Institute of Oceanology, Chinese Academy of Sciences
Article Information
- MA Dongdong, LI Yongfu, FU Haifang
- Effect of high temperature on the balance between photosynthetic light absorption and energy utilization in Chlorella pyrenoidosa (Chlorophyceae)
- Journal of Oceanology and Limnology, 38(1): 186-194
- http://dx.doi.org/10.1007/s00343-019-8369-5
Article History
- Received Dec. 25, 2018
- accepted in principle Feb. 27, 2019
- accepted for publication Apr. 8, 2019
2 Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China;
3 Nantong Research and Development Center of Marine Science and Technology, Institute of Oceanology, Chinese Academy of Sciences, Nantong 226019, China;
4 Zhonglu Environmental and Engineering Assessment Center of Shandong Province, Jinan 250013, China
Microalgae has long been cultured to produce valuable compounds, such as protein, carotenoids, poly-unsaturated fatty acids, vitamins and steroids (Pulz and Gross, 2004; Sajilata et al., 2008; Yang and Kong, 2011; Zhang and Liu, 2016). Chlorella is one of the earliest microalgae for commercial development and widely cultured for production of health food and animal feed (Hsieh and Wu, 2009; Yang and Kong, 2011; Du et al., 2017). In outdoors, mass cultivation of Chlorella is often affected by environmental stresses, such as high pH, UV, strong light, especially high temperature (Converti et al., 2009; Zhao et al., 2015; Serra-Maia et al., 2016).
Photosynthesis is one of the most high temperature susceptible physiological processes in higher plants and algae (Berry and Björkman 1980; Wen et al. 2005; Zhang and Liu 2016). Previous studies suggest that high temperature stress can damage the PSII reaction centers and oxygen-evolving complex, inhibit PSII acceptor side and CO2 assimilation capacities (Havaux, 1993; Zhao et al., 2008; Li et al., 2009). Therefore, high temperature in outdoors may inhibit the growth of Chlorella. Screening for high temperature resistant Chlorella strains is important for outdoor cultivation of this species for health food and animal feed production.
A freshwater Chlorella pyrenoidosa (Chlorophyceae) strain has long been cultured in outdoors in Hainan Province of China. In Hainan Province, during the daylight hours, the highest temperature (water temperature) and maximal light intensity in C. pyrenoidosa cultures are 40℃ and 2 300 μmol photons/(m2·s) in summer, respectively (Fig. 1). During most of the daylight hours, C. pyrenoidosa cells were exposed to high temperature (> 35℃). It shows that this C. pyrenoidosa strain exhibits strong tolerance to high temperature. However, the mechanisms of high temperature resistance of C. pyrenoidosa have not been clarified.

|
| Fig.1 The diurnal changes in light intensity of sunlight and in temperature of C. pyrenoidosa culture in an outdoor open raceway pond in summer |
In this study, the effect of high temperature on photosynthesis in C. pyrenoidosa was investigated. Meanwhile, the photoprotective mechanisms of C. pyrenoidosa at high temperature were also explored. A better understanding of thermal effects on photosynthesis and photoprotection can help optimize the productivity of the microalgal cultures.
2 MATERIAL AND METHOD 2.1 MicroalgaeChlorella pyrenoidosa (Chlorophyceae) (Xu et al., 2019) was grown in liquid BG-11 medium (Rippka et al., 1979). The culture conditions were 30℃ and a 14 h:10 h light/dark cycle at a light intensity of 500 μmol photons/(m2·s), with illumination provided from the top by cool-white fluorescent lamps. During the growth periods, the cultures were manually shaken two to three times a day to avoid sticking.
2.2 Cultivation of C. pyrenoidosa and measurement of cell growthChlorella pyrenoidosa previously grown at 30℃ was cultured in liquid BG-11 medium up to a final concentration of (10–12)×106 cells/mL in photobioreactors (working volume 500 mL) at 30, 35, 38, or 41℃. The growth conditions were 14 h:10 h light/dark cycle at a light intensity of 500 μmol photons/(m2·s). The cell growth was determined by counting the cell numbers using a 37XB inverted microscope (Shanghai, China). Specific growth rate was calculated with the following equation: R=(lnNt–lnN0)/t, where R represents the specific growth rate; Nt is the cell number after seven days of incubation; N0 is the initial cell number of C. pyrenoidosa; and t is the incubation period.
2.3 High temperature treatmentExponentially growing cells previously grown at 30℃ were used in this experiment. The glass cuvettes (250 mL) containing 100 mL cells (4.0×107 cells/mL) were placed into a water bath and incubated for 110 min at 30, 35, 38, or 41℃ in the dark, and then exposed to 500 μmol photons/(m2·s) at different temperatures for 10 min to activate photosynthetic photochemical quenching, Calvin cycle, nonphotochemical quenching, and antioxidant enzymes. The measurements were performed after high temperature treatments.
