
DOI:10.1023/A:1003231628456 |
Kuijper B, Hoyle R B. 2015. When to rely on maternal effects and when on phenotypic plasticity?. Evolution, 69(4): 950-968.
DOI:10.1111/evo.12635 |
Lansing A I. 1947. A transmissible, cumulative, and reversible factor in aging. J. Gerontol., 2(3): 228-239.
DOI:10.1093/geronj/2.3.228 |
Lints F A, Hoste C. 1977. The Lansing effect revisited. Ⅱ-Cumulative and spontaneously reversible parental age effects on fecundity in Drosophila melanogaster. Evolution, 31(2): 387-404.
DOI:10.1111/j.1558-5646.1977.tb01020.x |
Mcintyre G S, Gooding R H. 2000. Effects of maternal age on larval competitiveness in house flies. Heredity, 85(5): 480-489.
DOI:10.1046/j.1365-2540.2000.00787.x |
Mousseau T A, Fox C W. 1998. The adaptive significance of maternal effects. Trends Ecol. Evol., 13(10): 403-407.
DOI:10.1016/S0169-5347(98)01472-4 |
Opit G P, Throne J E. 2007. Influence of maternal age on the fitness of progeny in the rice weevil, Sitophilus oryzae(Coleoptera:Curculionidae). Environ. Entomol., 36(1): 83-89.
DOI:10.1603/0046-225X(2007)36[83:IOMAOT]2.0.CO;2. |
Plaistow S J, Shirley C, Collin H, Cornell S J, Harney E D. 2015. Offspring provisioning explains clone-specific maternal age effects on life history and life span in the water flea, Daphnia pulex. Am. Nat., 186(3): 376-389.
DOI:10.1086/682277 |
Raveh S, Vogt D, Kölliker M. 2016. Maternal programming of offspring in relation to food availability in an insect (Forficula auricularia). Proc. Roy. Soc. B Biol. Sci., 283(1828): 20152936.
DOI:10.1098/rspb.2015.2936 |
Rougier C, Pourriot R. 1977. Aging and control of the reproduction in Brachionus calyciflorus (Pallas)(Rotatoria). Exp. Gerontol., 12(3-4): 137-151.
DOI:10.1016/0531-5565(77)90022-5 |
Schröder T. 2005. Diapause in monogonont rotifers. Hydrobiologia, 546(1): 291-306.
DOI:10.1007/s10750-005-4235-x |
Serra M, Snell T W, King C E. 2004. The timing of sex in cyclically parthenogenetic rotifers. In: Moya A, Font E eds. Evolution: From Molecules to Ecosystems. Oxford University Press, Oxford. p.135-146.
|
Snell T W, Boyer E M. 1988. Thresholds for mictic female production in the rotifer Brachionus plicatilis (Muller). J.Exp. Mar. Biol. Ecol., 124(2): 73-85.
DOI:10.1016/0022-0981(88)90112-8 |
Snell T W, Kubanek J, Carter W, Payne A B, Kim J, Hicks M K, Stelzer C P. 2006. A protein signal triggers sexual reproduction in Brachionus plicatilis (Rotifera). Mar.Biol., 149(4): 763-773.
DOI:10.1007/s00227-006-0251-2 |
Snell T W. 1998. Chemical ecology of rotifers. Hydrobiologia, 387-388(0): 267-276.
DOI:10.1023/A:1017087003334 |
Stelzer C P, Snell T W. 2003. Induction of sexual reproduction in Brachionus plicatilis (Monogononta, Rotifera) by a density-dependent chemical cue. Limnol. Oceanogr., 48(2): 939-943.
DOI:10.4319/lo.2003.48.2.0939 |
Stelzer C P. 2011. The cost of sex and competition between cyclical and obligate parthenogenetic rotifers. Am. Nat., 177(2): E43-E53.
DOI:10.1086/657685 |
Storm J J, Lima S L. 2010. Mothers forewarn offspring about predators:a transgenerational maternal effect on behavior. Am.Nat., 175(3): 382-390.
DOI:10.1086/650443 |
Sun D, Niu C J. 2012. Maternal crowding can enhance the propensity of offspring to produce mictic females in the rotifer Brachionus calyciflorus. J. Plankton Res., 34(8): 732-737.
DOI:10.1093/plankt/fbs044 |
Tollrian R. 1995. Predator-induced morphological defenses:costs, life history shifts, and maternal effects in Daphnia pulex. Ecology, 76(6): 1691-1705.
DOI:10.2307/1940703 |
Uller T, English S, Pen I. 2015. When is incomplete epigenetic resetting in germ cells favoured by natural selection?Proc. Roy. Soc. B Biol. Sci., 282(1811): 20150682.
DOI:10.1098/rspb.2015.0682 |
Uller T, Nakagawa S, English S. 2013. Weak evidence for anticipatory parental effects in plants and animals. J. Evol.Biol., 26(10): 2161-2170.
DOI:10.1111/jeb.12212 |
Uller T. 2008. Developmental plasticity and the evolution of parental effects. Trends Ecol. Evol., 23(8): 432-438.
DOI:10.1016/j.tree.2008.04.005 |
Yin X W, Zhao N X, Wang B H, Li W J, Zhang Z N. 2015. Transgenerational and within-generational induction of defensive morphology in Brachionus calyciflorus(rotifera):importance of maternal effect. Hydrobiologia, 742(1): 313-325.
DOI:10.1007/s10750-014-1995-1 |
 2019, Vol. 37
2019, Vol. 37


