Institute of Oceanology, Chinese Academy of Sciences
Article Information
- WANG Shuping, ZHANG Yuan, HE Jia, JIA Xiaobo, LIN Jianing, LI Meng, WANG Qinglin
- Molecular analyses of bacterioplankton communities with highly abundant Vibrio clades: a case study in Bohai Sea coastal waters
- Journal of Oceanology and Limnology, 37(5): 1638-1648
- http://dx.doi.org/10.1007/s00343-019-8210-1
Article History
- Received Aug. 19, 2018
- accepted in principle Dec. 13, 2018
- accepted for publication Feb. 25, 2019
2 Institute for Advanced Study, Shenzhen University, Shenzhen 518000, China;
3 College of Animal Science and Technology, Hebei Normal University of Science and Technology, Qinhuangdao 066000, China
Bacterioplankton accounts for a large proportion of the species and the genetic diversity in the ocean (McArthur, 2006). Analysis of the bacterioplankton community composition is helpful to understand the potential impact of environmental changes on biogeochemical cycling and other critical ecosystem services (Rotini et al., 2017). Therefore, bacterioplankton is a promising indicator of ecosystem health in terms of biodiversity, the presence of pathogens and metabolic properties (Goodwin et al., 2017).
Vibrios belong to Gammaproteobacteria and are gram-negative (Hoffmann et al., 2010). In recent years, many studies have shown that the genus Vibrio is widely distributed in the marine environment (Oberbeckmann et al., 2011; Vezzulli et al., 2016; Westrich et al., 2016). It functions to remineralize organic matter (Fukami et al., 1985) and has a large impact on the biogeochemical cycling of key nutrients and trace metals (Westrich et al., 2016). Beyond that, some Vibrio species are pathogenic to humans and have been the subject of particular attention from researchers (Oberbeckmann et al., 2011). A long-term study found that Vibrio-related infections have occurred in the northern Europe and the Atlantic coast of the United States over the past 54 years, and the number of documented human cases parallels the observed increase in marine Vibrios (Vezzulli et al., 2016). It is speculated that the emergence of Vibrio as an etiological agent of the disease may increase over the next few years as ocean warming occurs (Paillard et al., 2004). The knowledge of an increase in Vibrio abundance may be useful for risk assessments of bacterioplankton blooms and understanding its potential roles in natural ecosystems.
The Bohai Sea is one of four Chinese marginal seas and is considered one of the most polluted marine areas in China (Wu et al., 2013; Gao et al., 2014). Mariculture mainly farming Argopectens irradias was undertaken in the shallow seawater from close inshore (one nautical mile) to ten nautical miles in the coastal Bohai Sea. The self-cleaning capacity of the Bohai Sea is limited because its connection with the outer ocean is narrow and the water-exchange rate is low. The Bohai Sea may serve as a large model system to study microbial eco-functionality and the biogeochemical cycling of pollutants in the presence and absence of intensive and extensive anthropogenic perturbations (Dang et al., 2013).
At present, there have been some publications on the distribution and abundance of bacterioplankton in the Bohai Sea (Zhao et al., 2013; Sun et al., 2016; Yu et al., 2018). Specific habitat and environmental conditions may promote the formation of unique bacterioplankton taxa; however, the distributions of major bacterioplankton taxa and their influential factors in Bohai Sea coastal waters remain poorly understood. Even if many Vibrios are endemic to coastal waters, there is only one study available on the distribution and abundance of pathogenic Vibrio species, including V. parahaemolyticus, V. alginolyticus, V. fluvialis, and V. harviyi in the Bohai Sea coastal environment (Peng et al., 2010). In this study, we characterized the bacterioplankton abundance and community composition in Bohai Sea coastal waters and investigated the relationships between the major taxa groups, alpha diversity, and environmental parameters. In addition, the detection of highly abundant Vibrio clades will allow us a new perspective of its ecological significance in Bohai Sea coastal waters.
2 MATERIAL AND METHOD 2.1 Study sites and sample collectionThe sampling sites were determined near a coastal aquaculture zone in the Bohai Sea. The maximum depth of the studied sites was no more than 10 m, especially the depth of site 1, which was only 3 m. Seawater samples of 10 L in triplicate at 0.5, 3, and 5 m depths were collected at each location near the coastal aquaculture zone of the Bohai Sea on May 12, 2016. Details of sampling are shown in Fig.S1 and Table S1. The triplicated water samples were mixed homogeneously and filtered immediately through a four-layer sterile mesh to remove organisms and debris of ≥30 mm diameter size. The filtrates were then passed through a 0.22-μm pore size polycarbonate membrane (Millipore, Billerica, MA, USA). The membranes were placed into sterile 5 mL freezer tubes and stored at -80℃ before DNA extraction.
The water temperature (Temp), dissolved oxygen (DO), pH, electrical conductivity (EC), total dissolved solids (TDS), and salinity were measured in situ with a YSI6600 profiler (YSI, Yellow Springs, OH, USA). The latitude and longitude coordinates were measured in the field using a portable GPS Explorist-200 (Magellan, Santa Clara, CA, USA). The concentrations of dissolved inorganic nitrogen (DIN) (ammonium, nitrate, and nitrite) and phosphate in the seawater samples were measured with a continuous flow analyzer (San++, SKALAR, Holland). The geographical and environmental parameters are also listed in Table S1.
2.2 DNA extraction and MiSeq-based 16S rRNA gene high-throughput sequencingAccording to the instructions of the FastDNA Spin Kit (MP Biomedicals, Solon, OH, USA), the total DNA was isolated directly from the membranes by an integrated mechanical and chemical procedure. The DNA was purified, dissolved in 30 μL ddH2O and stored at -20℃. The quality and quantity of DNA were confirmed by agarose gel electrophoresis and spectrophotometrically quantified with a NanoDrop ND 2000 (Thermo Fisher Scientific, Waltham, MA, USA).
The primers 515F (5′-GTGCCAGCMGCCGCGGTAA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) were used to amplify the V4 variable region of the 16S rRNA gene (Caporaso et al., 2012; Wear et al., 2018). The PCR amplification was performed as described by Caporaso et al. (2010). The amplicons were sequenced into 250-bp paired-end reads by the Illumina MiSeq platform at the Personal Biotechnology Company (BGI Genomics, Shenzhen, China).