2.4 Chlorophyll content measurementAfter high temperature treatment and 10 min of illumination, microalgal chlorophyll was extracted with 80% acetone, and was analyzed at 663.6 and 646.6 nm using a UV-120 spectrophotometer (Shimadzu, Kyoto, Japan) according to Porra (2002).
2.5 Chlorophyll a fluorescence transient measurementChlorophyll a fluorescence (OJIP) transients of microalgae were measured using a Handy PEA fluorimeter (Hansatech, Norfolk, UK). The transients were induced by red light measuring approximately 3 000 μmol photons/(m2·s) provided by an array of three light-emitting diodes (LEDs, peak 650 nm). All measurements were taken with 5 min dark-adapted cells (fully dark-adapted cells). The OJIP transients were analyzed by the JIP-test (Strasser et al., 2000, 2004; Yusuf et al., 2010; Zhang et al., 2017), using the following original data: (1) the fluorescence intensity at 20 μs, considered to be F0 when all photosystem (PS) Ⅱ reaction centers were open; (2) the maximal fluorescence intensity, Fm (FP, at about 300 ms), assuming that the excitation intensity was high enough to close all of the PSII reaction centers; and (3) the fluorescence intensities at 2 ms (J-step, FJ) and 30 ms (I-step, FI). The following parameters were used for the quantification of PSII behavior:
(1) The maximal photochemical efficiency of PSII, Fv/Fm=(Fm–F0)/Fm.
(2) The relative variable fluorescence at J-step, VJ=(FJ–F0)/(Fm–F0).
(3) The light absorption per active reaction center (ABS/RC), the trapping of excitation energy per active reaction center (TR/RC) and the electron transport per active reaction center (ET/RC).
(4) The maximal amplitude of fluorescence in the I-P phase of the OJIP transient, AIP=(FP–F0)/(FI–F0).
2.6 Measurements of modulated chlorophyll fluorescence parametersThe efficiency of excitation capture by PSII (Fv′/Fm′), photochemical quenching coefficient (qP), actual photochemical efficiency of PSII (ΦPSII), photosynthetic linear electron transport rate (ETR) and non-photochemical quenching (NPQ) were measured with a FMS-2 pulse-modulated fluorometer (Hansatech, Norfolk, UK) according to Zhang et al. (2014) and Xie et al. (2016). The actinic light at 500 μmol photons/(m2·s) was offered by the light source. The measurements of minimum (F0) and maximum (Fm) fluorescence of microalgae were done in the dark. Following the onset of actinic light irradiation, the steady state fluorescence level (FS), the light-adapted minimum (F0′) and maximum (Fm′) fluorescence of microalgae were recorded when the chlorophyll fluorescence attained steady-state levels.
The following parameters were then calculated: (1) Fv′/Fm′=(Fm′–F0′)/Fm′; (2) qP=(Fm′–FS)/(Fm′–F0′) (3) ΦPSII=1–FS/Fm′; (4) ETR=ΦPSII×PFD×A×0.5, where A is the proportion of incident light that is absorbed by cell suspensions, and was determined according to Xie et al. (2016); (5) NPQ=(Fm–Fm′)/Fm′.
2.7 Enzyme assaysThe activities of ribulose-1, 5-bisphosphate carboxylase/oxygenase (Rubisco), NADPglyceraldehyde-3-phosphate dehydrogenase (NADPGAPDH), fructose-1, 6-bisphosphatase (FBPase), superoxide dismutase (SOD), ascorbate peroxidase (APX) and catalase (CAT) were extracted and analyzed according to Chen et al. (2008) and Zhang et al. (2017). About 106 cells were ground in 1.5 mL extraction buffer containing 50 mmol/L Hepes-KOH (pH 7.5), 10 mmol/L MgCl2, 2 mmol/L ethylenediaminetetraacetic acid (EDTA), 10 mmol/L dithiothreitol (DDT), 1% (w/v) polyvinylpyrrolidone and 0.3% Triton X-100. The extract was centrifuged at 13 000×g for 5 min at 4℃, and the supernatant was used immediately for enzyme assays.
Rubisco activity was measured in a mixture of 0.5 mmol/L ribulose-1, 5-bisphosphate (RuBP), 100 mmol/L Bicine (pH 8.0 at 25℃), 25 mmol/L KHCO3, 20 mmol/L MgCl2, 3.5 mmol/L ATP, 5 mmol/L phosphocreatine, 5 units glyceraldehydes- 3-phosphate dehydrogenase, 5 units 3-phosphoglyceric phosphokinase, 17.5 units creatine phosphokinase and 0.25 mmol/L NADH. NADH oxidation was monitored at 340 nm.
NADP-GAPDH activity was determined in a mixture of 100 mmol/L Tricine-KOH (pH 8.0), 4 mmol/L 3-phosphoglycerate, 5 mmol/L ATP, 10 mmol/L MgCl2, 0.2 mmol/L NADPH and 20 units 3-phosphoglyceric phosphokinase. The reaction was initiated by adding enzyme extract.
