Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHUANG Longchuan, ZHANG Chi, LIU Qun, YE Zhenjiang
- Age, growth, reproductive biology, color pattern, and ontogenetic divergence of two closely related rockfishes (Sebastes koreanus and S. nudus) off the coasts of the Shandong Peninsula, China
- Journal of Oceanology and Limnology, 38(1): 204-225
- http://dx.doi.org/10.1007/s00343-019-8303-x
Article History
- Received Oct. 17, 2018
- accepted in principle Jan. 8, 2019
- accepted for publication May. 10, 2019
The live-bearing genus Sebastes (rockfish) is one of the most speciose genera of marine fishes with approximately 110 species known worldwide, the majority of which are distributed in temperate to Arctic waters throughout the North Pacific (Hyde and Vetter, 2007; Nelson et al., 2016). Most of the rockfishes are important components of both commercial and recreational fisheries within their range (Love et al., 2002). Although the large assemblage of Sebastes has been proposed to be the modern result of an ancient explosive speciation event (Johns and Avise, 1998), speciation mechanisms may still be active in Sebastes (Alesandrini and Bernardi, 1999). Many closely related species within the genus show only low levels of genetic divergence, and several examples of natural hybridization among them have been reported, suggestive of incipient speciation or recent speciation events (Roqueset et al., 2001; Narum et al., 2004; Hyde and Vetter, 2007; Kai and Nakabo, 2008; Artamonova et al., 2013).
The scarcity of geographical barriers to gene flow in marine ecosystems poses a challenge to the traditional view of speciation, with its emphasis on geographical isolation (Palumbi, 1994). Fully allopatric speciation can occur across barriers such as the Isthmus of Panama (Lessios and Cunningham, 1990), but these features are not common enough to explain all marine speciation events (Puebla, 2009; Ingram, 2010). Despite the recent examples of selfrecruitment (e.g., Johansson et al., 2008), the prevalence of pelagic larval phases in marine taxa provides ample potential for gene flow covering long oceanic distances on a time scale of a few thousands of years (Lessios et al., 1998; Bowen et al., 2001; Mora and Sale, 2002; Craig et al., 2006; Lessios and Robertson, 2006; Shanks, 2009). Ecological speciation (reproductive isolation resulting from divergent natural selection) seems to be widespread in marine taxa (Rocha et al., 2005; Puebla, 2009; Ingram, 2010) due to its potential to operate within any taxonomic group, under any geographic mode and involve diverse types of interactions between organisms and their environment (Orr and Smith, 1998; Doebeli and Dieckmann, 2000; Schluter, 2001; Rundle and Nosil, 2005). Many closely related Sebastes occur in broad sympatry (Orr et al., 2000; Love et al., 2002; Jin, 2006). Therefore, the considerable diversity in the absence of geographical barriers has sparked interest in the factors promoting speciation in Sebastes (Alesandrini and Bernardi, 1999; Hyde and Vetter, 2007; Hyde et al., 2008; Ingram, 2010; Tuset et al., 2016).
As speciation in marine taxa remains poorly understood (Palumbi, 1994), it is difficult to pinpoint the mechanisms of speciation in Sebastes. However, the extensive literature on ecological speciation in the lake-dwelling cichlid fishes (e.g., Endler, 1983; Allender et al., 2003; Seehausen and Schluter, 2004) provide valuable insights for investigating the speciation mechanisms of Sebastes, as both Sebastes and cichlids are renowned for their outstanding species richness and color diversity, as well as numerous co-occurring species (Alesandrini and Bernardi, 1999; Maan and Sefc, 2013). As in several cichlid assemblages, depth segregation and coloration differences suggested that ecological and sexual selection may play important roles in this system (Endler, 1983; Alesandrini and Bernardi, 1999; Allender et al., 2003; Kocher, 2004; Seehausen and Schluter, 2004). Several studies have suggested that closely related species and color morphotypes of rockfishes undergoing speciation differ primarily in color pattern, which is in turn usually associated with depth distribution (e.g., Hyde et al., 2008; Artamonova et al., 2013). Previous evolutionary studies have paid considerable attention to the role of bathymetric segregation in the speciation of rockfishes (Hyde et al., 2008; Ingram, 2010; Artamonova et al., 2013). Unexpectedly, despite the increasing reports of clear coloration differences between closely related Sebastes (e.g., Hyde et al., 2008; Artamonova et al., 2013), there is hardly any empirical research that has investigated how coloration acts on the speciation and adaptive evolution of Sebastes or how coloration and other potential speciation factors, e.g., bathymetric segregation, can interact.
Numerous studies in some speciose families of teleosts, e.g., Cichlidae and Poeciliidae, have suggested that body color can play important roles in social communication, competition, mate choice, predation and foraging and are therefore subject to potentially strong selective pressures (Endler, 1983; Godin and Dugatkin, 1996; Couldridge and Alexander, 2002; Godin and McDonough, 2003; Maan and Sefc, 2013). Moreover, there is growing evidence that strongly divergent selections generated through predator-prey interactions (predation and foraging) could lead to rapid adaptive divergence in body shape, especially the fitness traits closely related to survival and body condition such as foraging morphology, predator defense morphology, etc. (Langerhans et al., 2004; Andersson et al., 2006; Eklöv and Svanbäck, 2006; Domenici et al., 2007; Langerhans, 2009). Therefore, though many closely related species and color morphotypes within Sebastes show only low levels of genetic divergence (Roques et al, 2001; Narum et al., 2004; Hyde and Vetter, 2007; Hyde et al., 2008; Kai and Nakabo, 2008; Artamonova et al., 2013), the color-driven divergent selection generated through predator-prey interactions (Langerhans et al., 2004; Andersson et al., 2006; Eklöv and Svanbäck, 2006; Domenici et al., 2007; Langerhans, 2009) may also lead to rapid adaptive divergence in body shape of rockfishes and, consequently, accelerate their speciation and adaptive radiation. However, the strength of the color-driven divergent selection may vary, because in many teleosts, color patterns vary not only between species but also within a species (e.g., sexual dichromatism and polychromatism), as well as within individuals (e.g., nuptial coloration), depending on their age and sexual maturity (Macedonia et al., 2002; Leclercq et al., 2010; Maan and Sefc, 2013). Thus, in terms of understanding the potential mechanism of color-driven speciation in Sebastes, it is important to ascertain and analyze some aspects of the biology of closely related species or color morphotypes of Sebastes such as age, growth, maturity, coloration and its variation, as well as some morphological traits likely related to survival and body condition.
In this study, we selected a pair of very closely related rockfishes, Sebastes koreanus and S. nudus. Though not perfectly sympatric, the distributions of Sebastes koreanus and S. nudus are overlapping in the coastal areas of Shandong Peninsula and the southern part of the Korean Peninsula, according to previous records (Kai and Nakabo, 2013; Yu and Kim, 2014; Fang et al., 2015; Yu et al., 2015). After the pelagic larval and early juvenile stages, they dwell on demersal rocky reefs and display a migratory movement towards deeper waters during ontogeny, known as the "bigger-deeper" distribution pattern (e.g., Polloni et al., 1979), which is a common theme among rockfishes (Love et al., 1991; Jin, 2006). Sebastes koreanus and S. nudus are morphologically and meristically similar (Kai and Nakabo, 2013; Fang et al., 2015). Their color patterns, however, are distinct (Kai and Nakabo, 2013; Yu and Kim, 2014; Fang et al., 2015). Sebastes nudus appears to show a bolder color pattern (dark base color vs. conspicuous yellow markings) than Sebastes koreanus (light brown base color vs. dark brown bands or dots). Sebastes nudus shows much variation in its coloration among individuals (even within a population), mainly included in the size, number, and distribution of the conspicuous yellow markings (carotenoid pigments) on the body, head, and fins (Kai and Nakabo, 2013; Yu and Kim, 2014; present study). However, Sebastes koreanus shows little to no color variation among populations (Fang et al., 2015; Yu et al., 2015; present study) and even from juvenile to mature stages (Yu et al., 2015). The body color characteristics of Sebastes koreanus and S. nudus make them an ideal model to study the action of color-driven divergent selection on morphological traits in the rockfishes living in same environment and sharing a similar gene pool. Moreover, few other studies have attempted to analyze and compare the growth of these two Sebastes species, in part because of their problematic species identification. Recently, researchers have reviewed and re-described the two species and clarified their species identification (Kai and Nakabo, 2013; Fang et al., 2015). These efforts have provided an important basis for the reliable studies on the age and growth of these species.
Using specimens of S. koreanus and S. nudus collected off the coasts of Shandong Peninsula, China, the objectives of the present study were to: (ⅰ) ascertain their age and growth from otoliths; (ⅱ) investigate their reproductive seasonality; (ⅲ) investigate the ontogenetic, seasonal and sexual variations in the body color pattern of S. nudus; and (ⅳ) determine when divergence in some phenotypic traits occurs between the two closely related species. By understanding these details, we further hoped to infer a possible mechanism of speciation by natural selection in rockfishes.