2.3 Real-time PCR of total bacteria and Vibrio cladesThe total bacterial community and the Vibrios in each sample were quantified by real-time PCR. Realtime PCR with the primers 341F (5′-CCTACGGGNGGCWGCAG-3′) and 515R (5′- ATTCCGCGGCTGGCA-3′) was performed to assess the copy number of total bacterial 16S rRNA genes (Stackebrandt and Goodfellow, 1991; Yang et al., 2015). Genus-specific primers for Vibrio, 567F (5′-GGCGTAAAGCGCATGCAGGT-3′) and 680R (5′-GAAATTCTACCCCCCTCTACAG-3′), targeting the 16S rRNA genes were used for real-time PCR to assess the copy number of Vibrios (Thompson et al., 2004).
Clone sequencing was first used to construct standard curves. The PCR mixture was a 25-μL total volume containing: 0.5 μL primer set (2.5 mmol/L), 0.125 μL (5 U/μL) LA-Taq DNA polymerase, 2.5 μL of 10× LA-Taq Buffer, 2 μL dNTP mixture (2.5 mmol/L), 2 ng of DNA template, and 18.375 μL ddH2O. The PCR procedure was as follows: 95℃ for 3 min, 35 cycles at 95℃ for 30 s, 55℃ for 30 s and 72℃ for 30 s, and 72℃ for 10 min. The PCR products were purified, ligated into a pMD18-T vector (TaKaRa, Japan) and transformed into Escherichia coli DH5α cells (Tiangen, Beijing, China). The 16S rRNA gene fragments in plasmids were sequenced with an ABI3730 DNA Sequencer (Sangon Biotech, Shanghai, China). Successfully inserted plasmid DNA was extracted using a MiniBEST Plasmid Purification Kit Ver.4.0 (TaKaRa, Japan), and the plasmid concentration was determined with a NanoDrop ND 2000 (Thermo Fisher Scientific, Waltham, MA, USA).
An Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) was used to perform the real-time PCR. By plotting the cycle threshold values (Ct) versus the log10 of the copy number of 16S rRNA genes, the standard curve (equivalent to 103–108 copies of 16S rRNA genes per reaction volume) of real-time PCR was generated. According to the standard curve with known concentrations of template DNA, real-time PCR was performed to quantify the environmental samples. The amplification system was as follows: 10 μL SYBR Premix Ex TaqTM, 0.3 μL of each primer (10 μmol/L), 1 μL of total DNA, and 8.4 μL sterilized ddH2O. The real-time PCR procedure was carried out in accordance with the manufacturer's instructions (TaKaRa, Japan).
2.4 Data analysisLow-quality or ambiguous reads were removed, paired-end reads from the original sequences were merged, and chimeric sequences were discarded and checked using QIIME Version 1.9.158 (Caporaso et al., 2010). Clean tags were clustered into OTUs at a 97% similarity level (Stackebrandt and Goebel, 1994). To perform downstream analyses, the OTU matrix was normalized by setting the uniform sequence number to 25 460 for each sample. Taxonomic assignment of OTUs was performed using QIIME (Caporaso et al., 2010) against the SILVA v132 reference database (Yilmaz et al., 2014).
The rarefaction curve was generated using R software (V3.1.1) by plotting the observed species numbers versus the number of sequences sampled. To detect the differences between samples, the genera that had a relative >0.5% in at least one sample were utilized to make a heatmap. To estimate the alpha diversity of bacterial communities, multiple indices, including the observed species index, Chao index, Ace index, Shannon index, and Simpson index, were generated by the Mothur (V1.31.2).
The Pearson's Correlation was employed to evaluate the effect of environmental parameters, including longitude, latitude, seawater depth, seawater temperature, DO, pH, EC, TDS, salinity, phosphate, nitrate, nitrite, and ammonium, on the abundance of major taxonomic groups and the alpha diversity indices. A two-tailed test was performed to test the differences using SPSS 19.0 (SPSS, Chicago, IL, USA). P < 0.05 was considered statistically significant. Vibrio abundances were estimated by normalizing the specific 16S rRNA gene copy number using the average 16S rRNA gene copy number of Vibrios (nine, Acinas et al., 2004).
3 RESULT 3.1 Environmental characteristics and the bacterial 16S rRNA gene copy numbersThe environmental parameters, including latitude, longitude, seawater depth, seawater temperature, DO, pH, EC, TDS, salinity, NO2-, PO43-, NH4-, NO3-, are shown in Table S1. The seawater temperature, DO, pH, EC, TDS and salinity showed no obvious fluctuation among the different samples. The concentrations of NO2-, PO43-, NH4-, and NO3- in samples S1-3m were higher than in the other samples. The copy number of the 16S rRNA gene ranged from 1.2×108 to 6.8×109 copies/L (Fig. 1). The number of 16S rRNA gene copies in the deeper seawater was greater than that in the surface seawater (Fig. 1).
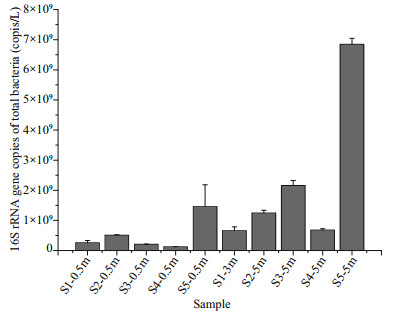
|
| Fig.1 The 16S rRNA gene copy numbers of total bacterioplankton in different samples The results are expressed as the mean±SE of three independent experiments. |
After denoising and chimera checking the raw sequences, 274 634 high quality paired-end clean tags with an average length of ~253 bp were generated. The number of high-quality sequences per sample ranged from 25 460 to 30 800 (mean 27 463, SD 1 895). A total of 485 OTUs were obtained from all these sequences with a cutoff of 97% sequence identity.
The rarefaction curve of 16S rRNA gene amplicons showed that all curves had overcome the exponential phase. Therefore, the number of sequences in each sample was sufficient to reflect the actual bacterioplankton diversity (Fig. 2). The rarefaction curve indicated that the samples S1-3m, S2-5m, and S3-5m samples had less biodiversity than the other samples.

