Institute of Oceanology, Chinese Academy of Sciences
Article Information
- DU Guangxun, QU Lingyun, SHANG Kun, SUN Chengjun, WANG Chen, GAO Ping
- Ciliate Uronema marinum is the causative agent of scuticociliatosis in farm raised turbot Scophthalmus maximus
- Journal of Oceanology and Limnology, 37(5): 1726-1735
- http://dx.doi.org/10.1007/s00343-020-8305-8
Article History
- Received Oct. 18, 2018
- accepted in principle Dec. 20, 2018
- accepted for publication Jan. 15, 2019
2 Laboratory for Marine Fisheries Science and Food Production Processes, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China;
3 College of Fisheries and Life Science, Dalian Ocean University, Dalian 116023, China
Turbot Scophthalmus maximus distributes mainly in Northeast Atlantic Ocean and can also be found in the most of Baltic Sea and is adapted to a benthic lifestyle (Figueras et al., 2016). Turbot is of great commercial value with an estimated aquaculture production of 11 000 tones in Europe in 2012. In China, turbot aquaculture has also generated great economic and social values and become one of the most important pillar industries of marine aquaculture since its introduction to China in 1992. However, a variety of turbot diseases ensued due to the rapid development of turbot aquaculture as well as the lack of disease prevention research and caused an annual loss of about 70 million dollars (Zhang et al., 2006). Among those diseases, scuticociliatosis is the most serious parasitic disease (Dyková et al., 2010; Harikrishnan et al., 2010). It occurs worldwide and often causes an outbreak of fatal infection (Piazzon et al., 2013; Stidworthy et al., 2014).
More than 20 species of scuticociliates have been shown facultative parasites that can destroy host tissues, such as Miamiensis avidus, Uronema marinum, and Anophryoides haemophila (Thompson and Moewus, 1964; Cheung et al., 1980; Cawthorn et al., 1996; Harikrishnan et al., 2010). The main clinical syndromes can be generally characterized as highly histophagous, systemic tissue destruction, and high mortality in cultured marine fish (Iglesias et al., 2001; Whang et al., 2013; Turgay et al., 2015; Cardoso et al., 2017). For example, Turgay et al. (2015) reported a scuticociliatosis case caused by M. avidus in cultured common dentex for the first time in 2015; the infected fish showed hemorrhaging on the surface, dorsal fin rot, accumulation of ascitic fluid, consequent distended abdominal cavity, and a swollen spleen.
In this study, infected turbots showing similar syndromes (such as cutaneous ulcers, exophthalmos, and darkened skin) from turbot farm in Laizhou City, Shandong, China, were examined. A species of ciliate was isolated from the brain of an infected turbot and has been identified as U. marinum based on morphological characterizations, 18S rRNA gene, and COX Ⅰ gene sequence analysis. We then artificially infected turbots with pure cultured U. marinum and these turbots developed similar syndrome of infection to those observed in the diseased ones in the aquaculture farm, confirming that this ciliate is the causative agent. To our best knowledge, this is the first report of scuticociliatosis caused by U. marinum in farm-raised turbots in China.
2 MATERIAL AND METHOD 2.1 Sample collectionIn June 2013, a turbot farm worker reported mass mortality among cultured juvenile turbots in Laizhou City. Eleven moribund turbots (mean total length: 10 cm; mean body weight: 15 g) were sampled from the turbot farm; they showed behavioral disorders (including repetitive movements such as heading up out of water, swimming upside down or rotating), a significant decline in appetite, decreased vitality, and slow reaction. In addition, the body skin was darkened, body surface mucus increased, ulcerative lesion occurred on the body surface, the fin bases reddened, and abdominal distension appeared in some individuals.
The moribund turbots were placed in a clean polyethylene bag (75 cm×45 cm) filled with seawater and were subsequently transported to the laboratory at the First Institute of Oceanography, Ministry of Natural Resources (MNR), Qingdao, China, in ~3 h over ~160 km in the expressway.
2.2 Histological examinationFresh tissues (skin, gill, liver, intestine, and brain) were cut off with a scalpel. The thickness of the tissue slices was thinner than 0.5 cm. Tissues were washed with physiological saline and rapidly fixed in Davidson's fluid for 24 h, then dehydrated through an ethanol series and embedded in paraffin wax, thereafter the paraffin blocks were sectioned at 5 μm in standard methodology. Tissue sections were examined under a light microscope (10×40) after being stained with haematoxylin and eosin (H & E) (Zhou et al., 2000; Qin et al., 2007; Ramos et al., 2007; Turgay et al., 2015).