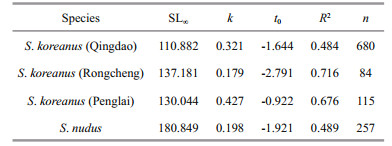
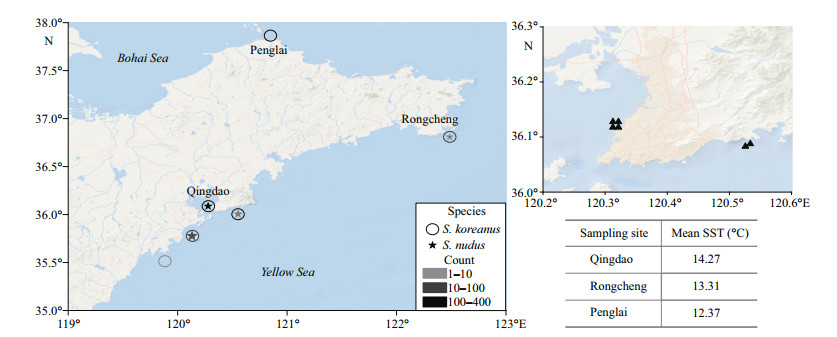
2 MATERIAL AND METHOD 2.1 Collection and preparationA total of 879 Sebastes koreanus and 257 S. nudus specimens were purchased live or, occasionally, freshly dead directly from local fishers from 2013 to 2016 (Table 1). The purchased fishes were fished by fish traps or occasionally, angling off the coasts of Shandong Peninsula, China (Fig. 1). Specimens for validating otolith annuli and reproductive seasonality analysis were sampled monthly from 2013 November to 2014 November in Qingdao, southern coast of Shandong Peninsula. To generally reflect the annual migration trends of the two species, we purchased the 24-hour catches in fish traps in the very nearshore of sample locations-1 and -2 in Qingdao (Fig. 1) (the water depth at the sites varies between 4 and 7 m, which is close to the shallower limit of distribution of the two species) for ≤3 times per month (Table 2). Specimens were also sampled seasonally off the coast of Rongcheng (eastern Shandong Peninsula) and Penglai (northern Shandong Peninsula) from 2015 to 2016 (Table 1). Species identification was carried out by morphological features as described by Kim et al. (2005) and Fang et al. (2015) for S. koreanus and Kai and Nakabo (2013) for S. nudus.

|
|
| Fig.1 The sampling sites for S. koreanus (enclosed star) and S. nudus (open circle) off the coasts of Shandong Peninsula, China Close triangles indicate the fixed fish traps in the very nearshore of sampling sites-1 and -2 in Qingdao. The mean sea surface temperatures (SSTs) from Jan 2013 to Dec 2016 off the coast of Qingdao, Rongcheng and Penglai (daily SST data were available at ftp://data.remss.com/SST/daily_v04.0/mw_ir/ and read by Python version 3.4) are given. |
|
For fish color analysis, to reduce, or possibly, avoid the possible body color variations caused by changes in feeding and illumination conditions (e.g., Oshima and Yokozeki, 1999; Kasai and Oshima, 2006; Alishahi et al., 2015) after the wild fish have been landed, we photographed the just caught S. nudus individuals as soon as possible outdoors in a shaded area. In view of the possible fading of body color following death, only live individuals (n=188 or 73.2% of S. nudus sample size) were selected to be photographed. Digital photographs were taken following methods suggested by Stevens et al. (2007), namely, the use of a high-resolution camera, the inclusion of a color standard in each image, the avoidance of file types that compress images, and manual control of exposure times and white balancing. We photographed the right and the left-side views of each individual alongside an X-Rite mini color checker card (X-Rite Inc.) in RAW format using a Nikon D90 digital camera (Nikon Inc.). The digital camera was fitted with a Nikon 24–85 mm zoom lens (Nikon Inc.) and attached to a tripod (Manfrotto Co.). Thin pins and bamboo skewers were used for fin spreading following the method suggested by Motomura and Ishikawa (2013). To reduce the reflected light spots from the body surface, we placed a semi-translucent white cloth around the specimen to soften the sunlight and wiped the body surface lightly while photographing (Motomura and Ishikawa, 2013).
All specimens of S. koreanus and S. nudus were measured, weighed, and sexed in the laboratory immediately upon collection. For each individual collected, total length (TL) and standard fish length (SL) were measured to the nearest mm, while total body weight (TW), eviscerated weight (EW), gonadal weight (GW) and gastrointestinal weight (GAW) were measured to the nearest 0.01 g. Here, the GAW included the weight of the gut itself as well as the weight of the gut contents. Caudal fin length (CFL) was taken as the TL minus the SL. Pectoral fin length (PFL), longest dorsal spine length (DSL) and mouth breadth (MB) were measured to the nearest mm in 111 S. koreanus and 156 S. nudus. Traits were selected based on their likely relationship to foraging and/or swimming ability (CFL, PFL, MB), as well as predator defense (DSL) (e.g., Malmquist, 1992; Ruzzante et al., 2003, 2011). The sex of each individual was determined by visual observation of the gonads. In small specimens, gonads were observed by a light microscope to aid sex determination. Only adults were selected to determine reproductive and feeding seasonality. Reproductive seasonality was indicated by the gonadosomatic index (GSI) calculated as: GSI=GW×100/EW. Timing of reproduction is often related to feeding seasonality of adults, allowing needed energy reserves (Guillemot et al., 1985). Hence, monthly variation in feeding condition was indicated by the digestosomatic index (DSI) calculated as: DSI=GAW×100/EW. The eviscerated weight was used because it is a more adequate body mass indicator than total weight, as it is not affected by individual variation in the mass of the digestive tract, liver and reproductive organs (Peres and Vooren, 1991).
All sagittae were dissected, cleaned, air-dried at room temperature and stored in plastic tubes for subsequent examination. We used the right otolith for age determination when it was available and the left when the right was not utilizable.
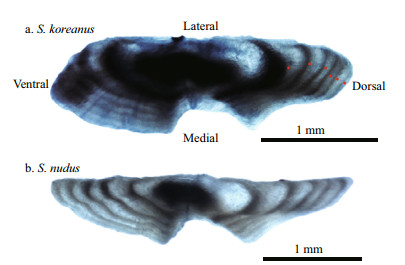
2.2 Age determination and growth analysisSagittae were embedded in epoxy resin and crosssectioned transversely to a thickness of approximately 800 μm with an Isomet low-speed diamond bladed saw, before mounting on glass slides. These sections were polished with a fine grit polishing pad and velvet polishing pad (Buehler) until the core was distinctly visible. Then, the otolith sections were viewed and imaged with a digital camera fitted to a Nikon SMZ1000 dissecting microscope under transmitted light at 40× magnification (Fig. 2). The images were then enhanced in Adobe Photoshop CS5 Extended as described by Campana et al. (2015) to maximize annuli visibility prior to age determination.
|
| Fig.2 Photographs of cross-section otolith of S. koreanus (a) (6-year-old female, 96 mm SL) and S. nudus (b) (5-year-old male, 134 mm SL) Red dots denote annulus. |
Annuli (outer margins of the opaque zone) were read twice (one month apart) in a random order without prior knowledge of the size or species. Deviations were counted a third time. Only counts with at least two agreements were used in subsequent analyses.
Annulus formation was verified by marginalincrement analysis technique (e.g., Kadison et al., 2010). For all age-2 and older fish, the marginal increment ratio (MIR) was calculated as:
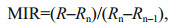
where R is the radius (the distance between the focus and the otolith edge), Rn is the distance between the focus and the last annual band and Rn-1 is the distance between the focus and the penultimate annual band. Once MIRs were obtained for all of the otoliths, the monthly mean values were plotted separately for S. koreanus and S. nudus to determine the annual patterns of band formation. For age-1 fish, which had no second annulus, the expected distance between the first and second annuli was calculated as described by Kadison et al. (2010). The marginal increment ratios for all fish age-1 and older were pooled and plotted as monthly means to verify and determine the timing of annulus formation.
Relative ages derived from otolith ageing were converted to absolute ages according to the reproductive seasonality, the date of capture, and the period of annulus formation (see Results). The von Bertalanffy growth function was fitted to the lengthat-age data by species, population and sex:
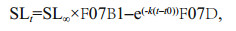
where SL∞ is the asymptotic length, k is the growth coefficient, and t0 is the hypothetical age at zero length. The analysis of the residual sum of squares (ARSS) (Chen et al., 1992) was employed to compare growth curves between populations, species and sexes. In addition, the ratio of male to female SL (SL ratio) was used as an index of sexual size dimorphism (Lenarz and Echeverria, 1991). Here, we used the average SL of mature fish measured in this study to calculate the SL ratio.
2.3 Bodycoloration analysisThe side-view images of S. nudus specimens were calibrated with the X-rite ColorChecker Passport software (V.1.0.2). For better measurement, we further enhanced the contrast between conspicuous yellow markings and their surroundings by converting the fish coloration to black-and-white mode within Adobe Photoshop CS5 Extended. Here, we selected Adobe Photoshop CS5 Extended software due to its flexible and strong performance in selection techniques (see Adobe Photoshop CS5 Help at https://helpx.adobe.com/photoshop/archive.html). The main steps in processing the image within the software are shown in Fig. 3.

