Institute of Oceanology, Chinese Academy of Sciences
Article Information
- CHAI Zhaoyang, HU Zhangxi, LIU Yuyang, TANG Yingzhong
- Proof of homothally of Pheopolykrikos hartmannii and details of cyst germination process
- Journal of Oceanology and Limnology, 38(1): 114-123
- http://dx.doi.org/10.1007/s00343-019-9077-x
Article History
- Received Mar. 21, 2019
- accepted in principle Apr. 26, 2019
- accepted for publication May. 15, 2019
2 Laboratory of Marine Ecology and Environmental Science, Qingdao National Laboratory of Marine Science and Technology, Qingdao 266237, China;
3 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China;
4 University of Chinese Academy of Sciences, Beijing 100049, China
Resting cysts play crucial roles in the ecology of dinoflagellates, and have been considered a vital attribute in dinoflagellate life cycles (Elbrächter, 2003), especially for the harmful algal blooms (HABs)-causing species. Dinoflagellates resting cysts are generally zygotic stage of the life cycle, accumulated in sediments, and resistant to harsh environments (Anderson and Wall, 1978; Anderson, 1989; Hallegraeff and Bolch, 1991; Matsuoka et al., 2003). Dinoflagellate resting cysts are associated with genetic recombination (Dale, 1986; Zingone et al., 2001; Bravo and Figueroa, 2014), initiation and termination of blooms (Anderson and Wall, 1978; Anderson and Morel, 1979), resistance to adverse environments (Anderson and Wall, 1978; Anderson, 1989; Brosnahan et al., 2017), protection from viruses, grazers, or parasite attacks (Rengefors et al., 1998; Zingone et al., 2001; Bravo and Figueroa, 2014), and biogeographical divergence of populations (Hallegraeff and Bolch, 1991; Smayda, 2007; Tang and Gobler, 2012, 2015). About 200 out of approximately 2 400 extant dinoflagellate species have been reported to produce cysts, and more than 20 of these cyst-producing dinoflagellates cause HABs (Nehring, 1993; Head, 1996; Matsuoka et al., 2003).
Dinoflagellate cells form resting cysts and sediment out of water column generally during the bloom termination phase (Figueroa et al., 2010). Formation of resting cysts is mostly coupled to sexual reproduction, with rare exceptions (Taylor, 1980; Pfiester and Anderson, 1987). Therefore, the sexual reproduction of dinoflagellates is of great importance for the understanding of HAB ecology. Sexual reproduction occur either by self-fertilization (i.e. fusion of gametes from genetically identical strains) or through outcrossing (i.e. fusion of gametes from different genetic strains) (Pfiester, 1984; Pfiester and Anderson, 1987; Figueroa et al., 2010). These processes are respectively referred to as homothallism (i.e. sexual mating and cyst formation occurs within a monoclonal culture) and heterothallism (i.e. sexual mating and cyst formation requires two different clonal strains, often referred as plus and minus types). Although the advantage of genetic recombination is supposedly lost in homothallism, other advantages of homothallic sexuality (e.g. DNA repair, less harmful effects of mutations in the offspring), might be kept in those homothallic species (Bernstein et al., 1985; Burt, 2000). More importantly, cells of homothallic species have much higher probability to encounter gametes with reduced energy expenditure, while heterothallic species need to find the compatible mating type, which makes homothally an advantage for the homothallic species in terms of the maintenance and succession of populations (Steidinger and Garccés, 2006; Figueroa et al., 2010). Homothally is thus believed to have evolved from heterothally (Goodenough, 1985). Those dinoflagellates having sexuality are either homothallic or heterothallic, while, occasionally, some species have been documented to be both homothallic and heterothallic (Blackburn et al., 2001; Blackburn and Parker, 2005; Figueroa et al., 2006; Steidinger and Garccés, 2006). Harmful algal species could select homothallism for maintaining populations if the habitat does not concentrate cells for sustaining blooms (Garcés et al., 2002; Figueroa and Bravo, 2005).
The athecate (unarmored) dinoflagellate Pheopolykrikos hartmannii (Zimmermann) Matsuoka and Fukuyo was initially described as Polykrikos hartmannii 90 years ago (Zimmermann, 1930) and redescribed as Pheopolykrikos hartmannii by Matsuoka and Fukuyo (1986) based on three features that differentiated it from Polykrikos (i.e. the same vs. different numbers of zooids and nuclei, the presence vs. absence of solitary cells in the life cycle, and photosynthesis vs. heterotrophy). Hoppenrath et al. (2010) transferred it back to Polykrikos based mainly on the ultrastructure of "taeniocyst-nematocyst complex", although phylogenetic analyses of the small subunit and large subunit rDNA may not support this transfer. However, Tang et al. (2013) disagreed to this re-classification and suggested to conserve it as Pheopolykrikos hartmannii because this species is genetically and morphologically distinct from other two common species of Polykrikos (P. schwartzii and P. kofoidii), such as the differences in the apical groove, the connection of zooids, and the continuity of sulcus/sulci for all zooids, plus the abovementioned three features stressed by Matsuoka and Fukuyo (1986). This species has been reported in different geographic regions of the world, including motile cell-based records and cyst-based records. The motile cell-based records of P. hartmannii include China (Huang and Dong, 2001), Mexico (Gárate-Lizárraga et al., 2008), Korea (Kim et al., 2008), Japan (Fukuyo et al., 1990) and USA (Badylak and Phlips, 2004; Hoppenrath et al., 2009; Tang et al., 2013), while the cyst-based records include India (Godhe et al., 2000), Japan (Matsuoka, 1985; Matsuoka and Fukuyo, 1986), Korea (Pospelova and Kim, 2010), USA (Bringué et al., 2014), Marmara and Black Sea (Mudie et al., 2017), and Canada (Pospelova et al., 2010; Price and Pospelova, 2011). Most earlier studies identified this species mainly using light microscopy (Matsuoka, 1985; Matsuoka and Fukuyo, 1986; Godhe et al., 2000; Huang and Dong, 2001; Badylak and Phlips, 2004; Gárate-Lizárraga et al., 2008; Kim et al., 2008; Hoppenrath et al., 2009), while Tang et al. (2013) observed the morphology of both vegetative cells and resting cysts using both LM and SEM based on laboratory cultures isolated from New York, USA. This study also reported blooms and ichthyotoxicity of P. hartmannii. In addition, based on the fact that the two clonal cultures did not produce cysts respectively but formed cysts easily once they were mixed together, they concluded that P. hartmannii is heterothallic (Tang et al., 2013).