|
| Fig.2 Rarefaction curve of 16S amplicons based on unique OTUs derived from MiSeq 16S rRNA gene amplicon data |
The taxonomic composition of the bacterioplankton community was described based on the OTUs. A total of 122 genera in 15 different phyla and some unclassified bacteria were found in these samples. The majority of bacterioplankton identified were Proteobacteria and Bacteroidetes, which comprised approximately 72.0% and 18.9% of the whole library, respectively (Fig. 3a). Gammaproteobacteria and Alphaproteobacteria were the most abundant classes in Proteobacteria. The Gammaproteobacteria included 111 OTUs with a relative abundance ranging from 32.0% (S4-5m) to 87.1% (S1-3m), followed by Alphaproteobacteria, which included 119 OTUs with a relative abundance ranging from 3.9% (S1-3m) to 32.8% (S4-5m) (Fig. 3b). The second predominant phylum was Bacteroidetes, with a relative abundance ranging from 5.3% (S1-3m) to 28.8% (S2-0.5m), and Flavobacteria was the most predominant class of Bacteroidetes (16. 8%) (Fig. 3b).The most abundant OTU in all of the samples belonged to the genera Pseudoalteromonas, Vibrio, and Octadecabacter, which consisted of (17.2±10.4)%, (11.1±8.9)% and (10.4±5.8)% of the total valid sequences, respectively. Obvious variations of Pseudoalteromonas, Vibrio and Octadecabacter were observed among different samples (Fig. 4). Notably, the abundances of Pseudoalteromonas and Vibrio in S1-3m, S2-5m, and S3-5m were much higher than those in S1-0.5m, S2- 0.5m, and S3-0.5m.
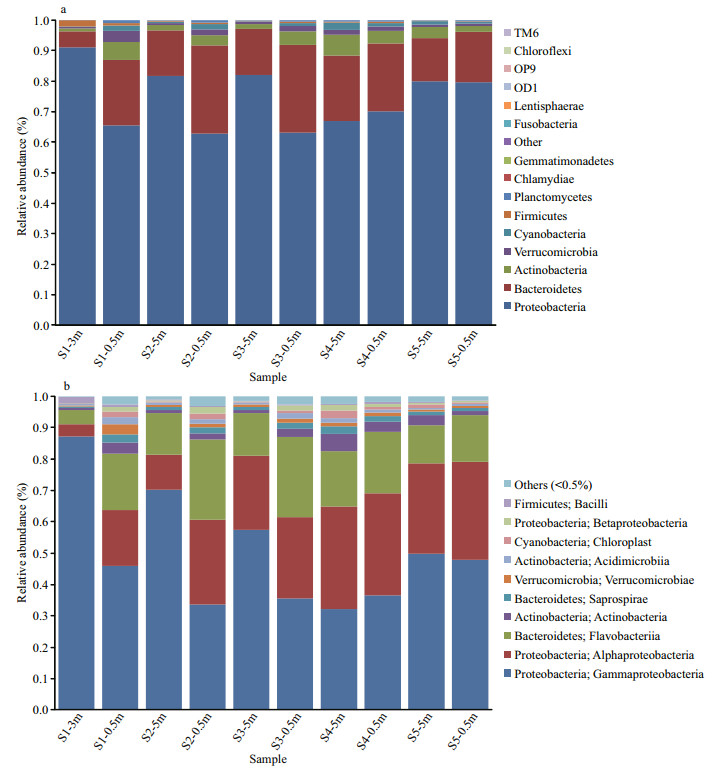
|
| Fig.3 Taxonomic classification of bacterial tags retrieved from different samples at phylum (a) and class levels (b) from 16S rRNA gene high-throughput sequences |
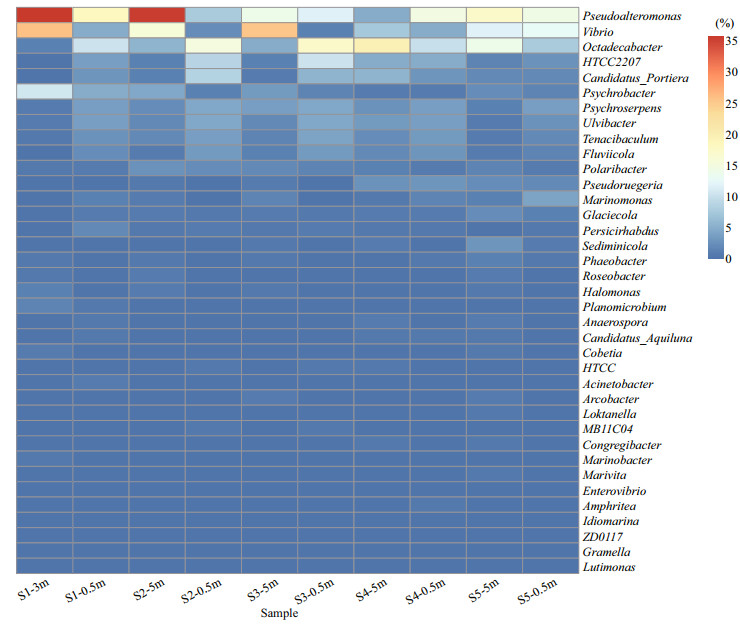
|
| Fig.4 The relative abundances of different bacterial genera from different locations The relative abundance of genera is reflected by the color of scale. Red-orange colors indicate the relative higher abundances in the samples, blue colors indicate the relative lower abundances in the samples. Percentage indicates relative abundance of each genus in each sample. |
Our dataset contained three Vibrio OTUs. Realtime PCR results show that high copy numbers of the Vibrio 16S rRNA gene existed in all of the samples. The copy number of the Vibrio 16S rRNA genes ranged from 2.6×106 to 6.3×108 copies/L (Fig. 5). The Vibrio cell concentration (/L) was evaluated as the quotient of 16S rRNA gene copy number divided by the average 16S rRNA gene copy number of Vibrio. The average 16S rRNA gene copy number of Vibrio strains was nine, which was obtained from a previous study (Acinas et al., 2004). The predicted Vibrio cell numbers and the proportion of their relative abundance in the different samples are listed in Table S3. The predicted cell numbers of Vibrios ranged from approximately 2.9×105 cells/L to 7.0×107 cells/L. The average relative proportion of Vibrios in all samples was 11.1%, and the highest proportions were 26% in S1-3m and 25% in S3-5m.