2.3 Isolation of ciliatesOne diseased fish was placed on the anatomical plate in an aseptic operating table, and the brain tissues were dissected and placed in sterilized seawater with a few drops of boiled fresh turbot gravy to be used as the bait for ciliate propagation. The culture was initially maintained at 17℃ for 7 days. At the end of this period, a few drops of the culture were sampled for microscopy and live ciliates were subcultured in 20 mL sterilized seawater containing 10 grains of rice at 17℃. The isolated ciliate was named LZLP2013.
2.4 Morphological characterizationAll data are from one cultivated clone. Living cells were studied using an oil immersion objective and differential interference contrast microscopy. Protargol impregnation was performed as Wilbert's Protargol Method (Wilbert, 1975). Counts and measurements of silvered specimens were performed at a magnification of 1 000×. The classification system refers to Corliss (1979).
2.5 Molecular methodsThe ciliates were enriched by 3 000 r/min centrifugation for 2 min. Genomic DNA was extracted from cells using OMEGA Tissue DNA Kit (D3396- 01) according to the manufacturer's instructions, and extracted genomic DNA was stored at -20℃ for subsequent use.
Universal primer sets (Euk A: 5′-AACCTGGTTGATCCTGCCAGT-3′; Euk B: 5′-TGATCCTTCTGCAGGTTCACCTAC-3′) were used to amplify a fragment (about 1 500 bp) of the 18S rRNA gene (Medlin et al., 1988). PCR was performed in a total volume of 60 μL. The PCR amplification protocol was pre-denaturation at 94℃ for 2 min, 35 cycles with 30 s at 94℃, 1 min at 55℃ and 2 min at 72℃, followed by 7 min at 72℃ (Ofelio et al., 2013).
The species-specific primer sets of U. marinum (OX09-144: 5′-AACATAGAGCATATAGAGAGTACTCTAA-3′; OX09-144: 5′-TTCATCCAGCTGTTGTTAATGT-3′) were used to amplify a fragment (about 280 bp) of mitochondrial cytochrome c oxidase 1 (COX Ⅰ). PCR was performed in a total volume of 60 μL. The PCR amplification protocol was predenaturation at 94℃ for 2 min, 30 cycles of 94℃ for 30 s, 50℃ for 30 s, 72℃ for 40 s, and a final extension at 72℃ for 5 min (Whang et al., 2013).
2.6 Sequence analysesPCR products were visually evaluated by electrophoresis on 1% agarose gel. Purified PCR amplicon was sequenced through by Sangon Biotech Co., Ltd. 3 times. The sequences of 18S rRNA gene and COX Ⅰ gene were submitted to NCBI and BLASTed in GenBank. The 18S rRNA and COX Ⅰ sequences from other 12 species of ciliates were respectively retrieved from NCBI. Phylogenetic trees based on the 18S rRNA and COX Ⅰ sequences were constructed in MEGA 6.0. All 18S rRNA and COX Ⅰ gene sequences were respectively aligned using MUSCLE (Edgar, 2004) and Bayesian Inference Criterion identified the Tamura-Nei model with invariable rates as the best-fit nucleotide substitution model for Neighbor-joining analysis (Tamura et al., 2013).
2.7 Experimental infectionTo confirm that the isolate ciliate LZLP2013 was the causative agent responsible for the scuticociliatosis, we performed an experimental infection. We set up 3 groups, each having 15 fishes in a tank (40 cm×30 cm×20 cm) filled with 15-L seawater. Turbots (average total length: 10 cm; average body weight: 15 g) were kept in tank feeding with 2.25-g pellet feed every day for 2 weeks prior to ciliate infection. Three groups of 15 fish were scratched on the skin and exposed to 8×103 ind./mL of cloned LZLP2013 strain of U. marinum for 24 h. A control group of 15 fish was scratched without exposure to ciliates. The seawater temperature was maintained at 17–18℃.
To detect the presence of ciliates in the infected turbots, microscopy and PCR were used. Gill and muscle tissue samples were removed with a pair of sterile scissors, and genomic DNA was extracted from these tissues using OMEGA Tissue DNA Kit (D3396- 01). Then species-specific primers (OX09-144: 5′-AACATAGAGCATATAGAGAGTACTCTAA-3′; OX09-144: 5′-TTCATCCAGCTGTTGTTAATGT-3′) of U. marinum were used to amplify the COX Ⅰ gene from these DNA samples using the same PCR protocol described above. PCR amplicons were purified and sequenced to verify the identity of the sequences as previously mentioned.