|
| Fig.3 Steps in processing the image within Adobe Photoshop CS5 Extended a. import the original image; b. select and fill the background with 50% grey; c. select and fill the yellow markings with a more contrastive and higher saturated color, e.g., brilliant blue; d. select and fill the fish surface outside the markings with black color; e. select and fill the markings with white color and finally, save the processed image as a maximum quality JPEG file. |
For each processed image, the total area of the markings (white area) and the total area of fish surface outside the markings (black area) were measured using Image-Pro Plus (V.6.0). The yellow marking ratio (YMR, the ratio between the total area of yellow markings and the total body surface area) was calculated as:

where Awhite is the white area value, and Ablack is the black area value in the processed image.
A paired-samples t-test of YMR derived from left side-view images and right side-view images indicated no significant difference (t=-0.617, P > 0.05). Then, the left side-view images were processed once again in a random order one month later. A pairedsamples t-test of YMR derived from the two separate handlings revealed no significant difference (t=0.224, P > 0.05). Hence, the YMR value derived from the first handling of the left side-view image of each individual was used in subsequent analyses.
Two-way analyses of variance (ANOVA) were performed to examine the age-group×month, sex×agegroup and sex×month interactions on the YMR, separately. When no significant interaction term was detected by the two-way ANOVA (P > 0.05), the simple main effect of each factor (age-group, month and sex) was analyzed separately by one-way ANOVA. Significant one-way ANOVA was followed by a Duncan's test for multiple comparisons. To investigate the possible seasonal sexual dimorphism in coloration, the sexual difference in the mean YMR value within each month was assessed, respectively, using independent-samples Student's t-test. The mean YMR values were plotted by age to display the variation trend. Then, the monthly mean YMR values for male, female and sex-pooled S. nudus were plotted separately.
2.4 Morphological trait analysisTo evaluate the scaling relationship for each morphological variable with body size, we calculated separate reduced major axis (RMA) regressions for CFL, PFL, DSL and MB on SL for each of the species examined. Reduced major axis regression is the most appropriate method for the evaluation of structural relationships between variables when both are subject to measurement error (Rayner, 1985; LaBarbera, 1989). All data were log10 transformed prior to analysis, allowing the exponent of each scaling relationship to be determined as the slope of its RMA regression. The RMA regression was applied using the RMA Software for Reduced Major Axis Regression (V. 1.17) by Bohonak (2004). Deviations from isometry and homogeneity of slopes between the species were examined by inspection of the 95% confidence intervals of the slope estimates.
The morphological variables measured above were used to obtain 4 morphometric ratios (CFL/SL, PFL/ SL, DSL/SL and MB/SL) that are considered potentially important for describing the swimming performance, predator avoidance or hunting capacity of fish (Wañkowski, 1979; Ringler, 1983; Blouw and Hagen, 1984; Fisher and Hogan, 2007; Nanami, 2007; Leavy and Bonner, 2009). As there were no significant sex×age-group interactions on the 4 morphological ratios within each species (two-way ANOVA, in all cases, S. koreanus P > 0.05 and S. nudus P > 0.05), the sex-pooled data were used in subsequent analyses. To examine the ontogenetic trajectories of the 4 morphometric ratios, we plotted the mean ratios by age within each species. To determine the age at which interspecific divergence in these ratios begins to appear, Student's t-tests were used to assess the interspecific differences of each ratio by age.
All statistical analyses were performed with a significance level of 0.05 using SPSS (V.19.0). Prior to each ANOVA and t-test, the variables were tested for normality (Kolmogorov-Smirnov test) and homogeneity of variance (Levene's test) and data were log-transformed if necessary.
3 RESULT 3.1 Reproductive and feeding seasonalityOnly adults sampled from Qingdao were used to determinate reproductive and feeding seasonality. In the 641 sexed S. koreanus, the youngest mature male was age-1 (67 mm SL), and the youngest mature female was age-2 (71 mm SL). In the 255 sexed S. nudus, the youngest mature male was age-2 (100 mm SL), and the youngest mature female was age-2 (99 mm SL). For both species, all age-3 and older individuals were classified as mature.
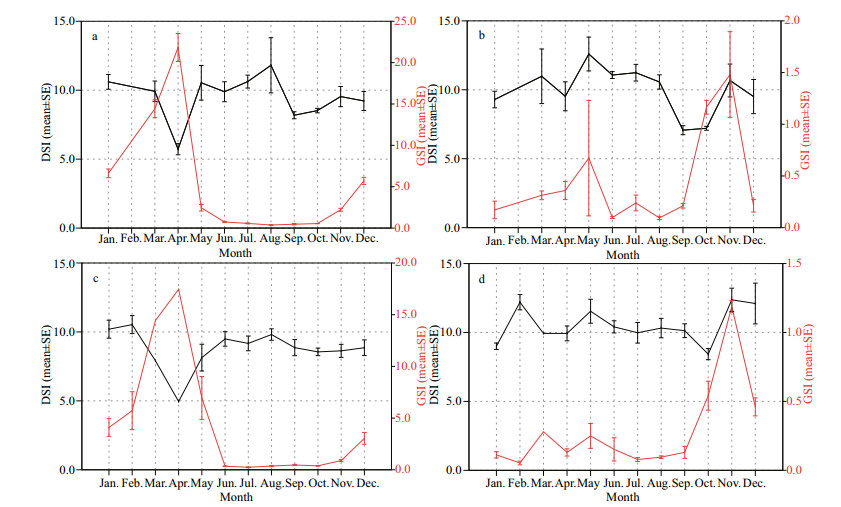
Monthly changes of GSI and DSI are plotted in Fig. 4. The monthly variations in the GSI for both species indicated that the peak parturition periods of both species were from April to May (Fig. 4a, c), and the peak copulation periods of both species were from November to December (Fig. 4b, d).
|
| Fig.4 Monthly variations in gonosomatic index (GSI) and digestosomatic index (DSI) of S. koreanus females (a), males (b), and S. nudus females (c), males (d) |
The mean DSI values of S. koreanus females (Fig. 4a) and S. nudus females (Fig. 4c) showed the minimum levels at the peak parturition periods and experienced different degrees of decline (very rapid for the former, moderate for the latter) just before their peak copulation periods. Moreover, the lowlevel period (just before the peak copulation period) of the mean DSI of S. koreanus males lasted approximately 2 months (September–October) (Fig. 4b) while the low-level period of S. nudus males lasted only approximately 1 month (October) (Fig. 4d).
3.2 Age and growth1 133 otoliths (99.8%) were used in the age and growth analyses because they were not only readable but also provided at least two agreements among separate readings. The dorsal face of the sectioned otoliths (Fig. 2) seemed to provide a better individualization of the growth increments owing to their greater sharpness, higher contrast between fast and slow growth bands and larger distance between them.
The plot of monthly mean MIR across all agegroups showed that the highest mean MIR occurred in May and lowest in July for both S. koreanus and S. nudus, suggesting that one growth ring formed on an annual basis for the two species (Fig. 5).

|
| Fig.5 Monthly mean marginal increment ratio (±SE) of otoliths for S. koreanus and S. nudus |
Having considered the peak parturition periods, 1st May was assigned as the birth date for both species in this study. Relative ages derived from ageing were then converted to absolute ages. Ages of S. koreanus ranged from 0.52 to 15.35 years, and ages of S. nudus ranged from 0.56 to 11.03 years.
No significant differences in growth curves were found between the sexes for S. nudus (ARSS, P > 0.05) and each population of S. koreanus (ARSS, in all cases, P > 0.05). However, the ARSS indicated that the sex-pooled growth curves of S. nudus and 3 populations of S. koreanus differed significantly from each other (in all cases, P < 0.05). Therefore, sexescombined von Bertalanffy growth curves are depicted in Fig. 6, and the growth parameters are shown in Table 3. The average ratio of male to female SL is 0.96 for S. koreanus and 0.98 for S. nudus.