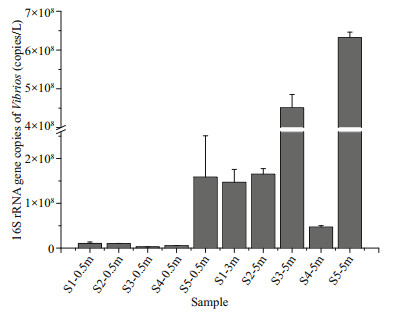
|
| Fig.5 The 16S rRNA gene copy numbers of Vibrios in different samples The results are expressed as the mean±SE of three independent experiments. |
The heatmap clearly shows the relationships between major bacterioplankton groups, alpha diversity and environmental factors (including latitude, longitude, depth, temperature, DO, pH, EC, TDS, salinity, NO2-, PO43-, NH4-, and NO3-) (Fig. 6). The genera Pseudoalteromonas and OTU1 (assigned to Pseudoalteromonas) were the most dominant taxa in all the samples. The environmental parameter PO43- was also positively related to the abundance of Pseudoalteromonas and OTU1 (P < 0.01). The abundance of Vibrio and Octadecabacter had no significant relationship with the environmental factors (P>0.05). The Sobs, Chao, Ace, Shannon and Simpson indices were used to evaluate the alpha diversity of the community in this study (Table S2). The environmental parameter PO43- was also negatively related to the Sobs and Shannon indices of the total bacterial communities (P < 0.05), and positively related to the Simpson index (P < 0.01).
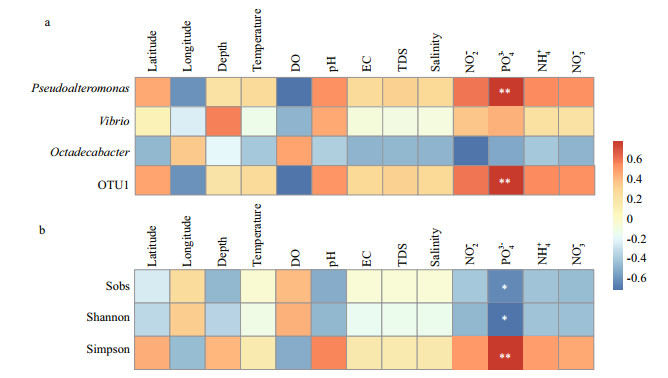
|
| Fig.6 Pearson's correlations between the most significant taxa and environmental parameters are shown in thi Color legend shows correlation coefficients. ** means correlation is significant at the 0.01 level (2-tailed); * means correlation is significant at the 0.05 level (2-tailed). |
In this study, seawater samples were collected from natural seawater at a maximum depth of 5 m because the depth in this region is no greater than 10 m, while the mean depth of the entire Bohai Sea is only 18 m (Lin et al., 2006). Culture-independent studies are essential for determining the number and relative abundance of different bacterial groups present in microbial communities because < 1% of bacteria in nature can be cultured with currently available methods, and uncultivable microbial species are widespread in the world's oceans (Cottrell and Kirchman, 2000). The rapid development of highthroughput 16S rRNA gene sequencing has enabled a fine-tuned assessment of the microbial taxonomic composition (Shendure and Ji, 2008; Ding et al., 2015). When accurate detection and abundance estimation is critical, 16S rRNA gene amplicon sequencing coupled with a real-time PCR method is a workable strategy, as applied in this study (Goodwin et al., 2017).
The 16S rRNA gene copies of bacterioplankton in the deeper seawater samples were, on average, 3.6- fold greater that in the upper seawater. The higher 16S rRNA gene copy numbers in samples from deeper waters could reflect higher bacterial abundance due to resuspension of bacteria from the sediment, or result from a shift in composition driven by bacterial taxa with higher 16S copies per cell, as suggested by higher abundances of Vibrios. The detection of 485 OTUs from 15 bacterioplankton phyla using highthroughput sequencing gave a more comprehensive view of the bacterioplankton community. Proteobacteria were the predominant bacterial phylum, and Gammaproteobacteria and Alphaproteobacteria were the predominant classes. Proteobacteria are notable as abundant free-living bacterioplankton both in coastal and open-ocean habitats (DeLong et al., 1993; Yang et al., 2015). Williams et al. (2013) revealed that the growth of Gammaproteobacteria in the presence of algal organic matter degraded Flavobacteria in the surface water of coastal East Antarctica. In this study, a high abundance of Gammaproteobacteria was found representing 49.5% of the total abundance. The overwhelming dominance of Gammaproteobacteria could be due to multiple factors, such as the role of other bacterial groups and its affinity for substrates (Ghosh and Bhadury, 2018). In addition, the phylum Bacteroidetes, which is common in aquatic ecosystems, was highly abundant in all the samples in this study (Abell and Bowman, 2005). Bacteroidetes are commonly assumed to be specialized in degrading high molecular weight compounds and to have a growth preference when attached to particles, surfaces or algal cells (Fernández-Gómez et al., 2013). Although the 16S rRNA gene sequences of Verrucomicrobia did not constitute more than 3% in global estuaries, their presence in almost all the estuaries indicates functional significance (Ghosh and Bhadury, 2018). Verrucomicrobia, which represented 1.3% of the total abundance in this study, is known for its dependence on organic matter and can be important in carbon cycling (Canfora et al., 2014). Furthermore, Firmicutes may have the capacity to remove excess nitrates from the environment via denitrification, dissimilatory nitrate reduction to ammonium or both (Sun et al., 2016). Pseudoalteromonas was the most abundant genus in the whole bacterial assemblages. Members of the genus Pseudoalteromonas have fundamental roles in influencing biofilm formation in various marine econiches; predator-like interactions within the microbial loop; influencing settlement, germination, and metamorphosis of various invertebrate and algal species; and may be adopted by marine flora and fauna as defensive agents (Bowman, 2007). The genus Octadecabacter was proposed by Gosink et al. (1997) and found widely distributed in global oceans (Brinkhoff et al., 2008). Vollmers et al. (2013) found that the genomes of Octadecabacter arcticus and Octadecabacter antarcticu exhibit a large number of transposable elements and a new subgroup of xanthorhodopsins, which was one type of rhodopsin for light energy harvest (Zhang et al., 2019). However, the ecological roles of Octadecabacter need to be explored in the future.