However, in a clonal culture of P. hartmannii established from a plankton sample taken in Jiaozhou Bay, Qingdao, China, resting cysts were observed, suggesting the species may be homothallic. Therefore, we conducted further experiments in this regard and here report our evidence for the homothally of P. hartmannii. In addition, we also report a detailed observation of the cyst germination processes, particularly with an interesting observation of an amoeboid stage of the germling during a short releasing process (~15 s). We believe our observations as presented provide further insights into the biology and ecology of P. hartmannii, particularly in terms of understanding the recurrence of blooms and ubiquitous distribution of the species.
2 MATERIAL AND METHOD 2.1 Collection of sample and culture establishmentA monoclonal culture of P. hartmannii (strain No.PHJZB 1705) was established by micro-pipetting a single cell from the samples collected in Jiaozhou Bay, Qingdao, China in 2017. Since the vegetative cells of the species exist as two-cell chains most of the time, the culture was established from such a two-cell chain. The culture was grown in autoclaved, 0.22 μmfiltered natural seawater with a salinity of 33 enriched with f/2-Si medium (Guillard, 1975). The culture was maintained at 21℃ in an incubator with a photoperiod of 12 h׃12 h (light׃dark) with an irradiance of ~100 μmol photons/(m2·s) illuminated by fluorescent lights. A mixture of 10 000 μg/mL streptomycin and 10 000 I.U. penicillin (Solarbio, Beijing, China) was added into the medium immediately before inoculation (final concentration 2%) to inhibit bacterial growth.
2.2 Molecular identification of the isolatesThe DNA extraction, PCR amplification and sequencing were the same to our previous study (Hu et al., 2018). The obtained sequence was deposited in GenBank with an accession number MK610716.
2.3 Production and observation of resting cystsAfter the initial observation of cysts at the bottom of culture plates, further observations of the monoclonal culture of PHJZB1705 were conducted using a six well cell culture plates with each well containing 10 mL f/2-Si medium which was added the antibiotics mixture (2% final concentration). The culture was grown into stationary phase after four weeks and were maintained for up to two to three months while the observations were performed. Observations for vegetative cells, cell pairs in division and sexual mating, planozygotes, and cyst morphology were accomplished under an upright microscope (BX53, Olympus, Japan) and inverted microscope (IX73, Olympus, Japan), and photographed with a digital camera (model Olympus DP80). For the observations of nuclei and chloroplasts, the vegetative cells and cysts were stained with SYBR Green (Solarbio, Beijing, China) prior to being observed with epifluoresence microscopy mode using the abovementioned digital camera.
In order to exclude the possibility that the initial two cells in the two-celled colony from which we established the clonal culture were diploids (although with an extremely low possibility), we then established a new clonal culture by isolating one of the two daughter cells after a newly germinated cyst (i.e. germling) had its first meiotic division (we assumed that the first cell division of a germling is meiotic). This new clonal culture was studied whether it could also produce cysts or not as was done for the initial culture.
2.4 Observations of germination time series of resting cystsIn order to test whether or not the cysts produced in clonal cultures can germinate, individual cysts were isolated by micro-pipetting from the culture plates that had been inoculated two to three months. The observations of germination time series of resting cysts were the same as described in our previous studies (Tang and Gobler, 2012, 2015). In brief, cysts in individual wells of a 24-well culture plate were observed and photographed every other day under the inverted microscope, with a longer observation when an obvious activity or morphological development was noticed.
3 RESULT 3.1 Molecular identification of the isolateA partial large subunit rDNA sequence (1 435 bp, accession No. MK610716) was obtained from the clonal culture of P. hartmannii, which was blasted with BLASTn in GenBank and 99.70% (1 308 bp/1 312 bp) and 99.69% (1 298 bp/1 302 bp) identical to two strains of this species collected from the Forge River, New York, USA (accession Nos. HQ834209 and HQ834210), respectively, confirmed the identity of our isolate as P. hartmannii. The phylogenetic analyses of the LSU rDNA sequences using maximum likelihood (ML) generated a well supported non-branching cluster composed of P. hartmannii sequences from China, USA, and Korea, demonstrating further the identity of the Jiaozhou Bay isolate as P. hartmannii (Fig. 1). Morphological observation of the vegetative cells (Fig. 2a, and others not shown) found all diagnostic features in morphology being consistent to previous descriptions in Matsuoka and Fukuyo (1986) and Tang et al. (2013).