3 RESULT 3.1 Disease characterizationThe syndromes of these moribund fishes collected from Laizhou are very similar to those scuticociliatosis reported (Fig. 1a). In the later stage of infection, exophthalmos was found in some turbots (Fig. 1b). We therefore performed microscopy to examine if ciliates are present in infected tissues. Smears were made from ulcers, gills, and brains of the diseased fish. Turbots infected with ciliates presented ulcerative lesions on the body surface, and microscopic examination showed a large number of motile ciliates at the lesions (Fig. 1c), it had a sunflower-seed-like shape and was densely covered with cilia (Fig. 1d). Taken together, we confirmed that the disease was scuticociliatosis.
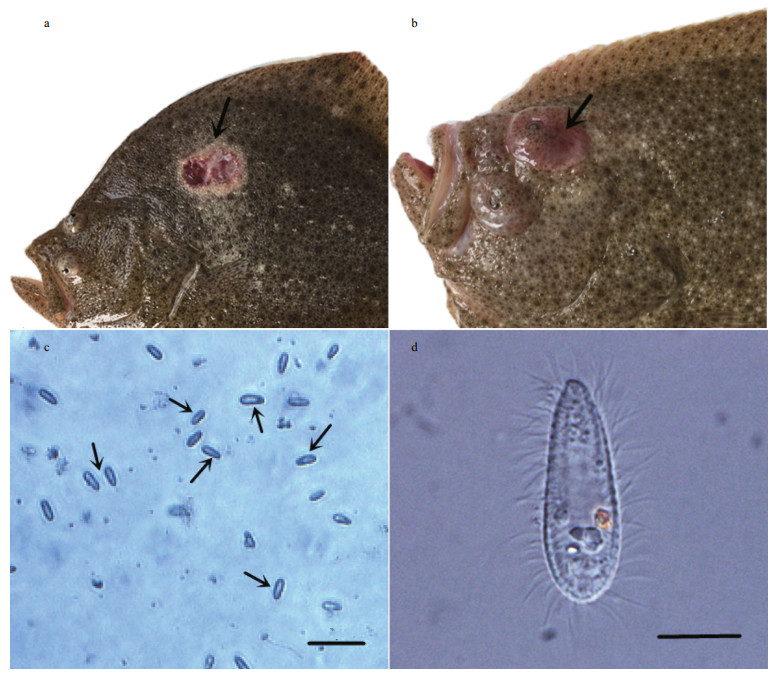
|
| Fig.1 Signs of turbots infected with ciliates a. ulcerative lesions on the body surface; b. exophthalmos, arrows indicate the foci; c. smears made from infected tissues, arrows indicate the ciliates, bar=50 μm; d. microscopical characteristics of ciliate, bar=10 μm. |
Except for the body surface, the skin, gill, liver, intestine, and brain of the diseased fish have been infected with a large number of ciliates, and the tissues became loose and vacuolated, as shown in the tissue sections.
As shown in the skin tissue sections, the normal skin tissue is tight (Fig. 2a). After the invasion of ciliates, the tissue changed obviously. Ciliates were abundant in subcutaneous tissue and muscle tissue, and the skin tissue became loose in which obvious vacuoles occurred. With the proliferation of ciliates, the skin tissue became necrotic, and the nuclei turned into pyknosis and became broken and dissolved (Fig. 2b). The gill sections showed that the normal gill filament is compact and regular (Fig. 2c). After being ciliate-diseased, the branchial pillar cells were destroyed and the epithelial cells were necrotic, causing gill filament deformation and strong dilatation of space (Fig. 2d). A normal fin tissue shall be compact and organized (Fig. 2e), while the ciliate-affected fin tissue showed fester at the side, caudal, and other places, plus bleeding and necrosis in subcutaneous connective tissue. Moreover, a large number of ciliates were found in the ulceration part (Fig. 2f). In liver section, normal liver tissues are closely packed (Fig. 2g), while the diseased ones had obvious necrosis and liver cells disappeared, disintegrated, and hollowed (Fig. 2h). In bowel section, a normal bowel villus structure shall be regular (Fig. 2i), and the diseased ones contained a large number of ciliates, resulting in necrosis in the intestinal mucosal epithelial cells, and the presence of voids in tissues (Fig. 2j). In brain section, the structure of a normal brain shall be compact (Fig. 2k), while the diseased ones are found having a large number of ciliates, and the brain tissues were disintegrated and hollowed (Fig. 2l).