FBPase was assayed in a mixture of 50 mmol/L Tris-HCl (pH 8.2), 10 mmol/L MgCl2, 1 mmol/L EDTA, 0.1 mmol/L fructose 1, 6-bisphosphate, 0.5 mmol/L NADP+, 4 units of phosphoglucoisomerase and 2 units of glucose-6-phosphate dehydrogenase. The reaction was initiated by adding enzyme extract.
SOD activity was assayed by monitoring the inhibition of the photochemical reduction of nitro blue tetrazolium (NBT). One milliliter reaction mixture contained 50 mmol/L potassium phosphate buffer (pH 7.8), 6.5 mmol/L methionine, 50 μmol/L NBT, 20 μmol/L riboflavin, 10 μmol/L EDTA-Na2 and 20 μL enzyme extract. The reaction mixture without enzyme was as control. All the reaction mixtures in small test tubes were mixed well in the dark, and then were irradiated for 3 min at 500 μmol photons/(m2·s) PFD. After the color of reaction mixture became blue black from yellow, absorbance at 560 nm of the reaction mixture was monitored.
APX activity was determined by monitoring the decrease in absorbance at 290 nm. Reaction mixture contained 50 mmol/L Hepes-KOH (pH 7.6), 0.1 mmol/L EDTA-Na2, 0.5 mmol/L ascorbate, 0.2 mmol/L H2O2 and enzyme extract. The reaction was initiated by adding H2O2.
CAT activity was determined by monitoring the decrease in absorbance at 240 nm. Reaction mixture contained 50 mmol/L potassium phosphate buffer (pH 7.0), 10 mmol/L H2O2 and enzyme extract. The reaction was initiated by adding H2O2.
2.8 Determination of metabolitesGlucose-6-phosphate (G6P), fructose-6-phosphate (F6P) and 3-phosphoglycerate (PGA) were extracted and analyzed according to Chen and Cheng (2003). About 106 cells were ground in 5% HClO4, and the homogenate was centrifuged at 16 000×g for 10 min. An aliquot of supernatant was neutralized with neutralizing buffer (1 mol/L triethanolamine containing 5 mol/L KOH) to pH 5-6. A pinch of activated charcoal was added and the mixture was kept in ice for 30 min and then centrifuged at 16 000×g for 10 min. The supernatant was used for the measurement of G6P, F6P, and PGA.
G6P and F6P were determined spectrophotometrically in reaction mixture containing sugar extraction, 100 mmol/L Hepes-KOH (pH 7.6), 4 mmol/L MgCl2, 0.2 mmol/L NAD+, 1 unit glucose- 6-phosphate dehydrogenase for G6P, then 1 unit glucosephosphate isomerase for F6P.
PGA was measured in reaction mixture containing sugar extraction, 40 mmol/L Hepes-KOH (pH 7.5), 0.2 mmol/L NADH, 5 mmol/L ATP, 5 mmol/L phosphocreatine, 10 units creatine phosphokinase, 5 units NADP-glyceraldehyde-3-phosphate dehydrogenase, 5 units 3-phosphoglyceric phosphokinase (PCK). PGA concentration was determined by the difference in absorbance at 340 nm before and after the addition of PCK.
2.9 Statistical analysisSPSS 17.0 was used for statistical analyses. The variation of each parameter was tested by the oneway analysis of variance (ANOVA) procedure. Least significant difference (LSD) was used to analyze differences between the measurements. Differences were considered significant at a probability level of P < 0.05.
3 RESULT AND DISCUSSION 3.1 Growth of C. pyrenoidosa exposed to various temperaturesThe cell numbers of C. pyrenoidosa enhanced gradually over time at all temperatures (30, 35, 38 and 41℃) during the first six days, and then remained stable (Fig. 2a). No significant difference was found among the cell numbers at 30, 35 and 38℃ during a seven-day incubation period. However, the C. pyrenoidosa cell numbers at 41℃ were lower than those at other three temperatures after four days of incubation (Fig. 2a). Compared with at 30℃, the specific growth rate did not change at 35 and 38℃ but decreased at 41℃ (Fig. 2b). These results suggest that this C. pyrenoidosa strain is resistant to high temperature, because it grew well at 38℃. Meanwhile, 41℃ is still suitable for growth of this strain, because 41℃ treatment just decreased instead of completely inhibited the enhancement of cell numbers during the incubation.