|
| Fig.6 Von Bertalanffy growth curves in standard length of S. koreanus and S. nudus |
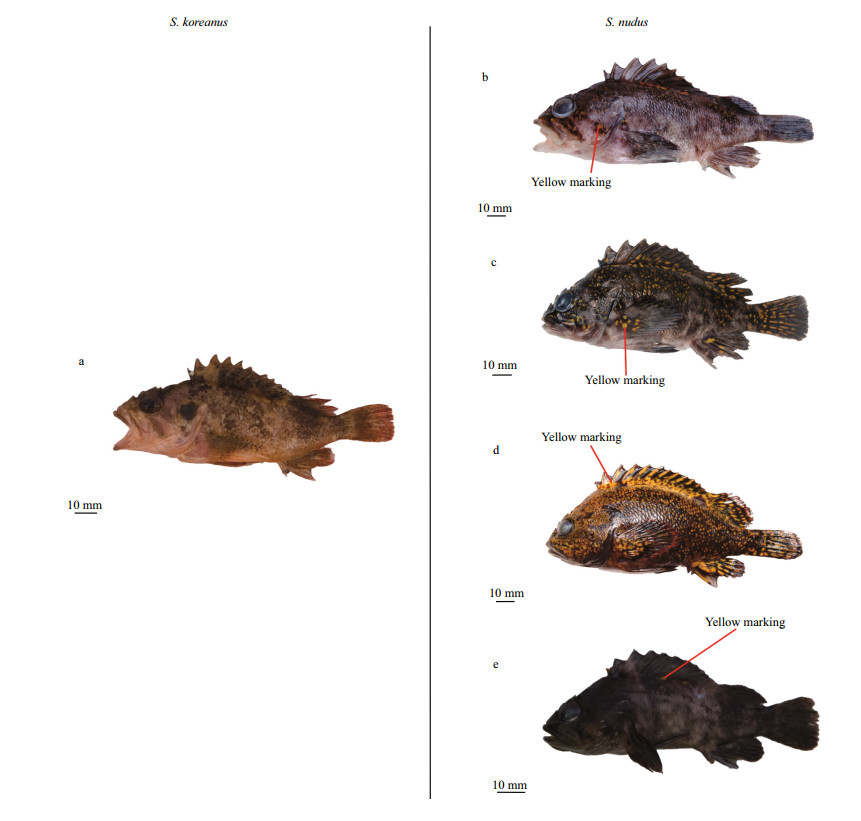
Sebastes nudus has a highly variable and conspicuous (dark base color vs. conspicuous yellow markings) color pattern (Kai and Nakabo, 2013; present study), differing from the stable and dull (light brown base color vs. dark brown bands or dots) color pattern of S. koreanus (Jin, 2006; Yu et al., 2015; present study) (Fig. 7). Interestingly, for S. nudus, irregular distinct yellow markings often extend onto head and pectoral, anal, caudal, and dorsal fins (Fig. 7c) but sometimes are indistinct (Fig. 7b & e), or oppositely, very conspicuous on almost the whole body except the ventral surface (Fig. 7d).
|
| Fig.7 Live body color morphotypes of S. koreanus and S. nudus a. S. koreanus, adult fish, 105 mm SL; b. S. nudus, adult fish, 122 mm SL, YMR=0.049; c. S. nudus, adult fish, 120 mm SL, YMR=0.097; d. S. nudus, adult fish, 117 mm SL, YMR=0.343; e. S. nudus, age-1 juvenile fish, 82 mm SL, YMR=0.001. |
Two-way ANOVAs indicated no significant agegroup×month (P > 0.05), sex×age-group (P > 0.05) and sex×month (P > 0.05) interaction effects on the YMR. Then, the one-way ANOVAs showed significant differences in YMR among the various age-groups (P < 0.05) and months (P < 0.05) and no significant differences between the sexes (P > 0.05). The mean YMR values of S. nudus were plotted by age in Fig. 8a. The age-1 group showed the smallest value while the age-3 showed the largest value. The mean YMR value rose rapidly from age-1 to age-2 and then declined gradually from age-3 to age-9. Multiple comparisons showed significant differences (Duncan's test, P < 0.05, respectively) between the age-1 group and the age-2~6 groups (Fig. 8a).
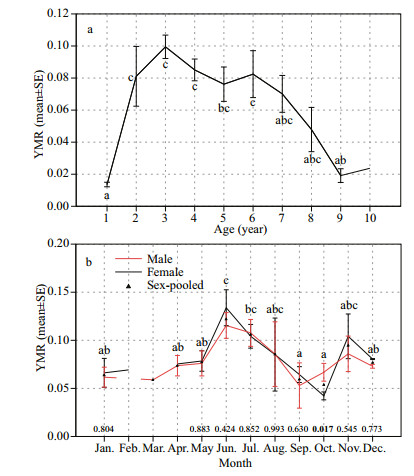
|
| Fig.8 Mean yellow marking ratio (±SE) by age-group of S. nudus (a); monthly mean yellow marking ratio (±SE) of male and female S. nudus and monthly mean yellow marking ratio of sex-pooled S. nudus (b) In (a), letters near the bars indicate significant differences between age-groups; those not sharing a common letter were significantly different (Duncan's test, P < 0.05); in (b), P-values based on a twotailed Student's t-test using the log-transformed data between sexes within each age-group are given on the top of the x-axis. Only significant values (P < 0.05) are in bold. P-values were not present in the February–April groups due to insufficient sample sizes for the K-S normality test. Letters near the bars indicate significant differences between monthly mean yellow marking ratio of sex-pooled S. nudus; those not sharing a common letter were significantly different (Duncan's test, P < 0.05). |
The monthly mean YMR values were plotted separately by sex in Fig. 8b. For both sexes, the monthly variations in the YMR data exhibited similar bimodal patterns with a major peak in June and a minor peak in November. The mean YMR values for both sexes increased gradually from January to May, rose rapidly from May to the major peak in June, and declined dramatically until September for males and October for females. Then, the mean YMR values for both sexes rose again to the minor peak in November and decreased in December. The Student's t-test indicated that significant difference (P < 0.05) between the sexes occurred only in October throughout the year (Fig. 8b).
3.4 Morphological trait analysisRegression analyses for both S. koreanus and S. nudus showed strong correlations between each of the 4 variables examined (CFL, PFL, DSL and MB) and SL (mean R2=0.857, Table 4, Fig. 9a, b, c, d). The intercept, slope and confidence intervals for each of the species are given in Table 4. The slope value of the CFL regression for S. nudus (1.047) is somewhat higher than the slope value of the CFL regression for S. koreanus (0.985), with both exhibiting isometry of CFL with respect to SL (95% CI of regression slope for each species overlaps predicted slope of 1 for isometry, Table 4). S. nudus exhibited positive allometry of PFL on SL (95% CI > 1, Table 4), whereas S. koreanus showed negative allometry of PFL on SL (95% CI < 1, Table 4). Similarly, S. nudus exhibited positive allometry of DSL on SL (95% CI > 1, Table 4), whereas S. koreanus showed negative allometry of DSL on SL (95% CI < 1, Table 4). Both species showed positive allometry of MB with respect to SL (95% CI > 1, Table 4). Interestingly, in all variables, S. nudus had higher slope values (Table 4).
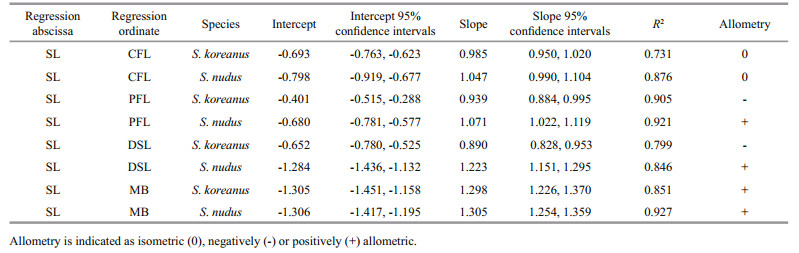
|
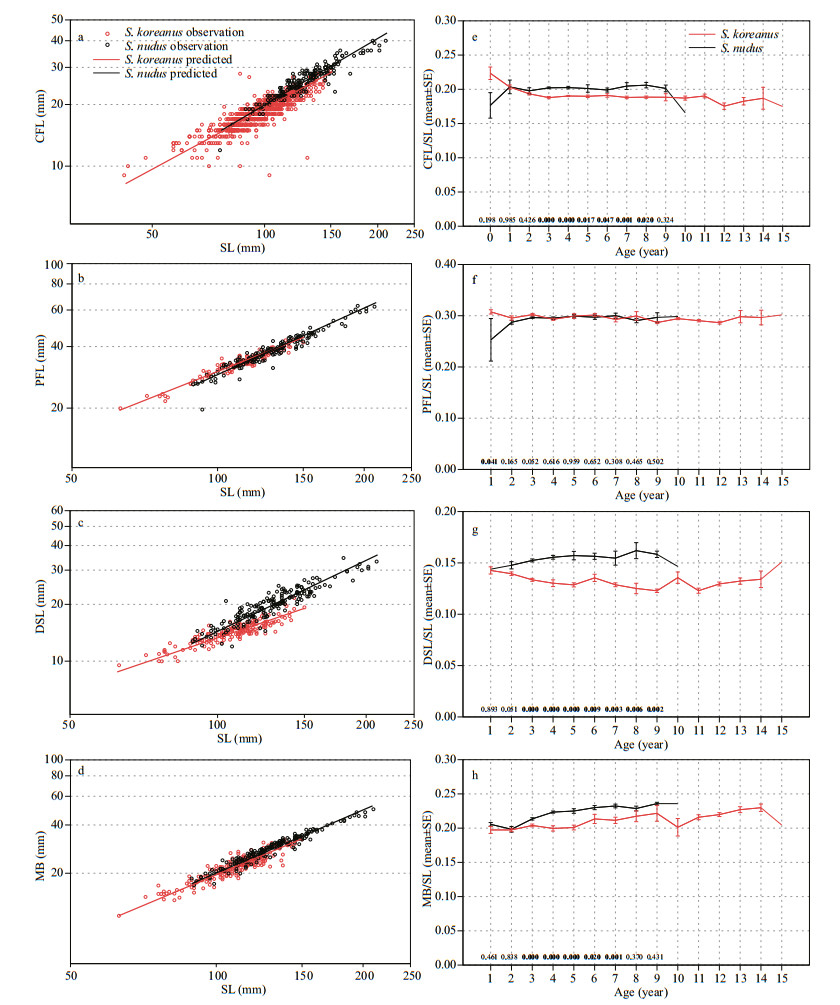
|
| Fig.9 Plots of log-log RMA regressions based on original body measurements, comparing scaling of CFL, PFL, DSL and MB on SL in S. koreanus and S. nudus (a–d) (see Table 2 for parameters of scaling equations); error bar plots of CFL/SL, PFL/SL, DSL/SL and MB/SL by age-group for S. koreanus and S. nudus (e–h) P-values based on a two-tailed Student's t-test between species within each age-group are given on the top of x-axis. Only significant values (P < 0.05) are in bold. P-values were not present in the 10-11 age-groups due to insufficient sample sizes for the K-S normality test. |
The age-related trends in morphometric ratios (CFL/SL, PFL/SL, DSL/SL and MB/SL) of S. koreanus and S. nudus were displayed in Fig. 9. For CFL/SL, S. koreanus and S. nudus showed two opposite trends during the early phases of growth (< age-3): a general decrease in S.koreanus and a general increase in S. nudus. However, no interspecific significant difference was detected in CFL/SL from age-0 to age-2 (Student's t-test, P > 0.05, Fig. 9e). S. nudus was characterized by significantly higher CFL/SL mean values beginning at age-3 and continuing through age-8 (Student's t-test, P < 0.05, Fig. 9e).