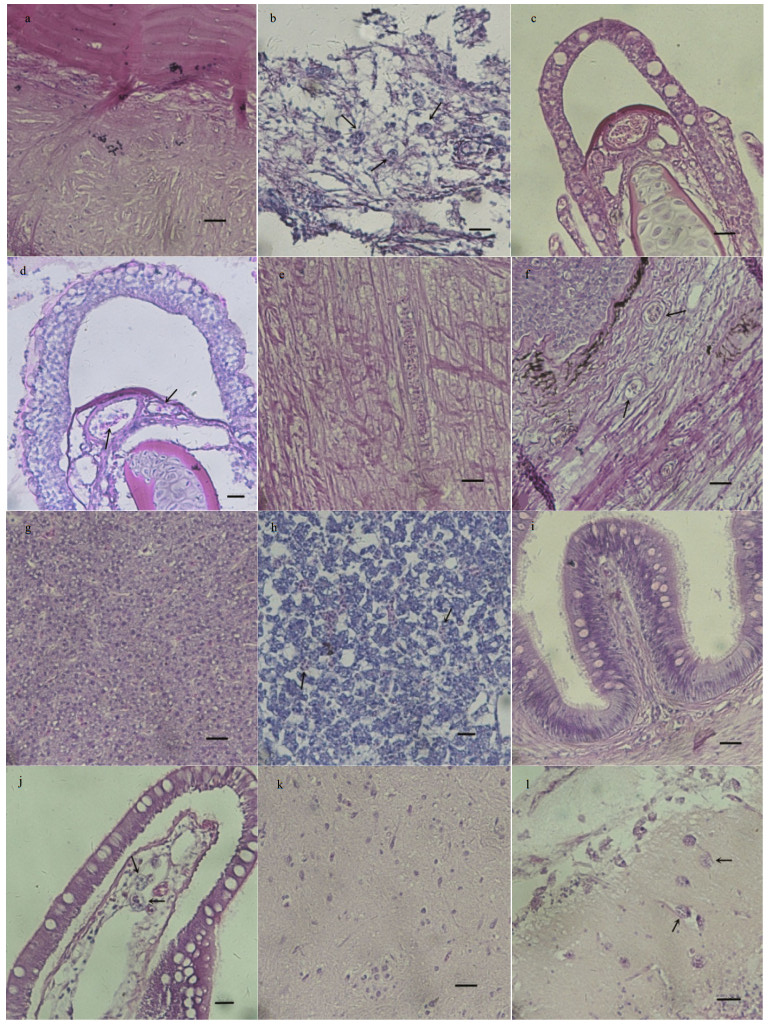
|
| Fig.2 Microscopic pathological changes of infected turbot a. normal skin tissue without ciliate; b. ciliates (arrows) in skin tissue of infected turbot, the skin tissue became loose; c. normal gill filament without ciliate; d. ciliates (arrows) caused gill filament deformation and strong dilatation of space; e. normal fin tissue without ciliate; f. ciliates in the fin of infected turbot; g. normal liver tissue without ciliate; h. ciliates (arrows) caused disintegration of liver tissues; i. normal bowel villus structure without ciliate; j. invasionof ciliates (arrows) led to necrosis of intestinal mucosal epithelial cells; k. normal brain tissue without ciliate; l. brain tissue disintegrated and became hollow due to the invasion of ciliates. Bar=25 μm. |
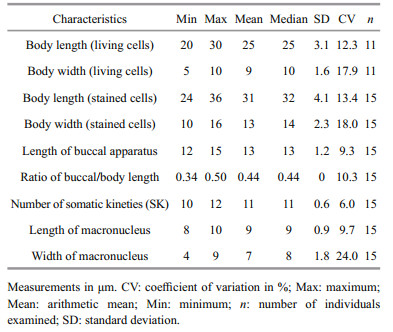
The main morphological features of this ciliate are as follows (morphological statistics are shown in Table 1).
|
Size of the body in vivo is about 20–30 μm by 5–10 μm, and it is elongated and elliptical in outline. The anterior end is truncated with a small apical plate, and posterior end is narrowly rounded. Buccal field occupies about half of body length, with cytostome centrally positioned on the ventral side. The pellicle is thin and strongly notched with reticulate ridges. Extrusomes are bar-shaped, about 2 μm, and sparsely arranged beneath pellicle. Cytoplasm is colorless to grayish, containing several refractile globules and bar-like crystals distributed at anterior and posterior portions. Single contractile vacuole caudally positioned, which is about 3–5 μm in diameter when fully expanded, pulsating at intervals of 30–40 s. Cilia is approximately 5–7 μm and densely arranged and it has a single caudal cilium, which is about 15 μm (Fig. 3a). The ciliate swims swiftly and rotated around its longitudinal axis of the body, and remains motionless for a long period when they are not disturbed.