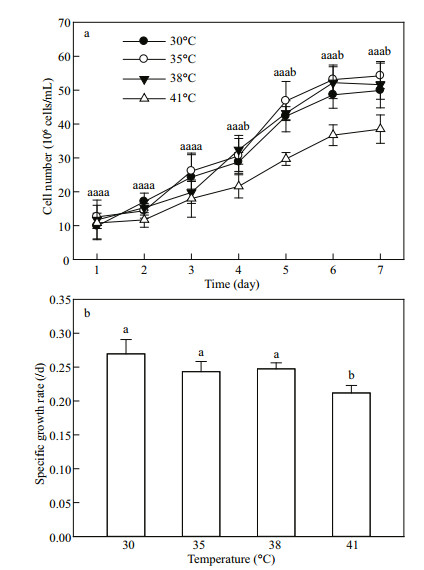
|
| Fig.2 Cell numbers (a) and specific growth rate (b) of C. pyrenoidosa exposed to various temperatures The data represent the mean±SD of five independent measurements. Different letters indicate significant difference between the treatments with various temperatures at P < 0.05, the same as this in other figures. |
No significant difference was found among the total chlorophyll contents at 30, 35, 38, and 41℃ (Fig. 3), indicating that high temperature treatment did not change the total chlorophyll content of C. pyrenoidosa.

|
| Fig.3 Effect of high temperature (35, 38 or 41℃) on total chlorophyll content in C. pyrenoidosa cells previously grown at 30℃ Exponentially growing cells previously grown at 30℃ were placed into a water bath and incubated for 110 min at 30, 35, 38, or 41℃ in the dark, and then exposed to 500 μmol photons/(m2·s) at different temperatures for 10 min. The data represent the mean±SD of five independent measurements. The letter 'a' in all histograms means that there is no significant difference among the four temperatures. |
The chlorophyll a fluorescence transient (OJIP transient) is one of the most useful tools to study photosynthetic behaviors (Strasser et al., 2000, 2004; Yusuf et al., 2010; Zhang et al., 2011; 2017). It is possible to detect changes in photosynthetic behaviors, including the light absorption, energy trapping, and electron transport. The shape of OJIP transient is very sensitive to environmental stresses, especially to high temperature (Chen et al., 2008; Li et al., 2009; Zhang and Liu, 2016). In this study, C. pyrenoidosa cells showed a typical OJIP transient at 30 and 35℃, and no significant difference was found between them (P > 0.05). However, 38 and 41℃ treatments changed the shape of OJIP transients (Fig. 4a), suggesting that high temperature significantly influenced photosynthetic behaviors.
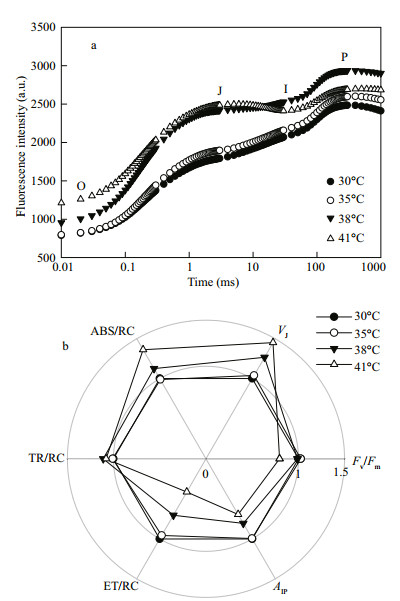
|
| Fig.4 Effects of high temperature (35, 38 or 41℃) on chlorophyll a fluorescence (OJIP) transient (a), as well as photosynthetic parameters derived from the analysis of OJIP transients (b) in C. pyrenoidosa cells previously grown at 30℃ Each transient represents the average of five independent measurements. The control value (30℃) was taken as 1, whereas the others in treatments were taken as the percentage of the control. |
In order to unravel the detailed influence of high temperature on photosynthetic behaviors, photosynthetic parameters of C. pyrenoidosa at different temperatures were calculated by analysis of the OJIP transients (Fig. 4b). Compared with at 30℃, the maximal photochemical efficiency of PSII (Fv/Fm) did not change at 35 and 38℃ but decreased at 41℃. The relative variable fluorescence kinetics at the J-step (VJ) increased with increased temperatures at temperatures higher than 35℃ in C. pyrenoidosa (Fig. 4b), indicating that high temperature (38 or 41℃) increased the ratio of QA-/QA (Strasser et al., 2000, 2004; Zhang et al., 2017), leading to an overreduction of the photosystem (PS) II acceptor side. The maximal amplitude of fluorescence in the I-P phase of the OJIP transient (AIP) reflects the pool size of end electron acceptors (Yusuf et al., 2010; Zhang and Liu, 2016; Zhang et al., 2017). The AIP did not change at 35℃ but decreased at 38 and 41℃ compared with at 30℃ (Fig. 4b), which suggests that high temperature decreased the pool size of photosynthetic end electron acceptors, resulting in an over-reduction of the PSI acceptor side (Kalachanis and Manetas, 2010; Yusuf et al., 2010).
No significant difference was found among the trapping of excitation energy per active reaction center (TR/RC) at 30, 35, 38 and 41℃ in C. pyrenoidosa (Fig. 4b). The light absorption per active reaction center (ABS/RC) increased; however, the electron transport per active reaction center (ET/ RC) decreased with temperatures increase at temperatures higher than 35℃. These results suggest that the balance between light absorption and electron transport was disturbed at temperatures higher than 35℃ in C. pyrenoidosa. Imbalance between light absorption and electron transport will lead to the over-excitation of PSII reaction centers (Vass, 2011; Zhang et al., 2011, 2017).