The 16S rRNA gene copies of bacterioplankton in the deeper seawater samples were, on average, 3.6- fold greater that in the upper seawater. The higher 16S rRNA gene copy numbers in samples from deeper waters could reflect higher bacterial abundance due to resuspension of bacteria from the sediment, or result from a shift in composition driven by bacterial taxa with higher 16S copies per cell, as suggested by higher abundances of Vibrios. The detection of 485 OTUs from 15 bacterioplankton phyla using highthroughput sequencing gave a more comprehensive view of the bacterioplankton community. Proteobacteria were the predominant bacterial phylum, and Gammaproteobacteria and Alphaproteobacteria were the predominant classes. Proteobacteria are notable as abundant free-living bacterioplankton both in coastal and open-ocean habitats (DeLong et al., 1993; Yang et al., 2015). Williams et al. (2013) revealed that the growth of Gammaproteobacteria in the presence of algal organic matter degraded Flavobacteria in the surface water of coastal East Antarctica. In this study, a high abundance of Gammaproteobacteria was found representing 49.5% of the total abundance. The overwhelming dominance of Gammaproteobacteria could be due to multiple factors, such as the role of other bacterial groups and its affinity for substrates (Ghosh and Bhadury, 2018). In addition, the phylum Bacteroidetes, which is common in aquatic ecosystems, was highly abundant in all the samples in this study (Abell and Bowman, 2005). Bacteroidetes are commonly assumed to be specialized in degrading high molecular weight compounds and to have a growth preference when attached to particles, surfaces or algal cells (Fernández-Gómez et al., 2013). Although the 16S rRNA gene sequences of Verrucomicrobia did not constitute more than 3% in global estuaries, their presence in almost all the estuaries indicates functional significance (Ghosh and Bhadury, 2018). Verrucomicrobia, which represented 1.3% of the total abundance in this study, is known for its dependence on organic matter and can be important in carbon cycling (Canfora et al., 2014). Furthermore, Firmicutes may have the capacity to remove excess nitrates from the environment via denitrification, dissimilatory nitrate reduction to ammonium or both (Sun et al., 2016). Pseudoalteromonas was the most abundant genus in the whole bacterial assemblages. Members of the genus Pseudoalteromonas have fundamental roles in influencing biofilm formation in various marine econiches; predator-like interactions within the microbial loop; influencing settlement, germination, and metamorphosis of various invertebrate and algal species; and may be adopted by marine flora and fauna as defensive agents (Bowman, 2007). The genus Octadecabacter was proposed by Gosink et al. (1997) and found widely distributed in global oceans (Brinkhoff et al., 2008). Vollmers et al. (2013) found that the genomes of Octadecabacter arcticus and Octadecabacter antarcticu exhibit a large number of transposable elements and a new subgroup of xanthorhodopsins, which was one type of rhodopsin for light energy harvest (Zhang et al., 2019). However, the ecological roles of Octadecabacter need to be explored in the future.
The genus Vibrio is one of the best model marine heterotrophic bacterial groups (Zhang et al., 2018). Although Vibrio is best known as a pathogen, only 12 species of the ~150 currently described species are associated with human disease. However, all Vibrio play potentially important roles in nutrient and chemical cycling in the marine environment (Grimes et al., 2009). In general, the Vibrio population is estimated to be ~1% of the total bacterioplankton in coastal waters using culture-independent methods (Thompson and Polz, 2006). The average abundance of the Vibrio population was 104 to 108 16S rRNA copies/L in estuarine and coastal waters (Zhang et al., 2018). However, this study revealed that the relative proportion of unnamed Vibrio in all samples was 11.1%, but it exceeded 25.0% in the S1-3m and S3- 5m samples, and there were relatively high 16S rRNA gene copy numbers ((2.6×106)–(6.3×108) copies/L). Research showed that the relative proportion of Vibrio increased from < 1.4% to 19.8% of the total bacterial community of the Looe Key Reef in Florida when Saharan dust nutrients promoted a Vibrio bloom (Westrich et al., 2016). Westrich et al. (2018) also revealed that Vibrio populations, which are rare in pelagic waters, significantly increase in abundance with the arrival of Saharan dust. Gilbert et al. (2012) found that one single Vibrio sp. occupied 54% of the total 16S rRNA genes sequences when the largest bacterial bloom occurred during August 2003. However, this Vibrio was relatively rare and had an abundance of 0–2% for the rest of the time series. It was speculated that organic nutrients secreted by Chaetoceros compressus might have promoted the Vibrio bloom (Gilbert et al., 2012). Farmer et al. (2005) revealed that unnamed Vibrios, which relied on organic matter as carbon and energy sources, occupied a large proportion of the marine environment.
To our best knowledge, this is the first study to demonstrate that the Vibrio 16S rRNA gene can be found in very high copy numbers and has a high relative proportion in Bohai Sea coastal waters. Several studies have concluded that marine host and vector organisms can promote the survival of Vibrios and influence their distribution in the environment (Pruzzo et al., 2005; Eiler et al., 2006; Worden et al., 2006). However, it is not easy to determine whether there is a direct correlation between the distribution of Vibrios with the nearby aquaculture zone, which mainly farms the bivalves Argopecten irradians. In addition, the abundance of Vibrio clades may consume a wide range of organic carbon sources. Therefore, the extent of organic sources should be thoroughly investigated in the future.
Vibrio can shift from a minor component of the marine bacterial community to a locally predominant member in a short time frame due to various conditions, and the bacterial community structure can be altered dramatically (Takemura et al., 2014; Westrich et al., 2016). Vibrio enrichment of surface water is common in the presence of high concentrations of pollutants and pharmaceutical waste, even within different habitat types (Grimes et al., 1984; DeLorenzo et al., 2016; Lydon et al., 2017). In this study, Vibrio comprised a fairly large proportion of the total bacterioplankton community in seawater, and it can become a blooming taxon if favorable conditions prevail.