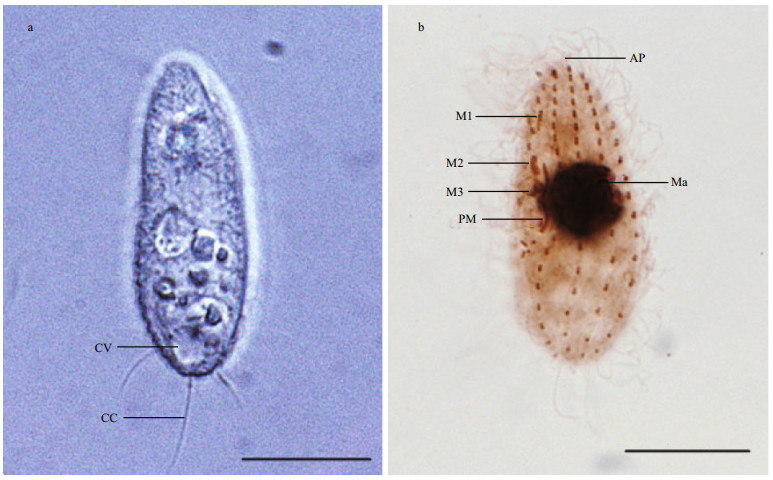
|
| Fig.3 Morphological characteristics of the ciliate a. general view and endoplasm of the living ciliate, bar=10 μm (CC: caudal cilium; CV: contractile vacuole); b. ciliate stained with protargol staining method showing apical plate, buccal apparatus, nuclear apparatus, somatic kineties, bar=10 μm (AP: apical plate; M1: membranelle1; M2: membranelle 2; M3: membranelle 3; PM: paroral membrane; Ma: macronucleus). |
Figure 3b shows the silver staining of ciliature. There were 10–12 somatic kineties from the entire length of the cell to the apical end around a small glabrous apical plate that composed of dikinetids in the anterior three-fourth of body length, while loosely-arranged monokinetids in posterior portion.
Buccal apparatus is the typical style of Uronema sp. Membranelle 1 locates one-sixth of body length from anterior end and it composes of about 5–7 kinetosomes arranged in a row. Membranelle 2 is slightly longer than membranelle 1, it comprises of two longitudinal rows of kinetosomes and positiones approximately one-third of body length from anterior. Membranelle 3 is the shortest and it composes of about 10 basal bodies. Paroral membrane located at the right side of the buccal cavity with zigzag rows of basal bodies, extending anteriorly to about the middle of membranelle 2. Scutica with 3 pairs of basal bodies is closely behind the posterior end of the paroral membrane, arranged in Y-shape. Contractile vacuole pore positions at the end of second somatic kinety.
Based on the results of intravital observation and silver staining, referred to the Corliss classification system, the systematic classification status of this scuticociliate is as follow:
Ciliophora Doflein, 1901
Oligohymenophora de Puytorac et al., 1974
Scuticociliatida Small, 1967
Philasterina Small, 1967
Philasteridae Kahl, 1931
Uronema Dujardin, 1841
Uronema marinum Dujardin, 1841.
3.4 Phylogenetic analysisThe 18S rRNA gene of the ciliate LZLP2013 was amplified and sequenced (GenBank accession number: MF418591), the sequence is 1 580 bp in length and it has only 4 different bases compared with those of U. marinum (GQ259746.1). They shared 99% identity in the sequence. The 18S rRNA of this ciliate was also compared with those of 12 related ciliates. Phylogenetic analysis revealed that LZLP2013 (MF418591) was the closest to that of U. marinum (GQ259746.1) with high confidence (100%), which further identified the phylogenetic taxonomic status of this ciliate (Fig. 4a).