Relative to PFL/SL, S. koreanus had a significant higher mean value than S. nudus at age-1 (Student's t-test, P < 0.05, Fig. 9f) while no significant interspecific difference was detected from age-2 to age-9 (Student's t-test, P > 0.05, Fig. 9f).
No significant interspecific difference was detected in DSL/SL at age-1 and age-2 (Student's t-test, P > 0.05, Fig. 9g). However, S. nudus was characterized by significantly higher DSL/SL mean values beginning at age-3 and continuing through age-9 (Student's t-test, P < 0.05, Fig. 9g).
Relative to MB/SL, both species showed a general increase with age (Fig. 9h). No significant interspecific difference was detected at age-1 and age-2 (Student's t-test, P > 0.05, Fig. 9h). S. nudus was characterized by significantly higher MB/SL mean values beginning at age-3 and continuing through age-7 (Student's t-test, P < 0.05, Fig. 9h). No statistically significant interspecific difference was found at age-8 and age-9 (Student's t-test, P > 0.05, Fig. 9h), but S. nudus still had higher mean values than S. koreanus.
4 DISCUSSIONIn the present study, S. koreanus specimens were collected easily and steadily by local fisheries off the south, east and north coasts of Shandong Peninsula. Similarly, S. nudus specimens were collected easily and steadily by local fisheries off the south coast of Shandong Peninsula. However, S. nudus were collected very rarely off the east coast of Shandong Peninsula and never off the north coast of Shandong Peninsula during the sample period. These findings are consistent with previous reports on the distributions of the two Sebastes (Kai and Nakabo, 2013; Fang et al., 2015; Yu et al., 2015).
To our knowledge, this study is the first validation of age determination from otoliths of S. koreanus and S. nudus and adds important biological knowledge to age, growth, reproductive biology and color pattern of these species. Excellent precision in independent ring counts on sectioned otoliths in our study provides accurate age estimates. The annual periodicity of formation of growth ring on otoliths was confirmed in the two species, which enabled analysis of their age and growth. In general, marginal increment analysis showed that annulus formation occurred once a year between June and July (early summer) in both species, suggesting that water temperatures likely correlate with the pattern of marginal increment growth.
Our results indicate that S. nudus grows to a larger size than S. koreanus, but that the longevities of the two species can be similar. Significant differences in growth curves were observed among the 3 populations of S. koreanus. S. koreanus exhibits a relatively shallow depth distribution (2–30 m) and a narrow latitudinal distribution (Fang et al., 2015; Yu et al., 2015) where environmental and biological factors may have a greater influence on its populations. Several studies on Sebastes explored the relationships between growth parameters (k and SL∞) and latitude or water temperature. Boehlert and Kappenman (1980) and Love and Westphal (1981) noted that the growth coefficient increased with latitude in S. serranoides and S. diploproa. Pearson and Hightower (1990) reported increase in asymptotic length and decrease in growth coefficient of S. entomelas with increasing latitude. In the present study, given the latitudes and mean ambient water temperatures of the 3 populations of S. koreanus (Fig. 1), we found there was no apparent latitudinal cline in growth parameters. However, the most southern (Qingdao) S. koreanus population showed the smallest SL∞ value of 110.882 cm and a high k value of 0.321/a (although this k value is somewhat lower than the Penglai k value of 0.427/a). According to previous studies, the southern coastal waters of Shandong Peninsula seems to be the southern border of the distributions of both S. koreanus and S. nudus (Kai and Nakabo, 2013; Fang et al., 2015; Yu et al., 2015). Therefore, the potential high temperature stress may impact the growth performance of the most southern (Qingdao) S. koreanus population, including increasing the growth rate of young fish, inducing earlier sexual maturity (see result, the youngest mature S. koreanus male caught in Qingdao was only age-1), as well as decreasing the asymptotic length. Moreover, since the Rongcheng population showed the highest SL∞ value and longevity (Fig. 6) among the 3 populations of S. koreanus, we speculated that this population may be under the lowest fishing pressure.
The adult size of female rockfish is often larger than males (Six and Horton, 1977; Love and Westphal, 1981; Love et al., 1990; Laidig et al., 2003). Although females may have a slightly greater growth parameter SL∞ than males, both species in the present study did not show clear sexual difference in growth curves based on the ARSS results. Territorial behaviour has now been observed for several demersal rockfishes (Larson, 1980; Hallacher and Roberts, 1985; Markevich, 1988; Shinomiya and Ezaki, 1991), and larger males tend to hold larger territories (Shinomiya and Ezaki, 1991). Lenarz and Echeverria (1991) suggested that the tendency for the ratio of male to female SL to be high for demersal species of Sebastes may be related to an increased tendency for territorial behaviour by demersal males. Hence, the demersal lifestyle of both S. koreanus and S. nudus may partly explain why their SL ratios were relatively high, like the average SL ratio of the demersal rockfishes (0.97) calculated by Lenarz and Echeverria (1991).
Both S. koreanus and S. nudus are cool-temperate fishes adopting the semi-settled lifestyle (Jin, 2006; Kai and Nakabo, 2013; Yu et al., 2015). Although no long-range movements or migratory patterns have been noted for the two rockfishes, they both tend to exhibit short-range, seasonal vertical migrations (Tang and Ye, 1990; Han and Lu, 2003; Ma and Hou, 2013) that may be interpreted as adaptations to the well-defined seasonal temperature fluctuations in temperate coastal waters. The large deep dwellers (i.e., the older fish) of the S. koreanus and S. nudus gradually migrated into the shallow areas with rising nearshore water temperature in spring and earlysummer (approximately March–June) (Table 2, Fig. 10). They gradually migrated back into their warmer, deeper habitats with declining nearshore water temperature in late autumn and winter (approximately October–next February) (Table 2, Fig. 10). Other than temperature, food availability is a possible cue for their seasonal vertical migrations because their main prey items, small-sized demersal fishes (e.g., gobies) and crustaceans, exhibit the similar seasonal migration pattern (Tang and Ye, 1990; Jin, 2006; Ma and Hou, 2013). It is highly plausible that their overwintering migrations also represent mating migrations, with peak periods of copulation of both species occurring in winter (November–December). In view of the bigger-deeper distribution patterns and seasonal shallow-deep migrations of both S. koreanus and S. nudus, we suggest that individuals tend to mate with fish of a similar size. In other words, the small-sized shallow dwellers may rarely encounter the large deep dwellers except during the nearshore aggregations in summer (May–July) (Table 2).
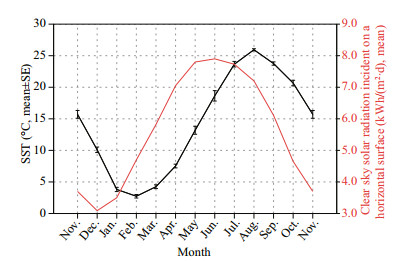
|
| Fig.10 Monthly mean sea surface temperatures (SST) from Nov. 2013 to Nov. 2014 off the coast of Qingdao Daily SST data were available at ftp://data.remss.com/SST/daily_v04.0/mw_ir/ and read by Python version 3.4) and monthly mean clear sky solar radiation incident on a horizontal surface at the coast of Qingdao in the 22-year period (Jul. 1983–Jun. 2005) (data were available at NASA Surface meteorology and Solar Energy: https://eosweb.larc.nasa.gov/. |
Based on analyses of YMR, we confirmed that S. nudus changes its body color with age and season. The age-1 juvenile fish showed a clearly lower YMR than the older fish, while the age-3 fish showed the highest YMR (Fig. 8a). Interestingly, the youngest mature fish were age-2, and all fish were mature by age-3 for S. nudus. These facts suggested that the bold color pattern may begin to fully express when the young fish reach first maturity.