Chlorella pyrenoidosa cells grown in outdoors are often subject to high light, especially at noon (Fig. 1). Under high light, the acceptor side of PSI and the PSII reaction centers are major sites of active oxygen species (AOS) production in chloroplasts (Niyogi, 1999, 2000; Vass, 2011; Zhang et al., 2017). Compared with at 30 and 35℃, the over-reduction of the PSI acceptor side and the over-excitation of PSII reaction centers at 38 and 41℃ in C. pyrenoidosa (Fig. 4b) would inevitably enhance the production of AOS, resulting in photodamage of photosynthetic apparatus and inhibition of cell growth. Therefore, why did not the 38℃ treatment inhibit the growth of C. pyrenoidosa compared that of 30℃ (Fig. 2)?
3.3 The photochemical quenching and Calvin cycle at high temperatureFigure 5 shows the effects of high temperature (35, 38, or 41℃) on photochemical quenching parameters, Calvin cycle (carbon assimilation) enzyme activities and Calvin cycle metabolite levels in C. pyrenoidosa. Compared with at 30℃, the efficiency of excitation capture by PSII (Fv′/Fm′), photochemical quenching coefficient (qP), actual photochemical efficiency of PSII (ΦPSII) and photosynthetic linear electron transport rate (ETR) in C. pyrenoidosa were not changed at high temperature (35, 38, or 41℃) (Fig. 5a). The activities of ribulose-1, 5-bisphosphate carboxylase/oxygenase (Rubisco), NADPglyceraldehyde-3-phosphate dehydrogenase (NADPGAPDH) and fructose-1, 6-bisphosphatase (FBPase) were not significantly different at 30, 35, 38, and 41℃ (Fig. 5b). Meanwhile, 3-phosphoglycerate (PGA), fructose-6-phosphate (F6P) and glucose-6-phosphate (G6P) contents were not changed at high temperature (35, 38 or 41℃), compared with at 30℃ (Fig. 5c).
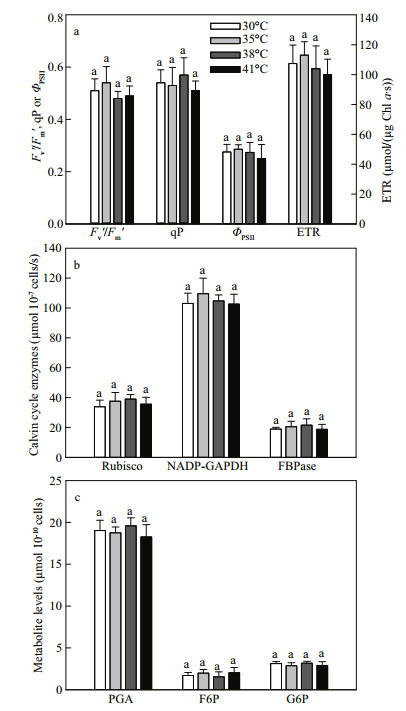
|
| Fig.5 Effects of high temperature (35, 38 or 41℃) on photochemical quenching parameters (a), Calvin cycle enzyme activities (b) and Calvin cycle metabolite levels (c) in C. pyrenoidosa cells previously grown at 30℃ The data represent the mean ± SD of five independent measurements. |
The electron transport and Calvin cycle are arranged in series in the photosynthesis, and the two processes activities should be coordinated in order to allow efficient photosynthesis (Chen et al., 2004; Allen et al., 2011). The inhibition of Calvin cycle would cause feedback suppression of photochemical quenching. In this study, the photosynthetic behaviors were changed (Fig. 4), but the photochemical quenching, activities of Calvin cycle enzymes (Rubisco, NADP-GAPDH and FBPase), contents of Calvin cycle metabolites (PGA, F6P and G6P) did not change (Fig. 5) at 38 and 41℃ compared with at 30℃. We concluded that carbon assimilation, not electron transport, is the main rate-limiting step in photosynthesis of C. pyrenoidosa at high temperature, and the carbon assimilation did not change at high temperature (38 or 41℃). The growth of microalgae mainly depends on photosynthetic carbon assimilation production (Torzillo et al., 1991). Therefore, why did the treatment at 41℃ decrease the growth of C. pyrenoidosa compared with other temperatures (Fig. 2)?
3.4 The photoprotective mechanisms at high temperatureThe over-reduction of PSI acceptor side and overexcitation of PSII reaction centers at high temperature can lead to generation of excess light energy in the light, which will accelerate the generation of AOS (Horton et al., 1996; Zhang et al., 2011). In order to escape from exposure to excess light, most plants have evolved various photoprotective mechanisms, such as non-photochemical quenching (NPQ) (Niyogi, 2000) and antioxidant enzymes (Mittler, 2002).