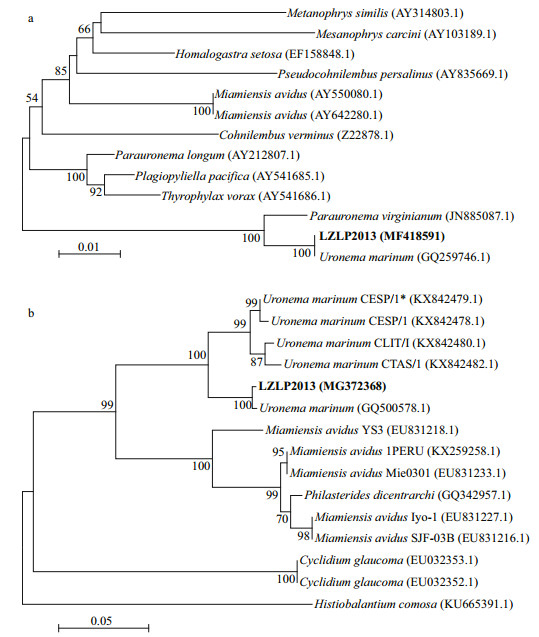
|
| Fig.4 Phylogenetic analysis of the ciliate a. the neighbor-joining tree of LZLP2013 based on 18S rRNA gene sequences; b. neighbor-joining tree of LZLP2013 based on COX Ⅰ gene sequences. |
The COX Ⅰ gene of this ciliate was amplified and sequenced (GenBank accession number: MG372368), the sequence length is 285 bp and it has only 1 different base compared with those of U. marinum (GQ500578.1). They shared 99% identity in sequence. The COX Ⅰ gene of this ciliate was compared with those of 14 related ciliates. The phylogenetic dendrogram was constructed based on the genetic distance analysis, which revealed that LZLP2013 (MG372368) and U. marinum (GQ500578.1) are clustered into one branch in high confidence (100%), which further identified the phylogenetic taxonomic status of this ciliate (Fig. 4b).
3.5 Infection experimentTo determine whether the isolate ciliate LZLP2013 was the causative agent responsible for scuticociliates in the turbot, we performed an infection experiment in a controlled laboratory setting. After 2 weeks, the skin of 80% turbots from experimental groups began to fester. After 3 weeks, turbots (the mortality rate was 53%) infected with LZLP2013 showed syndromes similar to those that we observed from diseased turbot collected from farms. Ulcers were observed at the skin scratches of turbots from experimental group (Fig. 5a); however, the skin scratches in the control group healed gradually (Fig. 5b). Microscopic examination of these skin ulcer scrapings from diseased fishes showed a heavy lead of ciliates (Fig. 5c). No ciliates were detected in the brain tissue. This may be due to the short duration of infection and the ciliates had not yet invaded into the brain of turbot. We also tried to detect the presence of the ciliates from those diseased tissues by performing diagnostic PCR targeting the COX Ⅰ gene. Results show that in all tissues detected, a specific band presents correspondingly to the expected size of COX Ⅰ gene and sequencing results confirmed that they were all COX Ⅰ genes (Fig. 5d). Taken together, our results show that the ciliate LZLP2013 was the causative agent responsible for scuticociliates in turbot.

|
| Fig.5 Experimental verification of infection a. ulcerative lesion on the body surface of turbot from the experimental group; b. healing wound on the body surface of turbot from the control group (arrows indicate the foci); c. ulcer muscle smear (arrows indicate the ciliates, bar=10 μm); d. results of the COX Ⅰ gene PCR for different samples: DNA marker (Lane 1), species-specific fragments for cloned LZLP2013 strain (Lanes 2, 3), muscle samples (Lanes 4, 5), and gill samples (Lanes 6, 7). |
Scuticociliates cause serious parasitic diseases in turbots in both China and Europe. Several species have been reported responsible for scuticociliatosis in turbots. For example, both Philasterides dicentrarchi and Mesanophrys carcini can infect turbot, the syndrome of infection are similar (Iglesias et al., 2001; Wang et al., 2005). From the isolated pathogenic scuticociliate taken from brain tissue of an infected turbot, we identified U. marinum. It was proved that the disease in the farm-raised turbots was scuticociliatosis supported by our microscopic and histochemical studies.
Identifying the causative agent is a critical step towards better understanding of the pathology of scuticociliatosis for developing an effective treatment strategy. Morphological characterization of this ciliate showed similar features to those described by Song et al. (2009). Phylogenetic analysis of the 18S rRNA and COX Ⅰ genes further identified the phylogenetic taxonomic status of this scuticociliate as U. marinum. To unequivocally show that the U. marinum clone LZLP2013 is the causative agent, we performed infection experiment. Healthy turbots were infected with laboratory grown ciliate and the turbots developed typical syndrome such as behavioral disorders, less food intake, and ulcerative lesions 3 weeks post infection. Microscopic examination and PCR diagnostic showed that the ciliate was present in the infected issues. Therefore, we strongly believed that the clone LZLP2013 we isolated was the causative agent. Clones from this U. marinum have been shown to be able to infect a variety of fishes such as olive flounders and New Zealand groupers (Jee et al., 2001; Kim et al., 2004; Anderson et al., 2009). However, no reports have shown that it can infect turbots in China. Therefore, our study represents the first report of scuticociliatosis in turbot caused by U. marinum in China.