For both sexes of S. nudus, the monthly variations in the YMR data exhibited similar bimodal patterns (Fig. 8b). Color changes in fish are often related to environmental stress, and light intensity is one of the most important factors regulating pigment distribution through hormone regulation (Fujii, 2000; Yasir and Qin, 2009). Additionally, the light intensity decreases along the depth gradient. In this study, the sunlight intensity in Qingdao reached its peak in June (Fig. 10) when the S. nudus migrated into the very nearshore from their relatively deep habitats (Table 2). Consequently, although the ambient light intensity of the S. nudus cannot be measured precisely, the ambient light intensity may reach the peak in June. Moreover, the major peak of monthly mean YMR also appeared in June (Fig. 8b). Taken together, these results support a general positive correlation between the ambient light intensity and the YMR. In view of the bigger-deeper distribution pattern of S. nudus, this positive correlation accounts well for the gradual decreases of the mean YMR by age-group after the first maturity (Fig. 8a).
Nuptial coloration is widespread among fishes and is generally more pronounced in the males (Andersson, 1994; Sköld et al., 2008). In Sebastes, sexual visual communication (via color patterns, visual sensitivity, etc.) may play a more important role in mating, as relatively elaborate courtship displays reflecting males' body condition were usually observed as an initial and essential stage of mate selection in rockfishes (Helvey, 1982; Shinomiya and Ezaki, 1991; Karageorge and Wilson, 2017). In this study, the monthly mean YMR of both sexes of S. nudus displayed a minor peak (Fig. 8b) during the peak copulation period in November (Fig. 4). Throughout the year, the monthly mean YMR of males was significantly higher than the monthly mean YMR of females only in October, which was just before the peak copulation period (Fig. 8b). Having considered this evidence, we speculated that S. nudus may show a typical carotenoid-based nuptial coloration. During the copulation season, the nuptial coloration may make the S. nudus more visually conspicuous and thus reduce the mate searching costs and maximize the efficiency of courtship displays in the relatively deep and murky environments. Previous observations confirmed that in Sebastes, males are relatively more active than females during the mating season (Helvey, 1982; Shinomiya and Ezaki, 1991; Eschmeyer et al., 2010). In view of the lowest-level period of DSI just before the peak copulation period (Fig. 4), we speculated that both S. koreanus and S. nudus males may stop feeding during the mating season. The males may spend more time and energy on mate searching and courtship activities rather than on food searching during this period. Within each sex, S. nudus showed a shorter low-level duration and smaller decrease in DSI than did S. koreanus during the copulation period (Fig. 4). Moreover, Li et al. (2014) recently studied the visual sense of 4 reef fishes and indicated that S. nudus may have better visual acuity and scotopic vision than S. koreanus. All these facts imply that the bolder nuptial coloration and better visual sense of S. nudus may together enhance the efficiency of mate searching and sexual selection and thus reduce the energy and time costs.
Although conspicuous coloration may confer mating advantages, it is also hypothesized to impose one or more fitness costs. In fact, several empirical studies involving sexual dichromatism, color morphotypes, or closely related species suggested that conspicuous coloration may inadvertently attract the attention of potential predators and thus may be costly in terms of increasing individual predation risk (e.g., Endler, 1983, 1992; Grafen, 1990; Johnstone, 1995; Godin and Dugatkin, 1996; Godin and McDonough, 2003). On the other end of predatorprey interactions, conspicuously colored individuals may also be visually conspicuous to their prey and thus suffer greater foraging difficulty (Grether and Grey, 1996; Macedonia et al., 2002; Baird, 2008). Hence, the conspicuously colored individuals of S. nudus may suffer more fitness costs such as higher predation risk and greater foraging difficulty than their closely related sympatric congener, S. koreanus.
Predator-prey interactions can generate divergent selection in several different ways and are presumed to represent a major source of evolutionary diversification (Kerfoot and Sih, 1987; Langerhans et al., 2004; Langerhans, 2007). Strong divergent selection generated through predator-prey interactions may contribute to rapid phenotypic plasticity and evolution of morphological traits that facilitate survival (Langerhans et al., 2004; Andersson et al., 2006; Eklöv and Svanbäck, 2006; Domenici et al., 2007; Langerhans, 2009). Predators often generate phenotypic selection favouring enhanced survival abilities of prey, such as better swimming performance (e.g., Langerhans et al., 2004), stronger anti-predator defense (e.g., Ruzzante et al., 2011), etc. Moreover, divergent selection imposed by differences in foraging difficulty may also cause adaptive divergence in foraging morphology such as feeding organs (e.g., Malmquist, 1992; Berner et al., 2008) and swimming organs (e.g., Berner et al., 2008; Sharpe et al., 2008), etc. The morphological traits that we have identified in comparisons of S. koreanus and S. nudus have several potential implications for locomotion, foraging and anti-predator defense. Sebastes are relatively active swimmers in scorpaenid fishes and primarily use their caudal structures and pectoral fins for propulsion and maneuvering (Chen, 1986; Jin, 2006). Like many other ambush predators of rockfish, both S. koreanus and S. nudus have rounded caudal fins. The expanded caudal area may increase the drag but increase the thrust-production for rapid acceleration in ambush predation and escape from potential predators (Massare, 1994). Therefore, both CFL/SL and PFL/SL are probably positively associated with swimming performance (Berbel-Filho et al., 2016). Based on these assumptions, the results of Student's t-tests in CFL/SL and PFL/SL suggested that relative to same-aged S. koreanus, S. nudus did not show a better swimming performance than S. koreanus during their juvenile stage (Fig. 9e & f). However, S. nudus tended to be a stronger swimmer than same-aged S. koreanus as they became mature adults (Fig. 9e & f). Both S. koreanus and S. nudus are equipped with sharp, hollow spines connected to venom glands. These species typically erect their dorsal spine in response to piscivorous predators (Jin, 2006). Hence, in the case of predation avoidance related to predator gape limitation, longer dorsal spines are the important adaptation to increase chances of survival (Abrahams, 2005; Ruzzante et al., 2011). Based on this assumption, the results of Student's t-tests in DSL/SL suggested that, relative to same-aged S. koreanus, S. nudus may suffer a higher predation risk beginning at age-3 (Fig. 9g). Both S. koreanus and S. nudus tend to swallow their prey whole (verified by visual observation) and thus are gape-limited predators (Jin, 2006). Generally, maximum prey size and foraging success are positively correlated with mouth size (especially, mouth breadth) in Sebastes (Roberts, 1979; Hallacher and Roberts, 1985; Anderson, 1994). Relative to same-aged S. koreanus, S. nudus was characterized by significantly greater mouth breadth beginning at age- 3 (Fig. 9h). These results, together with the results of CFL/SL, imply that clear differences in foraging may occur beginning at age-3.
However, our comparisons of scaling patterns between S. koreanus and S. nudus identified significant differences with respect to the scaling of PFL and DSL relative to SL (Table 4). Moreover, despite the similar scaling patterns of CFL and MB for the two species, S. nudus always have higher slope values (Table 4). These data suggested a strong selective pressure to enhance swimming performance, antipredator defense and foraging capacity in S. nudus. In view of the ontogenetic development of color pattern in S. nudus (Fig. 8), we suggest that the full expression of the bold color pattern, which begins at the age of first maturity, most likely triggers the strong selective pressure on S. nudus through predator-prey interactions. The divergences in swimming performance, anti-predator defense and foraging capacity, together with the differences in feeding intensity during copulation season, may account well for the divergence in the growth trajectories between S. koreanus and S. nudus.
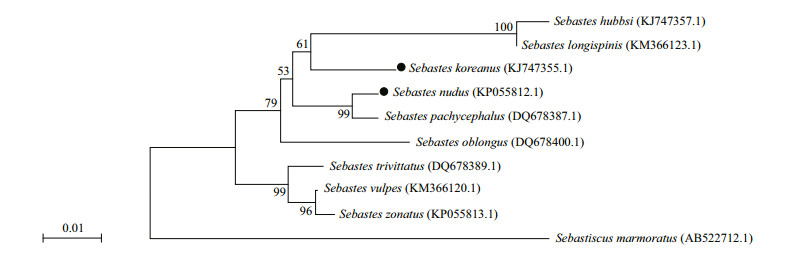
Although a recent taxonomic review has suggested that S. nudus and S. pachycephalus are sister species, their reproductive barrier is not yet completely effective, and their body color patterns are somewhat indistinguishable (Kai and Nakabo, 2013). No specimen of S. pachycephalus was encountered during our study. Moreover, there is no report of this species from the coasts of the Shandong Peninsula after its recent taxonomic review (Kai and Nakabo, 2013). Therefore, S. nudus may be the unique member from the Sebastes pachycephalus complex (Kai and Nakabo, 2013) inhabiting the coastal areas of Shandong Peninsula. However, the mitochondrial COI gene sequence analysis indicated that S. nudus maybe the species most closely related to S. koreanus among other closely related Sebastes species (Table 5, Fig. 11). In all, the dramatic difference in color pattern polymorphisms, the broadly overlapping distribution and the very close phylogenetic relationship together make S. koreanus and S. nudus an ideal model to investigate the role of color-driven divergent selection and ecological adaptation in the speciation of the Sebastes.