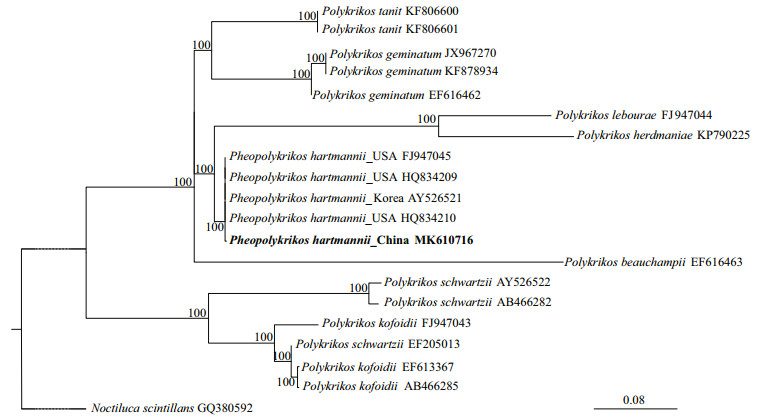
|
| Fig.1 Phylogeny inferred from partial large subunit rDNA sequence of P. hartmannii strain PHJZB1705 (MK610716) and other dinoflagellates sequences based on maximum likelihood (ML) GenBank accession numbers are shown after each taxon name in the tree. Maximum likelihood (ML) analyses were conducted with Ra×ML v7.2.6 (Stamatakis et al., 2008) on the T-REX web server (Boc et al., 2012) using the model GTR+G. Node support was assessed with 1 000 bootstrap replicates. Noctiluca scintillans was used as an outgroup. |
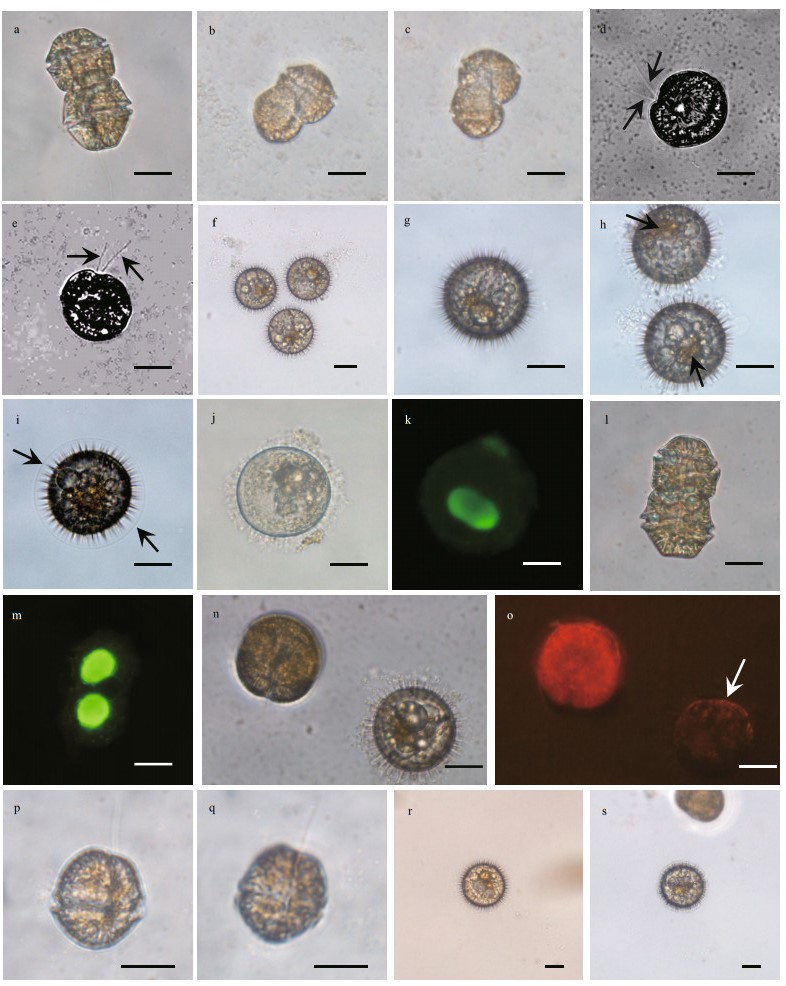
|
| Fig.2 Formation of resting cysts in P. hartmannii through sexual mating of gametes observed in the culture of PHJZB1705 a. a typical vegetative cell in two-celled chain; b, c. mating of gametes; d, e. planozygotes with two longitudinal flagella observed (arrows); f–h. mature resting cysts of P. hartmannii with red accumulation body (arrow); i. immature cysts covered with membrane (arrow); j, k. A SYBR Green-stained cyst showing a nucleus (green); l, m. a SYBR Green-stained vegetative cell in two-celled colony showing two nuclei (green); n, o. epifluorescence micrograph showing granular chloroplasts of a vegetative cell and a cyst (white arrow); p, q. one of the two daughter cells after the first cell division of the germling; r, s. new cysts produced from the newly established clonal culture. Scale bars=20 μm. |
Resting cysts of P. hartmannii were observed to form in the clonal culture via the mating of two isogametes (i.e. indistinguishable in morphology and size under the 400× magnification of LM), which was evidenced from two cells connected or fused in positions non-parallel to each other (Fig. 2b & c). A fused nucleus could be observed after mating was completed (Fig. 2d). After mating, planozygotes with two longitudinal flagella could remain in a vegetative state for several days prior to transforming to cysts (Fig. 2d & e), and the planozygotes moved more slowly than the vegetative cells (Supplementary video 1). Cysts were generally produced during the stationary stage of growth, i.e., four to five weeks after inoculation, although the time needed depended on the initial cell density.