Previous studies have revealed that seasonal changes (including winter, summer, autumn, and spring), diel variation (day and night), and spatial differences (including distances and depth) have strong effects on the microbial community structure (Gifford et al., 2014; Campbell et al., 2015). Alteration of the nutrient level and the freshwater input from terrestrial ecosystems can affect phytoplankton abundance and indirectly change the microbial community structure (Campbell et al., 2015; Rotini et al., 2017). We found that all the environmental factors except PO43- had no influence on the alpha diversity of the bacterial community and the dominant taxa. Zhao et al. (2013) concluded that the main factors influencing the growth of bacterioplankton in Bohai Bay were external pollution, phosphate concentration, and phytoplankton biomass. In this study, both the alpha diversity and major bacterioplankton group Pseudoalteromonas had significant correlations with the concentration of PO43-. The growth and activity of different microbial groups can be influenced by various concentrations of PO43-. A previous study showed that correlations between chemical parameters and particular bacteria indicated possible feedback interactions, such as the microbe-mediated release of phosphorus. Sinkko et al. (2011) revealed that bacteria contributing to phosphorus release were abundant in hypoxic coastal and open sea sediments where the high release of sediment phosphorus occurred at certain sites. However, understanding the mechanisms of PO43- on the abundance of Pseudoalteromonas is required in future studies. There was no obvious relationship between the abundance of Vibrios and the environmental parameters, and their correlation may depend on the ranges of environmental parameters examined (Zhang et al., 2018).
5 CONCLUTIONIn conclusion, this study provides an assessment of the bacterioplankton community abundance, composition and highly abundant Vibrio clades in Bohai Sea coastal waters. We hypothesize that bacterioplankton communities, including Proteobacteria, Bacteroidetes, Verrucomicrobia, and Firmicutes, can contribute to the degradation of organic matter, carbon cycling, and nitrogen cycling, which are parts of a complex functional network. Highly abundant Vibrio in the waters might have a significant role in marine organic-carbon cycling. The main environmental factor influencing the abundance of Pseudoalteromonas and the alpha diversity of the bacterial community was PO43-. More effort is needed to be conducted to elucidate the effect of environmental factors on dominant taxa and their mechanism. Given that the universal 16S rRNA gene cannot differentiate closely related species, the need for screening of candidate amplicons for Vibrio species-specific highthroughput sequencing libraries is urgent.
6 DATA AVAILABILITY STATEMENTUpon reasonable request, the data that support this study are available from the corresponding author. The assembled tag data for all 16S rRNA gene libraries were deposited in the NCBI database with accession Nos. SRS2686949, SRS2686950, SRS2686951, SRS2686952, SRS2686953, SRS2684038, SRS2684401, SRS2684423, SRS2684422, and SRS2686948.
Electronic supplementary materialSupplementary material (Supplementary Fig.S1 and Tables S1–S3) is available in the online version of this article at https://doi.org/10.1007/s00343-019-8210-1.
Abell G C J, Bowman J P. 2005. Ecological and biogeographic relationships of class Flavobacteria in the Southern Ocean. FEMS Microbiology Ecology, 51(2): 265-277.
|
Acinas S G, Marcelino L A, Klepac-Ceraj V, Polz M F. 2004. Divergence and redundancy of 16S rRNA sequences in genomes with multiple rrn operons. Journal of Bacteriology, 186(9): 2629-2635.
DOI:10.1128/JB.186.9.2629-2635.2004 |
Bowman J P. 2007. Bioactive compound synthetic capacity and ecological significance of marine bacterial genus Pseudoalteromonas. Marine Drugs, 5(4): 220-241.
DOI:10.3390/md504220 |
Brinkhoff T, Giebel H A, Simon M. 2008. Diversity, ecology, and genomics of the Roseobacter clade:a short overview. Archives of Microbiology, 189(6): 531-539.
DOI:10.1007/s00203-008-0353-y |
Campbell A M, Fleisher J, Sinigalliano C, White J R, Lopez J V. 2015. Dynamics of marine bacterial community diversity of the coastal waters of the reefs, inlets, and wastewater outfalls of southeast Florida. Microbiology Open, 4(3): 390-408.
|
Canfora L, Bacci G, Pinzari F, Lo Papa G, Dazzi C, Benedetti A. 2014. Salinity and bacterial diversity:to what extent does the concentration of salt affect the bacterial community in a saline soil?. PLoS One, 9(9): e106662.
DOI:10.1371/journal.pone.0106662 |
Caporaso J G, Kuczynski J, Stombaugh J, Bittinger K, Bushman F D, Costello E K, Fierer N, Peña A G, Goodrich J K, Gordon J I, Huttley G A, Kelley S T, Knights D, Koenig J E, Ley R E, Lozupone C A, McDonald D, Muegge B D, Pirrung M, Reeder J, Sevinsky J R, Turnbaugh P J, Walters W A, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QⅡME allows analysis of high-throughput community sequencing data. Nature Methods, 7(5): 335-336.
DOI:10.1038/nmeth.f.303 |
Caporaso J G, Lauber C L, Walters W A, Berg-Lyons D, Huntley J, Fierer N, Owens S M, Betley J, Fraser L, Bauer M, Gormley N, Gilbert J A, Smith G, Knight R. 2012. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. The ISME Journal, 6(8): 1621-1624.
DOI:10.1038/ismej.2012.8 |
Cottrell M T, Kirchman D L. 2000. Community composition of marine bacterioplankton determined by 16S rRNA gene clone libraries and fluorescence in situ hybridization. Applied and Environmental Microbiology, 66(12): 5116-5122.
DOI:10.1128/AEM.66.12.5116-5122.2000 |
Dang H Y, Zhou H X, Zhang Z N, Yu Z S, Hua E, Liu X S, Jiao N Z. 2013. Molecular detection of Candidatus Scalindua pacifica and environmental responses of sediment anammox bacterial community in the Bohai Sea, China. PLoS One, 8(4): e61330.
DOI:10.1371/journal.pone.0061330 |
DeLong E F, Franks D G, Alldredge A L. 1993. Phylogenetic diversity of aggregate-attached vs. free-living marine bacterial assemblages. Limnology and Oceanography, 38(5): 924-934.
|
DeLorenzo M E, Brooker J, Chung K W, Kelly M, Martinez J, Moore J G, Thomas M. 2016. Exposure of the grass shrimp, Palaemonetes pugio, to antimicrobial compounds affects associated Vibrio bacterial density and development of antibiotic resistance. Environmental Toxicology, 31(4): 469-477.