|
|
| Fig.11 Neighbor-joining tree based on mitochondrial COI gene sequences available in GenBank, showing the relationships among 9 closely related Sebastes species in clade NWPs (Sebastiscus marmoratus as an out group) The sequences were aligned using Clustal W, and the tree was constructed using the Kimura 2-parameter model and 1 000 bootstrap replications in the software MEGA 6.06. Parentheses indicate the NCBI registration number. |
It is becoming increasingly clear that, in conjunction with a solid understanding of their phylogenetic relationships, Sebastes spp. offers a unique opportunity to study speciation patterns in the marine realm, both modern and historic (Hyde and Vetter, 2007; Hyde et al., 2008; Ingram, 2010). In the present study, all the evidence strongly supports the hypothesis that both color pattern and bathymetric segregation play key roles in facilitating the speciation of demersal rockfishes. Color pattern polymorphisms are relatively common in Sebastes (e.g., Kai and Nakabo, 2013; Frable et al., 2015). The vast majority of this genus inhabit coastal or littoral zones (Nelson et al., 2016) that are well characterized by broad-spectrum sunlight and high luminous intensity. Sebastes have many visual predators (Mills et al., 2007), and themselves prey upon visually well-equipped organisms such as crustaceans and other fish (Guthrie, 1986). Hence, visual factor tends to play a more important role than any other sensory factors in inter- and intraspecific communication as well as predator-prey interactions and be an alternative force driving evolutionary process in Sebastes. A fish's boldness in coloration could reliably indicate its overall quality, as relatively conspicuous-colored fish tend to be better informed about potential predators, more likely to survive encounters with them, and feed at higher rates or more persistently than dull-colored fish. Bold coloration in rockfish thus appears to be an honest signal of high quality (Johnstone, 1995) such as high efficiency in mate searching, good swimming performance, good anti-predator defense and good foraging capacity. All these advantages may together make conspicuous-colored fish grow faster and attain a bigger size than dull-colored fish. Moreover, in view of the prevalence of the bigger-deeper phenomenon (Love et al., 1991), demersal lifestyle (Hyde and Vetter, 2007) and seasonal shallow-deep migration pattern (Tang and Ye, 1990) in Sebastes, we suggest that the conspicuous-colored adults are likely to migrate into deeper areas than dull-colored adults during mating period. The spatial separation (bathymetric segregation) of mating ground between color phenotypes may gradually develop through the strong divergent color-driven ecological and sexual selection and result in individuals reproducing and populations establishing in separate habitats. Migration of suboptimal phenotypes or competition between phenotypes may favour assortative mating, reducing gene flow and ultimately allowing speciation along the depth gradient (Doebeli and Dieckmann, 2003).
5 CONCLUSIONIn summary, this study first ascertained the age, growth, reproductive and feeding seasonality and color pattern of two very closely related rockfishes, S. koreanus and S. nudus. Based on the body color analyses, we confirmed that S. nudus changes its body color with age and season and fully expresses the carotenoid-based nuptial coloration when reaching first maturity. Moreover, our comparisons of morphological traits that are likely related to survival and quality of these fishes suggested that S. nudus might suffer higher predation risk and foraging difficulty beginning at age 3. In view of the ontogenetic development of the color pattern in S. nudus, we suggested that the full expression of nuptial coloration most likely triggers the strong selective pressure to enhance swimming performance, anti-predator defense and foraging capacity of S. nudus through predator-prey interactions. By understanding all these details, we further inferred a hypothesis that both color pattern and bathymetric segregation play key roles in facilitating the speciation of demersal rockfishes. We expect that in future, empirical research on color-driven speciation in Sebastes will become more integrative, and aspects of biology such as age, growth and reproductive biology should doubtlessly be integrated into this research. In this way, we can take full advantage of the Sebastes species flock as a tool for understanding the evolution of biological diversity.
6 DATA AVAILABILITY STATEMENTThe digital photographs of fish and otoliths are only available upon request to the corresponding author, Dr. YE Zhenjiang (yechen@ouc.edu.cn). All other data are available within the paper.
7 ACKNOWLEDGMENTWe are grateful to anonymous reviewers for providing constructive comments on the draft manuscript. All experimental procedures were done by following the general animal ethical guidelines of Institutional Animal Ethical Committee (IAEC).
Abrahams M. 2005. The physiology of antipredator behaviour:what you do with what you've got. Fish Physiology, 24: 79-108.
DOI:10.1016/S1546-5098(05)24003-7 |
Alesandrini S, Bernardi G. 1999. Ancient species flocks and recent speciation events:what can rockfish teach us about cichlids (and vice versa)?. Journal of Molecular Evolution, 49(6): 814-818.
|
Alishahi M, Karamifar M, Mesbah M. 2015. Effects of astaxanthin and Dunaliella salina on skin carotenoids, growth performance and immune response of Astronotus ocellatus. Aquaculture International, 23(5): 1 239-1 248.
DOI:10.1007/s10499-015-9880-0 |
Allender C J, Seehausen O, Knight M E, Turner G F, Maclean N. 2003. Divergent selection during speciation of Lake Malawi cichlid fishes inferred from parallel radiations in nuptial coloration. Proceedings of the National Academy of Sciences of the United States of America, 100(24): 14 074-14 079.
DOI:10.1073/pnas.2332665100 |
Anderson J T. 1994. Feeding ecology and condition of larval and pelagic juvenile redfish Sebastes spp. Marine Ecology Progress Series, 104: 211-226.
DOI:10.3354/meps104211 |
Andersson J, Johansson F, Söderlund T. 2006. Interactions between predator-and diet-induced phenotypic changes in body shape of crucian carp. Proceedings of the Royal Society B:Biological Sciences, 273(1585): 431-437.
DOI:10.1098/rspb.2005.3343 |
Andersson M. 1994. Sexual Selection. Princeton University Press, Princeton.
|
Artamonova V S, Makhrov A A, Karabanov D P, Rolskiy A Y, Bakay Y I, Popov V I. 2013. Hybridization of beaked redfish (Sebastes mentella) with small redfish (Sebastes viviparus) and diversification of redfish (Actinopterygii:Scorpaeniformes) in the Irminger Sea. Journal of Natural History, 47(25-28): 1 791-1 801.
DOI:10.1080/00222933.2012.752539 |
Baird T A. 2008. A growth cost of experimentally induced conspicuous coloration in first-year collared lizard males. Behavioral Ecology, 19(3): 589-593.
|
Berbel-Filho W M, Martinez P A, Ramos T P A, Torres R A, Lima S M Q. 2016. Inter-and intra-basin phenotypic variation in two riverine cichlids from northeastern Brazil:potential eco-evolutionary damages of São Francisco interbasin water transfer. Hydrobiologia, 766(1): 43-56.
DOI:10.1007/s10750-015-2440-9 |
Berner D, Adams D C, Grandchamp A C, Hendry A P. 2008. Natural selection drives patterns of lake-stream divergence in stickleback foraging morphology. Journal of Evolutionary Biology, 21(6): 1 653-1 665.
DOI:10.1111/j.1420-9101.2008.01583.x |
Blouw D M, Hagen D W. 1984. The adaptive significance of dorsal spine variation in the fourspine stickleback, Apeltesquadracus. Ⅲ. Correlated traits and experimental evidence on predation. Heredity, 53(2): 371-382.
DOI:10.1038/hdy.1984.94 |
Boehlert G W, Kappenman R F. 1980. Variation of growth with latitude in two species of rockfish (Sebastes pinniger and S. diploproa) from the Northeast Pacific Ocean. Marine Ecology Progress Series, 3: 1-10.
DOI:10.3354/meps003001 |
Bohonak A J. 2004. RMA: Software for Reduced Major Axis Regression.V.1.17. San Diego State University, San Diego, California.
|
Bowen B W, Bass A L, Rocha L A, Grant W S, Robertson D R. 2001. Phylogeography of the trumpetfishes (Aulostomus):ring species complex on a global scale. Evolution, 55(5): 1 029-1 039.
DOI:10.1554/0014-3820(2001)055[1029:POTTAR]2.0.CO;2 |
Campana S E, Valentin A E, MacLellan S E, Groot J B. 2015. Image-enhanced burnt otoliths, bomb radiocarbon and the growth dynamics of redfish (Sebastes mentella and S.fasciatus) off the eastern coast of Canada. Marine and Freshwater Research, 67(7): 925-936.
|
Chen L C. 1986. Meristic Variation in Sebastes (Scorpaenidae), with an Analysis of Character Association and Bilateral Pattern and Their Significance in Species Separation.NOAA Tech. Rep. NMFS 45, NMFS, Weston, TX. p.1-17.
|
Chen Y, Jackson D A, Harvey H H. 1992. A comparison of von Bertalanffy and polynomial functions in modelling fish growth data. Canadian Journal of Fisheries and Aquatic Sciences, 49(6): 1 228-1 235.
DOI:10.1139/f92-138 |
Couldridge V C K, Alexander G J. 2002. Color patterns and species recognition in four closely related species of Lake Malawi cichlid. Behavioral Ecology, 13(1): 59-64.
|
Craig M T, Hastings P A, Pondella II D J, Ross Robertson D, Rosales-Casián J A. 2006. Phylogeography of the flag cabrilla Epinephelus labriformis (Serranidae):implications for the biogeography of the Tropical Eastern Pacific and the early stages of speciation in a marine shore fish. Journal of Biogeography, 33(6): 969-979.