NPQ decreased with the increase of temperature at temperatures higher than 35℃ (Fig. 6), which suggests that non-photochemical quenching was not induced at high temperature to protect C. pyrenoidosa cells. Figure 7 shows the effects of high temperature (35, 38, or 41℃) on antioxidant enzyme activities in C. pyrenoidosa. The 35℃ treatment did not change activities of superoxide dismutase (SOD), ascorbate peroxidase (APX) and catalase (CAT). However, the SOD, APX, and CAT activities increased significantly at 38 and 41℃, and no significant difference was found between antioxidant enzyme activities at the two high temperatures. It suggests that antioxidant enzymes were activated at high temperature to protect C. pyrenoidosa cells from the generation of AOS.

|
| Fig.6 Effect of high temperature (35, 38 or 41℃) on nonphotochemical quenching (NPQ) in C. pyrenoidosa cells previously grown at 30℃ The data represent the mean±SD of five independent measurements. |
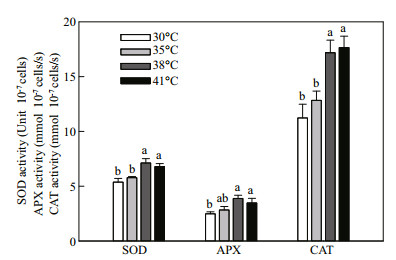
|
| Fig.7 Effects of high temperature (35, 38 or 41℃) on the activities of superoxide dismutase (SOD), ascorbate peroxidase (APX) and catalase (CAT) in C. pyrenoidosa cells previously grown at 30℃ The data represent the mean±SD of five independent measurements |
The possible phenomena in C. pyrenoidosa cells exposed to high temperature (38 or 41℃) are shown in Fig. 8. In contrast to 30℃, at 38℃, the PSI acceptor side was over-reduced and PSII reaction centers were over-excited after exposure to high light, leading to generation of AOS. However, under this condition, the activated antioxidant enzymes could remove completely the over-production of AOS; thereby protect C. pyrenoidosa cells from photodamage. Meanwhile, the photochemical quenching and carbon assimilation was not inhibited at 38℃. These are why 38℃ treatment did not inhibit the growth of C. pyrenoidosa compared with 30℃. Compared with 38℃, 41℃ treatment caused higher reduction of PSI acceptor side and higher excitation of PSII reaction centers, which would generate much more AOS under high light. However, the antioxidant enzymes did not increase at 41℃, in contrast to 38℃. Under this condition, the capacities of antioxidant enzymes were not enough to remove excess AOS, which would cause more severe photodamage when C. pyrenoidosa cells exposed to high light for a long time, although 41℃ also did not inhibit directly the photochemical quenching and carbon assimilation. Meanwhile, compared with that at 41℃, the higher NPQ at 38℃ could also efficiently remove excess light energy and protect C. pyrenoidosa cells from photodamage. These are why 41℃ treatment decreased the growth of C. pyrenoidosa compared with 38℃.
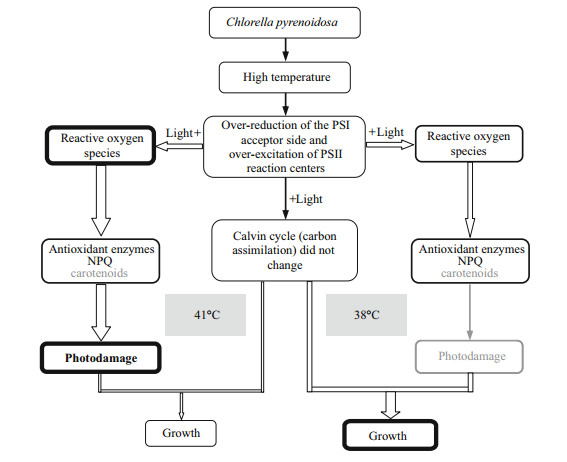
|
| Fig.8 Schemes of the possible phenomena in C. pyrenoidosa cells exposed to high temperature (38 or 41℃) |
Furthermore, carotenoids can also act as antioxidants by scavenging excess AOS induced by high light intensity and other stressors (e.g. high temperature). Meanwhile, the de novo biosynthesis of carotenoids in microalgal cells begins with the Calvin cycle (Huang et al., 2017). More studies are needed to explore the role of carotenoids at high temperature in C. pyrenoidosa.
4 CONCLUSIONThe growth of C. pyrenoidosa was not negatively affected at 35℃ or 38℃, but was inhibited significantly at 41℃ compared with 30℃. The balance between photosynthetic light absorption and energy utilization could be still maintained at 35℃. In contrast to 30℃, high temperature at 38℃ disturbed the balance between light absorption and energy utilization, leading to generation of active oxygen species (AOS). However, the activated antioxidant enzymes might remove completely the over-production of AOS; thereby protect C. pyrenoidosa cells from photodamage. 41℃ treatment caused more severe imbalance between light absorption and energy utilization than 38℃. However, the activities of antioxidant enzymes did not further increase at 41℃, which would cause severe photodamage under high light. It shows that this C. pyrenoidosa strain exhibits strong tolerance to high temperature as high as 38℃.