Mature resting cysts of P. hartmannii were light brown in color, contained a red body, and spherical (Fig. 2f–h). The surface of mature cysts were covered with many arrow-like spines exuded from inside of the cyst (Fig. 2f–h). Immature cysts were covered with a perfect spherical membrane (Fig. 2i), and the membrane disappeared when the resting cysts were mature. A single nucleus was observed in the resting cyst (Fig. 2j & k) and the chloroplasts disappeared in the resting cysts, judging from the absence of red autofluorescence of chlorophyll under fluorescent LM (Fig. 2n & o). These observations are consistent to previous works (Matsuoka and Fukuyo, 1986; Tang et al., 2013).
In the cyst-producing experiments designed to exclude the possibility that the initial two cells of the colony from which we established the clonal culture were diploids, we did observe formation of resting cysts (Fig. 2r & s) with the same processes and morphology as described above in the clonal culture that was established by isolating one of the two daughter cells (Fig. 2p & q) of a germling. It was about three weeks after the isolation of one of the two daughter cells when we observed the normal chain consisting of two cells. This result further confirms the culture isolated from Jiaozhou Bay is homothallic.
3.3 Observations of the germination process of resting cystsIndividually isolated resting cysts were successfully germinated under normal culturing conditions (21℃, 12 h: 12 h light: dark cycle, ~100 μmol quanta/(m2·s)) after 20–50 days (Figs. 2 & 3). At the first 4–10 days, the morphology of cyst contents transformed from round to ellipsoidal, and a space between cyst wall and cyst contents became distinguishable (Fig. 3a–c). After 10–30 days, the morphology of cyst contents continued to transform, and developed into a gymnodinoid cell with the cingulum being recognizable first (Fig. 3d & e). The contents became more granular and the pigmentation became denser with the progression of germination, and the germling was visually identical to vegetative cells (Fig. 3f). After germination, the wall of empty cysts was not pigmented.
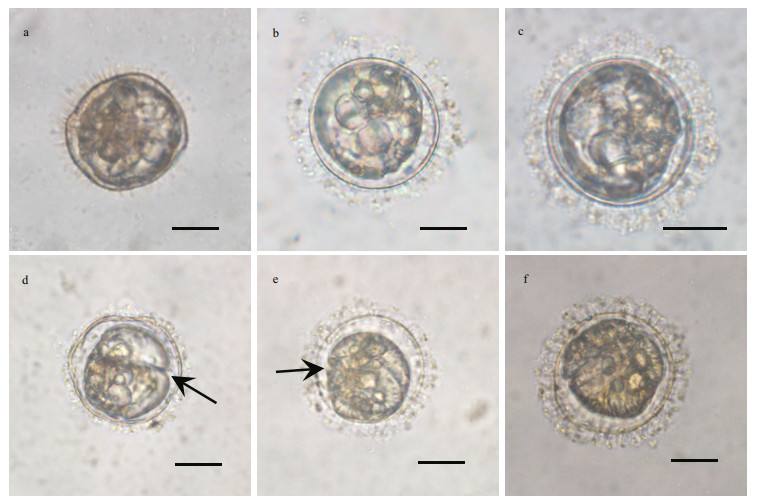
|
| Fig.3 Different stages of cyst germination processes within the cyst wall a. day 3; b. day 5; c. day 7; d. day 12 (arrow: cingulum); e. day 17 (arrow: cingulum); f. day 20. Scale bars=20 μm. |
A significant landmark of the germination process was observed at the transient period when the germling was breaking out of the cyst wall, which took about 15 s (Fig. 4a–j; Supplementary video 2). Before the germling broke through cyst wall, it rotated within the cyst wall and its morphology was highly plastic, i.e., it took an amoeboid morphology and changed to several different shapes within a second (Supplementary video 2). After about 7 s, the germling shape changed to ellipsoidal and began to squeeze through the archeopyle of cyst wall (Fig. 4g–j). When the germling was released, the distal end of the transverse flagellum appeared to be within the germling (Fig. 4j) and was drawn out from the joining location of the cingulum and sulcus within one second (Supplementary video 2). The transverse flagellum was helical, and did not encircle the cingulum of the germling (as seen in a typical vegetative cell) in a short time (Fig. 4k & l). The germling also has two longitudinal flagella (Fig. 4l & m; Supplementary videos 2 & 3), demonstrating its diploidity. The archeopyle was chasmic, being clearly visible after germination ended (Fig. 4n & o) and the same as described in Matsuoka and Fukuyo (1986).