5 CONCLUSIONParasitic diseases are a major threat to marine aquaculture globally. Accurate specific pathogen identification is important to guide the development of an effective treatment regime. In this study, we reported a new pathogen for turbot and determined that U. marinum is the causative pathogen, which paved the way for accurately diagnosing and treating this disease.
6 DATA AVAILABILITY STATEMENTThe 18S rRNA gene sequence of ciliate LZLP2013 is available from the National Center for Biotechnology Information under the accession number MF418591. The COX Ⅰ gene sequence of ciliate LZLP2013 is available from the National Center for Biotechnology Information under the accession number MG372368.
7 ACKNOWLEDGMENTThe LZLP2013 is morphologically identified by Prof. HU Xiaozhong and Dr. LIU Mingjian from the Laboratory of Protozoa, Ocean University of China.
Anderson S A, Hulston D A, McVeagh S M, Webb V L, Smith P J. 2009. In vitro culture and cryopreservation of Uronema marinum isolated from farmed New Zealand groper (Polyprion oxygeneios). Journal of Microbiological Methods, 79(1): 62-66.
DOI:10.1016/j.mimet.2009.07.022 |
Cardoso P H M, de Carvalho Balian S, Matushima E R, De Pádua S B, Martins M L. 2017. First report of scuticociliatosis caused by Uronema sp. in ornamental reef fish imported into Brazil. Revista Brasileira de Parasitologia Veterinaria, 26(4): 491-495.
DOI:10.1590/s1984-29612017031 |
Cawthorn R J, Lynn D H, Despres B, MacMillan R, Maloney R, Loughlin M, Bayer R. 1996. Description of Anophryoides haemophila n. sp. (Scuticociliatida:Orchitophryidae), a pathogen of American lobsters Homarus americanus. Diseases of Aquatic Organisms, 24(2): 143-148.
DOI:10.3354/dao024143 |
Cheung P J, Nigrelli R F, Ruggieri G D. 1980. Studies on the morphology of Uronema marinum Dujardin (Ciliatea:Uronematidae) with a description of the histopathology of the infection in marine fishes. Journal of Fish Diseases, 3(4): 295-303.
DOI:10.1111/j.1365-2761.1980.tb00400.x |
Corliss J O. 1979. The Ciliated Protozoa. Characterization, Classification and Guide to the Literature. Pergamon Press, Oxford, UK.
|
Dyková I, Tyml T, Kostka M, Pecková H. 2010. Strains of Uronema marinum (Scuticociliatia) co-isolated with amoebae of the genus Neoparamoeba. Diseases of Aquatic Organisms, 89(1): 71-77.
DOI:10.3354/dao02168 |
Edgar R C. 2004. MUSCLE:multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32(5): 1792-1797.
DOI:10.1093/nar/gkh340 |
Figueras A, Robledo D, Corvelo A, Hermida M, Pereiro P, Rubiolo J A, Gómez-Garrido J, Carreté L, Bello X, Gut M, Gut I G, Marcet-Houben M, Forn-Cuní G, Galán B, García J L, Abal-Fabeiro J L, Pardo B G, Taboada X, Fernández C, Vlasova A, Hermoso-Pulido A, Guigó R, Álvarez-Dios J A, Gómez-Tato A, Viñas A, Maside X, Gabaldón T, Novoa B, Bouza C, Alioto T, Martínez P. 2016. Whole genome sequencing of turbot (Scophthalmus maximus; Pleuronectiformes):a fish adapted to demersal life. DNA Research, 23(3): 181-192.
DOI:10.1093/dnares/dsw007 |
Harikrishnan R, Balasundaram C, Heo M S. 2010. Scuticociliatosis and its recent prophylactic measures in aquaculture with special reference to South Korea:taxonomy, diversity and diagnosis of scuticociliatosis:part Ⅰ Control strategies of scuticociliatosis:part Ⅱ. Fish & Shellfish Immunology, 29(1): 15-31.
DOI:10.1016/j.fsi.2010.02.026 |
Iglesias R, Paramá A, Alvarez M F, Leiro J, Fernández J, Sanmartín M L. 2001. Philasterides dicentrarchi (Ciliophora, Scuticociliatida) as the causative agent of scuticociliatosis in farmed turbot Scophthalmus maximus in Galicia (NW Spain). Diseases of Aquatic Organisms, 46(1): 47-55.