DOI:10.1002/tox.22060 |
Ding J J, Zhang Y G, Deng Y, Cong J, Lu H, Sun X, Yang C Y, Yuan T, Van Nostrand J D, Li D Q, Zhou J Z, Yang Y F. 2015. Integrated metagenomics and network analysis of soil microbial community of the forest timberline. Scientific Reports, 5: 7994.
DOI:10.1038/srep07994 |
Eiler A, Johansson M, Bertilsson S. 2006. Environmental influences on Vibrio populations in northern temperate and boreal coastal waters (Baltic and Skagerrak Seas). Applied and Environmental Microbiology, 72(9): 6004-6011.
DOI:10.1128/AEM.00917-06 |
Farmer J J, Janda J M, Brenner F W, Cameron D N, Birkhead K M. 2005. Genus I. Vibrio pacini 1854. In: Brenner D J, Kreig N R, Staley J T, eds. Bergey's Manual of Systematic Bacteriology. 2nd edn. Springer, New York.p.494-546.
|
Fernández-Gómez B, Richter M, Schüler M, Pinhassi J, Acinas S G, González J M, Pedrós-Alió C. 2013. Ecology of marine Bacteroidetes:a comparative genomics approach. The ISME Journal, 7(5): 1026-1037.
DOI:10.1038/ismej.2012.169 |
Fukami K, Simidu U, Taga N. 1985. Microbial decomposition of phyto-and zooplankton in seawater. I. Changes in organic matter. Marine Ecology Progress Series, 21: 1-5.
DOI:10.3354/meps021001 |
Gao X L, Zhou F X, Chen C T A. 2014. Pollution status of the Bohai Sea:an overview of the environmental quality assessment related trace metals. Environment International, 62: 12-30.
DOI:10.1016/j.envint.2013.09.019 |
Ghosh A, Bhadury P. 2018. Exploring biogeographic patterns of bacterioplankton communities across global estuaries. MicrobiologyOpen: e741.
DOI:10.1002/mbo3.741 |
Gifford S M, Sharma S, Moran M A. 2014. Linking activity and function to ecosystem dynamics in a coastal bacterioplankton community. Frontiers in Microbiology, 5: 185.
|
Gilbert J A, Steele J A, Caporaso J G, Steinbrück L, Reeder J, Temperton B, Huse S, McHardy A C, Knight R, Joint I, Somerfield P, Fuhrman J A, Field D. 2012. Defining seasonal marine microbial community dynamics. The ISME Journal, 6(2): 298-308.
|
Goodwin K D, Thompson L R, Duarte B, Kahlke T, Thompson A R, Marques J C, Caçador I. 2017. DNA sequencing as a tool to monitor marine ecological status. Frontiers in Marine Science, 4: 107.
DOI:10.3389/fmars.2017.00107 |
Gosink J J, Herwig R P, Staley J T. 1997. Octadecabacter arcticus gen. nov., sp. nov., and O. antarcticus, sp. nov., nonpigmented, psychrophilic gas vacuolate bacteria from polar sea ice and water. Systematic and Applied Microbiology, 20(3): 356-365.
|
Grimes D J, Johnson C N, Dillon K S, Flowers A R, Noriea Ⅲ N F, Berutti T. 2009. What genomic sequence information has revealed about Vibrio ecology in the ocean-a review. Microbial Ecology, 58(3): 447-460.
|
Grimes D J, Singleton F L, Colwell R R. 1984. Allogenic succession of marine bacterial communities in response to pharmaceutical waste. Journal of Applied Microbiology, 57(2): 247-261.
|
Hoffmann M, Fischer M, Ottesen A, McCarthy P J, Lopez J V, Brown E W, Monday S R. 2010. Population dynamics of Vibrio spp. associated with marine sponge microcosms. The ISME Journal, 4(12): 1608-1612.
|
Lin X P, Xie S P, Chen X P, Xu L L. 2006. A well-mixed warm water column in the central Bohai Sea in summer:effects of tidal and surface wave mixing. Journal of Geophysical Research:Oceans, 111(C11): C11017.
DOI:10.1029/2006JC003504 |
Lydon K A, Glinski D A, Westrich J R, Henderson W M, Lipp E K. 2017. Effects of triclosan on bacterial community composition and Vibrio populations in natural seawater microcosms. Elementa:Science of the Anthropocene, 5: 22.
|
McArthur J V. 2006. Microbial Ecology: An Evolutionary Approach. Elsevier, Amsterdam. 432p.
|
Oberbeckmann S, Wichels A, Maier T, Kostrzewa M, Raffelberg S, Gerdts G. 2011. A polyphasic approach for the differentiation of environmental Vibrio isolates from temperate waters. FEMS Microbiology Ecology, 75(1): 145-162.
|
Paillard C, Le Roux F, Borrego J J. 2004. Bacterial disease in marine bivalves, a review of recent studies:trends and evolution. Aquatic Living Resources, 17(4): 477-498.
DOI:10.1051/alr:2004054 |
Peng Z Q, Zhuang Z X, Huang R F, Lu Z Q. 2010. Distribution of pathogen in the Bohai sea in spring and summer. African Journal of Microbiology Research, 4(13): 1383-1390.
|
Pruzzo C, Gallo G, Canesi L. 2005. Persistence of vibrios in marine bivalves:the role of interactions with haemolymph components. Environmental Microbiology, 7(6): 761-772.
DOI:10.1111/j.1462-2920.2005.00792.x |
Rotini A, Manfra L, Spanu F, Pisapia M, Cicero A M, Migliore L. 2017. Ecotoxicological method with marine bacteria Vibrio anguillarum to evaluate the acute toxicity of environmental contaminants. Journal of Visualized Experiments, (123): e55211.
|
Shendure J, Ji H. 2008. Next-generation DNA sequencing. Nature Biotechnology, 26(10): 1135-1145.