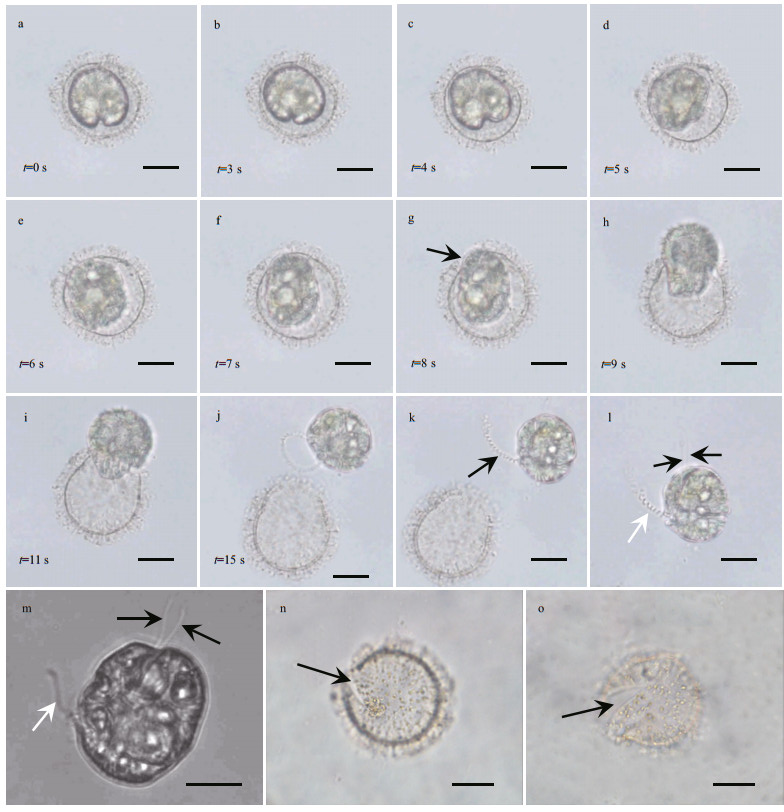
|
| Fig.4 A time-series observation for the germling about and after breaking through the cyst wall in 15 s a–f. shape transformations of germling; g–j. germling with an amoeboid morphology that was struggling out of the cyst wall; k. newly released germling with one helical transverse flagellum. The distal end of transverse flagellum appeared to be swiftly drawn out from the joint position of cingulum and sulcus (arrow); l, m. released germling with one transverse flagellum (white arrow) and two longitudinal flagella (black arrows); n, o. empty cysts after germination, showing a chasmic archeopyle (arrow). Scale bars=20 μm. |
What we observed in the morphology of P. hartmannii cysts produced under normal culturing conditions is similar to previous observations (Matsuoka and Fukuyo, 1986; Tang et al., 2013). The details of cyst germination processes, however, have not been reported for this species in the literature, although a gymnodinoid germling prior to releasing from the cyst wall was also observed in the species (Tang et al., 2013) and similar gymnodinoid germlings immediately after germination were reported in Gonyaulax digitalis (Wall and Dale, 1968) and Polykrikos kofoidii (Tillmann and Hoppenrath, 2013). The germination process was also similar to that of Margalefidinium polykrikoides (=Cochlodinium polykrikoides) (Tang and Gobler, 2012) and Akashiwo sanguinea (Tang and Gobler, 2015) in terms of the general gymnodinoid shape of the motile cell and development of motile cell within the cyst (e.g. gradual appearance of cingulum and sulcus, etc.), but it seemed the germination of P. hartmanni cysts took a shorter time around 20–50 days according to the present observation than the cysts of A. sanguinea (Tang and Gobler, 2015) and Gymnodinium aureolum (Tang et al., 2008), which took a time up to three months. The most interesting observation of the germination process was the amoeboid stage of germling breaking through the archeopyle. While we are not sure of its biological or taxonomical significance at the time, we believe it is of interest to observe the details of the cyst germination process with a complete time series and particularly the plasticity of amoeboid germling, which, to the best of our knowledge, has not been previously reported for this species and other cyst-producing dinoflagellate. It is also noteworthy that the cysts of P. hartmannii are produced sexually and homothallically, which is discussed below.
4.2 Ecological implications of the homothally of P. hartmanniiAs introduced before, the encystment of dinoflagellates is either via self-fertilization (homothallism) or through intercrossing (heterothallism) (Pfiester and Anderson, 1987; Figueroa et al., 2010). In this study, we confirmed that P. hartmannii isolated from Jiaozhou Bay, China can produce resting cysts homothallically and that the cysts were produced sexually. The confirmation of homothally contrasts to the conclusion of Tang et al. (2013) in which they explicitly stated P. hartmannii produces cysts heterothallically, as they observed cysts production only by crossing two clonal cultures (strains FR-3 and FR-4, both isolated from the Forge River, New York, USA), but cyst formation was never observed in both clonal cultures even after they had been cultured for a long period of time. While the partial LSU rDNA sequence of our isolate is almost identical to FR-3 and FR-4 (99.7%), we are not sure about whether this difference is caused by genetic differentiation of the two populations or a false phenomenon in their observation (i.e. conditions in the respective cultures might not be suitable for sexual mating or cyst production), although the latter is not likely the case. Most dinoflagellates having been documented of sexuality are either homothallic or heterothallic, however, a number of species have been documented to be both homothallic and heterothallic, such as Gymnodinium catenatum (Figueroa et al., 2006; Steidinger and Garccés, 2006), Scrippsiella trochoidea (Montresor et al. 2003), Alexandrium tamarense, Karenia brevis, Noctiluca scintillans (Blackburn and Parker, 2005), and now also including P. hartmannii. Compared to heterothallism, advantages of homothallic sexuality (e.g. DNA repair, less harmful effects of mutations in the offspring) might be kept in those homothallic species (Bernstein et al., 1985; Burt, 2000). It has been well known that homothallic species have higher probability in gametes encountering and thus less energy expenditure comparing to heterothallic species, therefore, homothally of P. hartmannii could make it much easier to have gametes mating, produce resting cysts, and the maintenance and succession of populations. Consequently, it may be possible to observe higher cyst density in sediments of areas where a homothallic population dominates, compared to the area dominated by a heterothallic population of P. hartmannii. This sexuality type of P. hartmannii may be one of the reasons accounting for its blooms and cosmopolitan distribution around the world.