DOI:10.3354/dao046047 |
Jee B Y, Kim Y C, Park M S. 2001. Morphology and biology of parasite responsible for scuticociliatosis of cultured olive flounder Paralichthys olivaceus. Diseases of Aquatic Organisms, 47(1): 49-55.
DOI:10.3354/dao047049 |
Kim S M, Cho J B, Kim S K, Nam Y K, Kim K H. 2004. Occurrence of scuticociliatosis in olive flounder Paralichthys olivaceus by Phiasterides dicentrarchi(Ciliophora:Scuticociliatida). Diseases of Aquatic Organisms, 62(3): 233-238.
DOI:10.3354/dao062233 |
Medlin L, Elwood H J, Stickel S, Sogin M L. 1988. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene, 71(2): 491-499.
DOI:10.1016/0378-1119(88)90066-2 |
Ofelio C, Blanco A, Roura Á, Pintado J, Pascual S, Planas M. 2013. Isolation and molecular identification of the scuticociliate Porpostoma notata Moebius, 1888 from moribund reared Hippocampus hippocampus (L.)seahorses, by amplification of the SSU rRNA gene sequences. Journal of Fish Diseases, 37(12): 1061-1065.
DOI:10.1111/jfd.12207 |
Piazzon M C, Leiro J, Lamas J. 2013. Fish immunity to scuticociliate parasites. Developmental & Comparative Immunology, 41(2): 248-256.
DOI:10.1016/j.dci.2013.05.022 |
Qin L, Wang Y G, Zhang L J, Dai J X. 2007. Histopathology of turbot associated with Mesanophrys carcini parasite. Acta Hydrobiologica Sinica, 31(5): 618-628.
(in Chinese with English abstract) |
Ramos M F, Costa A R, Barandela T, Saraiva A, Rodrigues P N. 2007. Scuticociliate infection and pathology in cultured turbot Scophthalmus maximus from the north of Portugal. Diseases of Aquatic Organisms, 74(3): 249-253.
DOI:10.3354/dao074249 |
Song W B, Warren A, Hu X Z. 2009. Free-living Ciliates in the Bohai and Yellow Seas, China. Science Press, Beijing, China. p.171-175.
(in Chinese)
|
Stidworthy M F, Garner M M, Bradway D S, Westfall B D, Joseph B, Repetto S, Guglielmi E, Schmidt-Posthaus H, Thornton S M. 2014. Systemic Scuticociliatosis(Philasterides dicentrarchi) in sharks. Veterinary Pathology, 51(3): 628-632.
DOI:10.1177/0300985813492800 |
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6:molecular evolutionary genetics analysis Version 6.0. Molecular Biology and Evolution, 30(12): 2725-2729.
DOI:10.1093/molbev/mst197 |
Thompson Jr J C, Moewus L. 1964. Miamiensis avidus n. g., n.sp., a marine facultative parasite in the ciliate order Hymenostomatida. Journal of Eukaryotic Microbiology, 11(3): 378-381.
DOI:10.1111/j.1550-7408.1964.tb01766.x |
Turgay E, Steinum T M, Gül A E, Karataş S. 2015. An outbreak of scuticociliatosis in cultured common dentex (Dentex dentex) in turkey. Bulletin-European Association of Fish Pathologists, 35(3): 104-111.
|
Wang Y G, Chen J J, Qin L. 2005. Mesanphrys carcini causing severe scuticociliatosis in farmed turbot Scophthalmus maximus in China. Journal of Fishery Sciences of China, 12(5): 594-601.
(in Chinese with English abstract) |
Whang I, Kang H S, Lee J. 2013. Identification of scuticociliates(Pseudocohnilembus persalinus, P. longisetus, Uronema marinum and Miamiensis avidus) based on the cox1 sequence. Parasitology International, 62(1): 7-13.
DOI:10.1016/j.parint.2012.08.002 |
Wilbert N. 1975. Eine verbesserte Technik der Protargolimprägnation für Ciliaten. Mikrokosmos, 64: 171-179.
|
Zhang Z, Wang Y G, Qin L, Li Q F. 2006. Epizootic investigation and study on challenge experiment of fin rot disease in cultured turbot (Scophthalmus maximus). Fisheries Science, 25(6): 271-274.
(in Chinese with English abstract) |
Zhou L, Zhan W B, Song W B, Song C H. 2000. Observations on the histopathology of the external ulcerative disease of Japanese flounder, Paralichthys olivaceus. Journal of Ocean University of Qingdao, 30(4): 593-597.
(in Chinese with English abstract) |
 2019, Vol. 37
2019, Vol. 37