5 DATA AVAILABILITY STATEMENTFull information developed from this study is available from the corresponding author upon reasonable request.
Allen J F, De Paula W B M, Puthiyaveetil S, Nield J A. 2011. A structural phylogenetic map for chloroplast photosynthesis. Trends in Plant Science, 16(12): 645-655.
DOI:10.1016/j.tplants.2011.10.004 |
Berry J, Björkman O. 1980. Photosynthetic response and adaptation to temperature in higher plants. Annual Review of Plant Physiology, 31: 491-543.
DOI:10.1146/annurev.pp.31.060180.002423 |
Chen H X, Li W J, An S Z, Gao H Y. 2004. Characterization of PSII photochemistry and thermostability in salt-treated Rumex leaves. Journal of Plant Physiology, 161(3): 257-264.
|
Chen L S, Cheng L L. 2003. Carbon assimilation and carbohydrate metabolism of 'Concord' grape (Vitis labrusca L.) leaves in response to nitrogen supply. Journal of the American Society for Horticultural Science, 128(5): 754-760.
DOI:10.21273/JASHS.128.5.0754 |
Chen L S, Li P M, Cheng L L. 2008. Effects of high temperature coupled with high light on the balance between photooxidation and photoprotection in the sun-exposed peel of apple. Planta, 228(5): 745-756.
DOI:10.1007/s00425-008-0776-3 |
Converti A, Casazza A A, Ortiz E Y, Perego P, Del Borghi M. 2009. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chemical Engineering and Processing:Process Intensification, 48(6): 1 146-1 151.
DOI:10.1016/j.cep.2009.03.006 |
Du H M, Ahmed F, Lin B, Li Z, Huang Y H, Sun G, Ding H, Wang C, Meng C X, Gao Z Q. 2017. The effects of plant growth regulators on cell growth, protein, carotenoid, PUFAs and lipid production of Chlorella pyrenoidosa ZF strain. Energies, 10(11): 1 696.
DOI:10.3390/en10111696 |
Havaux M. 1993. Characterization of thermal damage to the photosynthetic electron transport system in potato leaves. Plant Science, 94(1-2): 19-33.
DOI:10.1016/0168-9452(93)90003-I |
Horton P, Ruban A V, Walters R G. 1996. Regulation of light harvesting in green plants. Annual Review of Plant Physiology and Plant Molecular Biology, 47: 655-684.
DOI:10.1146/annurev.arplant.47.1.655 |
Hsieh C H, Wu W T. 2009. Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresource Technology, 100(17): 3 921-3 926.
DOI:10.1016/j.biortech.2009.03.019 |
Huang J J, Lin S L, Xu W W, Cheung P C K. 2017. Occurrence and biosynthesis of carotenoids in phytoplankton. Biotechnology Advances, 35(5): 597-618.
DOI:10.1016/j.biotechadv.2017.05.001 |
Kalachanis D, Manetas Y. 2010. Analysis of fast chlorophyll fluorescence rise (O-K-J-I-P) curves in green fruits indicates electron flow limitations at the donor side of PSII and the acceptor sides of both photosystems. Physiologia Plantarum, 139(3): 313-332.
|
Li P M, Cheng L L, Gao H Y, Jiang C D, Peng T. 2009. Heterogeneous behavior of PSII in soybean (Glycine max) leaves with identical PSII photochemistry efficiency under different high temperature treatments. Journal of Plant Physiology, 166(15): 1 607-1 615.
DOI:10.1016/j.jplph.2009.04.013 |
Mittler R. 2002. Oxidative stress, antioxidants and stress tolerance. Trends in Plant Science, 7(9): 405-410.
DOI:10.1016/S1360-1385(02)02312-9 |
Niyogi K K. 1999. Photoprotection revisited: Genetic and molecular approaches. Annual Review of Plant Physiology and Plant Molecular Biology, 50: 333-359.
|
Niyogi K K. 2000. Safety valves for photosynthesis. Current Opinion in Plant Biology, 3(6): 455-460.
DOI:10.1016/S1369-5266(00)00113-8 |
Porra R J. 2002. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynthesis Research, 73(1-3): 149-156.
|
Pulz O, Gross W. 2004. Valuable products from biotechnology of microalgae. Applied Microbiology and Biotechnology, 65(6): 635-648.