5 CONCLUSIONCollectively, we provided convincing evidence for the homothally of P. hartmannii and the first detailed visual evidence of the cyst germination process, particularly the amoeboid stage of germling, in this study. These observations provide further insight into the biology and ecology of P. hartmannii, particularly a mechanism that may partly account for the population dynamics and ubiquitous distribution of the species.
6 DATA AVAILABILITY STATEMENTThe authors declare that all data supporting the findings of this study are available within the article and its supplementary information files.
7 ACKNOWLEDGEMENTWe want to express our sincere gratitude to the anonymous reviewers for their constructive comments.
Anderson D M, Morel F M M. 1979. The seeding of two red tide blooms by the germination of benthic Gonyaulax tamarensis hypnocysts. Estuarine and Coastal Marine Science, 8(3): 279-293.
DOI:10.1016/0302-3524(79)90098-7 |
Anderson D M, Wall D. 1978. Potential importance of benthic cysts of Gonyaulax tamarensis and G. excavata in initiating toxic dinoflagellate blooms. Journal of Phycology, 14(2): 224-234.
DOI:10.1111/j.1529-8817.1978.tb02452.x |
Anderson D M. 1989. Cysts as factors in Pyrodinium bahamense ecology. In: Hallegraeff G M, Maclean J L eds. Biology, Epidemiology and Management of Pyrodinium Red Tides. The Fisheries Department, Ministry of Development, Brunei Darussalem, and International center for Living Aquatic Resources Management, Metro Manila. p.81-88.
|
Badylak S, Phlips E J. 2004. Spatial and temporal patterns of phytoplankton composition in subtropical coastal lagoon, the Indian River Lagoon, Florida, USA. Journal of Plankton Research, 26(10): 1.
|
Bernstein H, Byerly H C, Hopf F A, Michod R E. 1985. Genetic damage, mutation, and the evolution of sex. Science, 229(4719): 1.
|
Blackburn S I, Bolch C J S, Haskard K A, Hallegraeff G M. 2001. Reproductive compatibility among four global populations of the toxic dinoflagellate Gymnodinium catenatum (Dinophyceae). Phycologia, 40(1): 78-87.
DOI:10.2216/i0031-8884-40-1-78.1 |
Blackburn S I, Parker N. 2005. Microalgal life cycles: encystment and excystment. In: Anderson R A ed. Algal Culturing Techniques. Elsevier Academic Press, Burlington. p.399-417.
|
Boc A, Diallo A B, Makarenkov V. 2012. T-REX:a web server for inferring, validating and visualizing phylogenetic trees and networks. Nucleic Acids Research, 40(w1): w573-w579.
DOI:10.1093/nar/gks485 |
Bravo I, Figueroa R I. 2014. Towards an ecological understanding of dinoflagellate cyst functions. Microorganisms, 2(1): 11-32.
DOI:10.3390/microorganisms2010011 |
Bringué M, Pospelova V, Field D B. 2014. High resolution sedimentary record of dinoflagellate cysts reflects decadal variability and 20th century warming in the Santa Barbara Basin. Quaternary Science Reviews, 105: 86-101.
DOI:10.1016/j.quascirev.2014.09.022 |
Brosnahan M L, Ralston D K, Fischer A D, Solow A R, Anderson D M. 2017. Bloom termination of the toxic dinoflagellate Alexandrium catenella:vertical migration behavior, sediment infiltration, and benthic cyst yield. Limnology and Oceanography, 62(6): 2 829-2 849.
DOI:10.1002/lno.10664 |
Burt A. 2000. Perspective:sex, recombination, and the efficacy of selection-was Weismann right?. Evolution, 54(2): 337-351.
|
Dale B. 1986. Life cycle strategies of oceanic dinoflagellates. UNESCO Technical Papers in Marine Science, 49: 65-72.
|
Elbrächter M. 2003. Dinophyte reproduction:progress and conflicts. Journal of Phycology, 39(4): 629-632.
DOI:10.1046/j.1529-8817.2003.39041.x |
Figueroa R I, Bravo I, Garcés E, Ramilo I. 2006. Nuclear features and effect of nutrients on Gymnodinium catenatum (Dinophyceae) sexual stages. Journal of Phycology, 42(1): 67-77.