DOI:10.1038/nbt1486 |
Sinkko H, Lukkari K, Jama A S, Sihvonen L M, Sivonen K, Leivuori M, Rantanen M, Paulin L, Lyra C. 2011. Phosphorus chemistry and bacterial community composition interact in brackish sediments receiving agricultural discharges. PLoS One, 6(6): e21555.
DOI:10.1371/journal.pone.0021555 |
Stackebrandt E, Goebel B M. 1994. Taxonomic note:a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. International Journal of Systematic and Evolutionary Microbiology, 44(4): 846-849.
DOI:10.1099/00207713-44-4-846 |
Stackebrandt E, Goodfellow M. 1991. Nucleic acid Techniques in Bacterial Systematics. Wiley, New York. 329p.
|
Sun Y H, De Vos P, Heylen K. 2016. Nitrous oxide emission by the non-denitrifying, nitrate ammonifier Bacillus licheniformis. BMC Genomics, 17: 68.
DOI:10.1186/s12864-016-2382-2 |
Takemura A F, Chien D M, Polz M F. 2014. Associations and dynamics of Vibrionaceae in the environment, from the genus to the population level. Frontiers in Microbiology, 5: 38.
|
Thompson J R, Polz M F. 2006. Dynamics of Vibrio populations and their role in environmental nutrient cycling. In: Thompson F L, Austin B, Swings J eds. The Biology of Vibrios. ASM Press, Washington, DC. p.190-203.
|
Thompson J R, Randa M A, Marcelino L A, Tomita-Mitchell A, Lim E, Polz M F. 2004. Diversity and dynamics of a North Atlantic coastal Vibrio community. Applied and Environmental Microbiology, 70(7): 4103-4110.
DOI:10.1128/AEM.70.7.4103-4110.2004 |
Vezzulli L, Grande C, Reid P C, Hélaouët P, Edwards M, Höfle M G, Brettar I, Colwell R R, Pruzzo C. 2016. Climate influence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic. Proceedings of the National Academy of Sciences of the United States of America, 113(34): E5062-E5071.
DOI:10.1073/pnas.1609157113 |
Vollmers J, Voget S, Dietrich S, Gollnow K, Smits M, Meyer K, Brinkhoff T, Simon M, Daniel R. 2013. Poles apart:Arctic and Antarctic Octadecabacter strains share high genome plasticity and a new type of Xanthorhodopsin. PLoS One, 8(5): e63422.
DOI:10.1371/journal.pone.0063422 |
Wear E K, Wilbanks E G, Nelson C E, Carlson C A. 2018. Primer selection impacts specific population abundances but not community dynamics in a monthly time-series 16S rRNA gene amplicon analysis of coastal marine bacterioplankton. Environmental Microbiology, 20(8): 2709-2726.
DOI:10.1111/1462-2920.14091 |
Westrich J R, Ebling A M, Landing W M, Joyner J L, Kemp K M, Griffin D W, Lipp E K. 2016. Saharan dust nutrients promote Vibrio bloom formation in marine surface waters. Proceedings of the National Academy of Sciences of the United States of America, 113(21): 5964-5969.
DOI:10.1073/pnas.1518080113 |
Westrich J R, Griffin D W, Westphal D L, Lipp E K. 2018. Vibrio population dynamics in Mid-Atlantic surface waters during Saharan dust events. Frontiers in Marine Science, 5: 12.
DOI:10.3389/fmars.2018.00012 |
Williams T J, Wilkins D, Long E, Evans F, DeMaere M Z, Raftery M J, Cavicchioli R. 2013. The role of planktonic Flavobacteria in processing algal organic matter in coastal East Antarctica revealed using metagenomics and metaproteomics. Environmental Microbiology, 15(5): 1302-1317.
DOI:10.1111/1462-2920.12017 |
Worden A Z, Seidel M, Smriga S, Wick A, Malfatti F, Bartlett D, Azam F. 2006. Trophic regulation of Vibrio cholerae in coastal marine waters. Environmental Microbiology, 8(1): 21-29.
DOI:10.1111/j.1462-2920.2005.00863.x |
Wu Z X, Yu Z M, Song X X, Yuan Y Q, Cao X H, Liang Y B. 2013. Application of an integrated methodology for eutrophication assessment:a case study in the Bohai Sea. Chinese Journal of Oceanology and Limnology, 31(5): 1064-1078.
DOI:10.1007/s00343-013-2286-9 |
Yang C Y, Li Y, Zhou B, Zhou Y Y, Zheng W, Tian Y, Van Nostrand J D, Wu L Y, He Z L, Zhou J Z, Zheng T L. 2015. Illumina sequencing-based analysis of free-living bacterial community dynamics during an Akashiwo sanguine bloom in Xiamen sea, China. Scientific Reports, 5: 8476.
DOI:10.1038/srep08476 |
Yilmaz P, Parfrey L W, Yarza P, Gerken J, Pruesse E, Quast C, Schweer T, Peplies J, Ludwig W, Glöckner F O. 2014. The SILVA and "All-species Living Tree Project (LTP)" taxonomic frameworks. Nucleic Acids Research, 42(D1): D643-D648.
DOI:10.1093/nar/gkt1209 |
Yu S X, Pang Y L, Wang Y C, Li J L, Qin S. 2018. Distribution of bacterial communities along the spatial and environmental gradients from Bohai Sea to northern Yellow Sea. PeerJ, 6: e4272.
DOI:10.7717/peerj.4272 |
Zhang X H, Lin H Y, Wang X L, Austin B. 2018. Significance of Vibrio species in the marine organic carbon cycle-a review. Science China Earth Sciences, 61(10): 1357-1368.
DOI:10.1007/s11430-017-9229-x |
Zhang Y Q, Lin X, Shi X G, Lin L X, Luo H, Li L, Lin S J. 2019. Metatranscriptomic signatures associated with phytoplankton regime shift from diatom dominance to a dinoflagellate bloom. Frontiers in Microbiology, 10: 590.
DOI:10.3389/fmicb.2019.00590 |
Zhao H P, Tao J H, Li Q X, Yuan D K, Gao Q C. 2013. Microbial ecological characteristics in the Red TideMonitoring area of Bohai Bay. Journal of Hydroenvironment Research, 7(2): 141-151.
|
 2019, Vol. 37
2019, Vol. 37