DOI:10.1007/s00253-004-1647-x |
Rippka R, Deruelles J, Waterbury J B, Herdman M, Stanier R Y. 1979. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiolog, 111: 1-61.
|
Sajilata M G, Singhal R S, Kamat M Y. 2008. Fractionation of lipids and purification of γ-linolenic acid (GLA) from Spirulinaplatensis. Food Chemistry, 109(3): 580-586.
|
Serra-Maia R, Bernard O, Gonçalves A, Bensalem S, Lopes F. 2016. Influence of temperature on Chlorella vulgaris growth and mortality rates in a photobioreactor. Algal Research, 18: 352-359.
DOI:10.1016/j.algal.2016.06.016 |
Strasser R J, Srivastava A, Tsimilli-Michael M. 2000. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P eds. Probing Photosynthesis: Mechanism, Regulation and Adaptation. Taylor & Francis, London. p.445-483.
|
Strasser R J, Tsimilli-Michael M, Srivastava A. 2004. Analysis of the chlorophyll a fluorescence transient. In: Papageorgiou G C ed. Chlorophyll a fluorescence: a signature of photosynthesis. Kluwer Academic Publishers Press, Dordrecht. p.321-362.
|
Torzillo G, Sacchi A, Materassi R, Richmond A. 1991. Effect of temperature on yield and night biomass loss in Spirulina platensis grown outdoors in tubular photobioreactors. Journal of Applied Phycology, 3(2): 103-109.
DOI:10.1007/BF00003691 |
Vass I. 2011. Role of charge recombination processes in photodamage and photoprotection of the photosystem Ⅱ complex. Physiologia Plantarum, 142(1): 6-16.
DOI:10.1111/j.1399-3054.2011.01454.x |
Wen X G, Gong H M, Lu C M. 2005. Heat stress induces a reversible inhibition of electron transport at the acceptor side of photosystem Ⅱ in a cyanobacterium Spirulina platensis. Plant Science, 168(6): 1 471-1 476.
DOI:10.1016/j.plantsci.2005.01.015 |
Xie X J, Huang A Y, Gu W H, Zang Z R, Pan G H, Gao S, He L W, Zhang B Y, Niu J F, Lin A P, Wang G C. 2016. Photorespiration participates in the assimilation of acetate in Chlorella sorokiniana under high light. New Phytologist, 209(3): 987-998.
DOI:10.1111/nph.13659 |
Xu R, Zhang L T, Liu J G. 2019. The natural triterpenoid toosendanin as a potential control agent of the ciliate Stylonychia mytilus in microalgal cultures. Journal of Applied Phycology, 31(1): 41-48.
DOI:10.1007/s10811-018-1522-2 |
Yang Z, Kong F X. 2011. Enhanced growth and esterase activity of Chlorella pyrenoidosa (Chlorophyta) in response to short-term direct grazing and grazingassociated infochemicals from Daphnia carinata. Journal of Freshwater Ecology, 26(4): 553-561.
|
Yusuf M A, Kumar D, Rajwanshi R, Strasser R J, Tsimilli-Michael M, Govindjee, Sarin N B. 2010. Overexpression of γ-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress:physiological and chlorophyll a fluorescence measurements. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1797(8): 1 428-1 438.
DOI:10.1016/j.bbabio.2010.02.002 |
Zhang L T, Li L, Liu J G. 2014. Comparison of the photosynthetic characteristics of two Isochrysis galbana strains under high light. Botanica Marina, 57(6): 477-481.
|
Zhang L T, Liu J G. 2016. Effects of heat stress on photosynthetic electron transport in a marine cyanobacterium Arthrospira sp. Journal of Applied Phycology, 28(2): 757-763.
DOI:10.1007/s10811-015-0615-4 |
Zhang L T, Su F, Zhang C H, Gong F Y, Liu J G. 2017. Changes of photosynthetic behaviors and photoprotection during cell transformation and astaxanthin accumulation in Haematococcus pluvialis grown outdoors in tubular photobioreactors. International Journal of Molecular Sciences, 18(1): E33.
|
Zhang L T, Zhang Z S, Gao H Y, Xue Z C, Yang C, Meng X L, Meng Q W. 2011. Mitochondrial alternative oxidase pathway protects plants against photoinhibition by alleviating inhibition of the repair of photodamaged PSII through preventing formation of reactive oxygen species in Rumex K-1 leaves. Physiologia Plantarum, 143(4): 396-407.
DOI:10.1111/j.1399-3054.2011.01514.x |
Zhao B B, Wang J, Gong H M, Wen X G, Ren H Y, Lu C M. 2008. Effects of heat stress on PSII photochemistry in a cyanobacterium Spirulina platensis. Plant Science, 175(4): 556-564.
DOI:10.1016/j.plantsci.2008.06.003 |
Zhao F C, Tan X B, Zhang Y L, Chu H Q, Yang L B, Zhou X F. 2015. Effect of temperature on the conversion ratio of glucose to Chlorella pyrenoidosa cells:reducing the cost of cultivation. Algal Research, 12: 431-435.
DOI:10.1016/j.algal.2015.10.009 |
 2020, Vol. 38
2020, Vol. 38