DOI:10.1111/j.1529-8817.2006.00181.x |
Figueroa R I, Bravo I. 2005. A study of the sexual reproduction and determination of mating type of Gymnodinium nolleri(Dinophyceae) in culture. Journal of Phycology, 41(1): 74-83.
DOI:10.1111/j.1529-8817.2005.04045.x |
Figueroa R I, Rengefors K, Bravo I, Bensch S. 2010. From homothally to heterothally:Mating preferences and genetic variation within clones of the dinoflagellate Gymnodinium catenatum. Deep Sea Research Part II:Topical Studies in Oceanography, 57(3-4): 190-198.
DOI:10.1016/j.dsr2.2009.09.016 |
Fukuyo Y, Takano H, Chihara M, Matsuoka K. 1990. Red Tide Organisms in Japan-An Illustrated Taxonomic Guide.Uchida Rokakuho, Tokyo.
|
Gárate-Lizárraga I, Band-Schmidt C J, Grayeb del Alamo T. 2008. Myrionecta, Gyrodinium and Katodinium bloom in Gulf of California. Harmful Algae News, 37: 6-7.
|
Garcés E, Zingone A, Montresor M, Reguera B, Dale B. 2002.LIFEHAB: life histories of microalgal species causing harmful blooms. Office for the Official Publications of the European Communities, Luxembourg.
|
Godhe A, Karunasagar I, Karunasagar I, Karlson B. 2000. Dinoflagellate cysts in recent marine sediments from SW India. Botanica Marina, 43(1): 39-48.
|
Goodenough U. 1985. An essay on the origins and evolution of eukaryotic sex. In: Halvorns H, Monroy O A eds. The Origin and Evolution of Sex, Marine Biological Laboratory Lectures in Biology. Alan R. Liss, Inc., New York. p.123-140.
|
Guillard R R L. 1975. Culture of phytoplankton for feeding marine invertebrates. In: Smith W L, Chanley M H eds. Culture of Marine Invertebrate Animals. Springer, Boston. p.26-60.
|
Hallegraeff G M, Bolch C J. 1991. Transport of toxic dinoflagellate cysts via ships' ballast water. Marine Pollution Bulletin, 22(1): 27-30.
DOI:10.1016/0025-326X(91)90441-T |
Head M J. 1996. Modern dinoflagellate cysts and their biological affinities. In: Jansonius J, McGregor D C eds.Palynology: Principles and Applications. American Association of Stratigraphic Palynologists Foundation, Dallas. p.1 197-1 248.
|
Hoppenrath M, Bachvaroff T R, Handy S M, Delwiche C F, Leander B S. 2009. Molecular phylogeny of ocelloidbearing dinoflagellates (Warnowiaceae) as inferred from SSU and LSU rDNA sequences. BMC Evolutionary Biology, 9: 116.
DOI:10.1186/1471-2148-9-116 |
Hoppenrath M, Yubuki N, Bachvaroff T R, Leander B S. 2010. Re-classification of Pheopolykrikos hartmannii as Polykrikos (Dinophyceae) based partly on the ultrastructure of complex extrusomes. European Journal of Protistology, 46(1): 29-37.
DOI:10.1016/j.ejop.2009.08.003 |
Hu Z X, Deng Y Y, Li Y H, Tang Y Z. 2018. The morphological and phylogenetic characterization for the dinoflagellate Margalefidinium fulvescens (=Cochlodinium fulvescens) isolated from the Jiaozhou Bay, China. Acta Oceanologica Sinica, 37(10): 11-17.
DOI:10.1007/s13131-018-1295-0 |
Huang C J, Dong Q X. 2001. Taxonomic and biological studies on organisms causing a large scale red tide in Zhujiang River Estuary in spring, 1998 Ⅲ. Oceanologia et Limnologia Sinica, 32(1): 1-6.
|
Kim K Y, Iwataki M, Kim C H. 2008. Research Note:molecular phylogenetic affiliations of Dissodinium pseudolunula, Pheopolykrikos hartmannii, Polykrikos cf. schwartzii and Polykrikos kofoidii to Gymnodinium sensu stricto species (Dinophyceae). Phycological Research, 56(2): 89-92.
|
Matsuoka K, Fukuyo Y, Hallegraeff G M, Anderson D M, Cembella A D. 2003. Taxonomy of cysts. In: Hallegraeff G M, Anderson D M, Cembella A D eds. Manual on Harmful Marine Microalgae. UNESCO, Paris. p.563-592.
|
Matsuoka K, Fukuyo Y. 1986. Cyst and motile morphology of a colonial dinoflagellate Pheopolykrikos hartmannii(Zimmermann) comb. nov. Journal of Plankton Research, 8(4): 811-818.
DOI:10.1093/plankt/8.4.811 |
Matsuoka K. 1985. Archeopyle structure in modern gymnodinialean dinoflagellate cysts. Review of Palaeobotany and Palynology, 44(3-4): 217-231.
DOI:10.1016/0034-6667(85)90017-X |
Montresor M, Sgrosso S, Procaccini G, Kooistra W H C F. 2003. Intraspecific diversity in Scrippsiella trochoidea(Dinophyceae):evidence for cryptic species. Phycologia, 42(1): 56-70.
DOI:10.2216/i0031-8884-42-1-56.1 |
Mudie P J, Marret F, Mertens K N, Shumilovskikh L, Leroy S A G. 2017. Atlas of modern dinoflagellate cyst distributions in the Black Sea Corridor:from Aegean to Aral Seas, including Marmara, Black, Azov and Caspian Seas. Marine Micropaleontology, 134: 1-152.
DOI:10.1016/j.marmicro.2017.05.004 |
Nehring S. 1993. Mechanisms for recurrent nuisance algal blooms in coastal zones: resting cyst formation as lifestrategy of dinoflagellates. In: Sterr H, Hofstade J, Plag H P eds. Interdisciplinary Discussion of Coastal Research and Coastal Management Issues and Problems. Lang, Frankfurt/M. p.454-467.
|
Pfiester L A, Anderson D M. 1987. Dinoflagellate reproduction.In: Taylor F J R ed. The Biology of Dinoflagellates.Blackwell Scientific Publications, Oxford. p.621-629.
|
Pfiester L A. 1984. Sexual reproduction. In: Spector D L ed.Dinoflagellates. Academic Press, Massachusetts. p.181-200.
|
Pospelova V, Esenkulova S, Johannessen S C, O'Brien M C, Macdonald R W. 2010. Organic-walled dinoflagellate cyst production, composition and flux from 1996 to 1998 in the central Strait of Georgia (BC, Canada):a sediment trap study. Marine Micropaleontology, 75(1-4): 17-37.
DOI:10.1016/j.marmicro.2010.02.003 |
Pospelova V, Kim S J. 2010. Dinoflagellate cysts in recent estuarine sediments from aquaculture sites of southern South Korea. Marine Micropaleontology, 76(1-2): 37-51.
DOI:10.1016/j.marmicro.2010.04.003 |
Price A M, Pospelova V. 2011. High-resolution sediment trap study of organic-walled dinoflagellate cyst production and biogenic silica flux in Saanich Inlet (BC, Canada). Marine Micropaleontology, 80(1-2): 18-43.
DOI:10.1016/j.marmicro.2011.03.003 |
Rengefors K, Karlsson I, Hansson L A. 1998. Algal cyst dormancy:a temporal escape from herbivory. Proceedings of the Royal Society B Biological Sciences, 265(1403): 1 353-1 358.
DOI:10.1098/rspb.1998.0441 |
Smayda T J. 2007. Reflections on the ballast water dispersal -harmful algal bloom paradigm. Harmful Algae, 6(4): 601-622.
DOI:10.1016/j.hal.2007.02.003 |
Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RA×ML Web servers. Systematic Biology, 57(5): 758-771.
DOI:10.1080/10635150802429642 |
Steidinger K A, Garccés E. 2006. Importance of life cycles in the ecology of harmful microalgae. In: Granéli E, Turner J T eds. Ecology of Harmful Algae. Springer, Netherlands.p.37-49.
|
Tang Y Z, Egerton T A, Kong L S, Marshall H G. 2008. Morphological variation and phylogenetic analysis of the dinoflagellate Gymnodinium aureolum from a tributary of Chesapeake Bay. Journal of Eukaryotic Microbiology, 55(2): 91-99.
DOI:10.1111/j.1550-7408.2008.00305.x |
Tang Y Z, Gobler C J. 2012. The toxic dinoflagellate Cochlodinium polykrikoides (Dinophyceae) produces resting cysts. Harmful Algae, 20: 71-80.
DOI:10.1016/j.hal.2012.08.001 |
Tang Y Z, Gobler C J. 2015. Sexual resting cyst production by the dinoflagellate Akashiwo sanguinea:a potential mechanism contributing to the ubiquitous distribution of a harmful alga. Journal of Phycology, 51(2): 298-309.
DOI:10.1111/jpy.12274 |
Tang Y Z, Harke M J, Gobler C J. 2013. Morphology, phylogeny, dynamics, and ichthyotoxicity of Pheopolykrikos hartmannii (Dinophyceae) isolates and blooms from New York, USA. Journal of Phycology, 49(6): 1 084-1 094.
DOI:10.1111/jpy.12114 |
Taylor F J R. 1980. On dinoflagellate evolution. Biosystems, 13(1-2): 65-108.
DOI:10.1016/0303-2647(80)90006-4 |
Tillmann U, Hoppenrath M. 2013. Life cycle of the pseudocolonial dinoflagellate Polykrikos kofoidii(Gymnodiniales, Dinoflagellata). Journal of Phycology, 49(2): 298-317.
DOI:10.1111/jpy.12037 |
Wall D, Dale B. 1968. Modern dinoflagellate cysts and evolution of the Peridiniales. Micropaleontology, 14(3): 265-304.
DOI:10.2307/1484690 |
Zimmermann W. 1930. Neue und wenig bekannte Kleinalgen von Neapel Ⅰ-Ⅴ. Zeitschrift für Botany, 23: 419-442.
|
Zingone A, Garcés E, Wyatt T, Silvert B, Bolch C. 2002. The importance of life cycles in the ecology of harmful algal blooms. In: Garcés E, Zingone A, Montresor M, Reguera B, Dale B eds. LIFEHAB: Life Histories of Microalgal Species Causing Harmful Blooms. Office for the Official Publications of the European Community, Luxembourg, Office for the Official Publications of the European Community, p.134-137.
|
 2020, Vol. 38
2020, Vol. 38