DOI:10.1111/j.1365-2699.2006.01467.x |
Doebeli M, Dieckmann U. 2000. Evolutionary branching and sympatric speciation caused by different types of ecological interactions. The American Naturalist, 156(S4): S77-S101.
DOI:10.1086/303417 |
Doebeli M, Dieckmann U. 2003. Speciation along environmental gradients. Nature, 421(6920): 259-264.
DOI:10.1038/nature01274 |
Domenici P, Turesson H, Brodersen J, Brönmark C. 2007. Predator-induced morphology enhances escape locomotion in Crucian carp. Proceedings of the Royal Society B:Biological Sciences, 275(1631): 195-201.
|
Eklöv P, Svanbäck R. 2006. Predation risk influences adaptive morphological variation in fish populations. The American Naturalist, 167(3): 440-452.
DOI:10.1086/499544 |
Endler J A. 1983. Natural and sexual selection on color patterns in poeciliid fishes. Environmental Biology of Fishes, 9(2): 173-190.
DOI:10.1007/BF00690861 |
Endler J A. 1992. Signals, signal conditions, and the direction of evolution. The American Naturalist, 139: S125-S153.
DOI:10.1086/285308 |
Eschmeyer W N, Fricke R, Fong J D, Polack D A. 2010. Marine fish diversity:history of knowledge and discovery(Pisces). Zootaxa, 2525(1): 19-50.
DOI:10.11646/zootaxa.2525.1.2 |
Fang Y L, Kai Y, Yanagimoto T, Song N, Gao T X. 2015. A new record of Sebastes koreanus from China based on morphological characters and DNA barcoding. Chinese Journal of Oceanology and Limnology, 33(3): 590-596.
DOI:10.1007/s00343-015-4166-y |
Fisher R, Hogan J D. 2007. Morphological predictors of swimming speed:a case study of pre-settlement juvenile coral reef fishes. Journal of Experimental Biology, 210(14): 2 436-2 443.
DOI:10.1242/jeb.004275 |
Frable B W, Wagman D W, Frierson T N, Aguilar A, Sidlauskas B L. 2015. A new species of Sebastes (Scorpaeniformes:Sebastidae) from the northeastern Pacific, with a redescription of the blue rockfish, S. mystinus (Jordan and Gilbert, 1881). Fishery Bulletin, 113(4): 355-377.
DOI:10.7755/FB.113.4.1 |
Fujii R. 2000. The regulation of motile activity in fish chromatophores. Pigment Cell Research, 13(5): 300-319.
DOI:10.1034/j.1600-0749.2000.130502.x |
Godin J G J, McDonough H E. 2003. Predator preference for brightly colored males in the guppy:a viability cost for a sexually selected trait. Behavioral Ecology, 14(2): 194-200.
DOI:10.1093/beheco/14.2.194 |
Godin J G, Dugatkin L A. 1996. Female mating preference for bold males in the guppy, Poecilia reticulata. Proceedings of the National Academy of Sciences of the United States of America, 93(19): 10 262-10 267.
DOI:10.1073/pnas.93.19.10262 |
Grafen A. 1990. Biological signals as handicaps. Journal of theoretical biology, 144(4): 517-546.
DOI:10.1016/S0022-5193(05)80088-8 |
Grether G F, Grey R M. 1996. Novel cost of a sexually selected trait in the rubyspot damselfly Hetaerina americana:conspicuousness to prey. Behavioral Ecology, 7(4): 465-473.
DOI:10.1093/beheco/7.4.465 |
Guillemot P J, Larson R J, Lenarz W H. 1985. Seasonal cycles of fat and gonad volume in five species of northern California rockfish (Scorpaenidae:Sebastes). Fishery Bulletin, 83(3): 299-311.
|
Guthrie D M. 1986.Role of vision in fish behaviour. In: Pitcher T J ed. The Behaviour of Teleost Fishes. Springer, Boston.p.75-113.
|
Hallacher L E, Roberts D A. 1985. Differential utilization of space and food by the inshore rockfishes (Scorpaenidae:Sebastes) of Carmel Bay, California. Environmental Biology of Fishes, 12(2): 91-110.
DOI:10.1007/BF00002762 |
Han S W, Lu S X. 2003. Shandong Aquatic Products. Shandong Science and Technology Press, Jinan. (in Chinese)
|
Helvey M. 1982. First observations of courtship behavior in rockfish, genus Sebastes. Copeia, 1982(4): 763-770.
DOI:10.2307/1444084 |
Hyde J R, Kimbrell CA, Budrick J E, Lynn E A, Vetter R D. 2008. Cryptic speciation in the vermilion rockfish(Sebastes miniatus) and the role of bathymetry in the speciation process. Molecular Ecology, 17(4): 1 122-1 136.
DOI:10.1111/j.1365-294X.2007.03653.x |
Hyde J R, Vetter R D. 2007. The origin, evolution, and diversification of rockfishes of the genus Sebastes(Cuvier). Molecular Phylogenetics and Evolution, 44(2): 790-811.
DOI:10.1016/j.ympev.2006.12.026 |
Ingram T. 2011. Speciation along a depth gradient in a marine adaptive radiation. Proceedings of the Royal Society B:Biological Sciences, 278(1705): 613-618.
DOI:10.1098/rspb.2010.1127 |
Jin X. 2006. Fauna Sinica: Osteichthyes, Scorpaeniformes.Science Press, Beijing. (in Chinese)
|
Johansson M L, Banks M A, Glunt K D, Hassel-Finnegan H M, Buonaccorsi V P. 2008. Influence of habitat discontinuity, geographical distance, and oceanography on fine-scale population genetic structure of copper rockfish (Sebastes caurinus). Molecular Ecology, 17(13): 3 051-3 061.
DOI:10.1111/j.1365-294X.2008.03814.x |
Johns G C, Avise J C. 1998. Tests for ancient species flocks based on molecular phylogenetic appraisals of Sebastes rockfishes and other marine fishes. Evolution, 52(4): 1 135-1 146.
DOI:10.1111/j.1558-5646.1998.tb01840.x |
Johnstone R A. 1995. Sexual selection, honest advertisement and the handicap principle:reviewing the evidence. Biological Reviews, 70(1): 1-65.
DOI:10.1111/j.1469-185X.1995.tb01439.x |
Kadison E, D'Alessandro E K, Davis G O, Hood P B. 2010. Age, growth, and reproductive patterns of the great barracuda, Sphyraena barracuda, from the Florida Keys. Bulletin of Marine Science, 86(4): 773-784.
DOI:10.5343/bms.2009.1070 |
Kai Y, Nakabo T. 2008. Taxonomic review of the Sebastes inermis species complex (Scorpaeniformes:Scorpaenidae). Ichthyological Research, 55(3): 238-259.
DOI:10.1007/s10228-007-0029-7 |
Kai Y, Nakabo T. 2013. Taxonomic review of the Sebastes pachycephalus complex (Scorpaeniformes:Scorpaenidae). Zootaxa, 3 637(5): 541-560.
|
Karageorge K W, Wilson R R Jr. 2017. An integrative mating system assessment of a nonmodel, economically important Pacific rockfish (Sebastes melanops) reveals nonterritorial polygamy and conservation implications for a large species flock. Ecology and Evolution, 7(24): 11 277-11 291.
DOI:10.1002/ece3.3579 |
Kasai A, Oshima N. 2006. Light-sensitive motile iridophores and visual pigments in the neon tetra, Paracheirodon innesi. Zoological Science, 23(9): 815-819.
DOI:10.2108/zsj.23.815 |
Kerfoot C W, Sih A. 1987. Predation: Direct and Indirect Impacts on Aquatic Communities. University Press of New England, Hanover.
|
Kim I S, Choi Y, Lee C L, Lee Y J, Kim B Y, Kim J H. 2005.Illustrated Book of Korean Fishes. Kyo-Hak Publishing Co., Seoul, 615p.
|
Kocher T D. 2004. Adaptive evolution and explosive speciation:the cichlid fish model. Nature Reviews Genetics, 5(4): 288-298.
DOI:10.1038/nrg1316 |
LaBarbera M. 1989. Analyzing body size as a factor in ecology and evolution. Annual Review of Ecology and Systematics, 20: 97-117.
DOI:10.1146/annurev.es.20.110189.000525 |
Laidig T E, Pearson D E, Sinclair L L. 2003. Age and growth of blue rockfish (Sebastes mystinus) from central and northern California. Fishery Bulletin, 101(4): 800-808.
|
Langerhans R B, Layman C A, Shokrollahi A M, DeWitt T J. 2004. Predator-driven phenotypic diversification in Gambusia affinis. Evolution, 58(10): 2 305-2 318.
DOI:10.1111/j.0014-3820.2004.tb01605.x |
Langerhans R B. 2007. Evolutionary consequences of predation: avoidance, escape, reproduction, and diversification. In: Elewa A M T ed. Predation in Organisms: A Distinct Phenomenon. Springer, Berlin.p.177-220.
|
Langerhans R B. 2009. Trade-off between steady and unsteady swimming underlies predator-driven divergence in Gambusia affinis. Journal of Evolutionary Biology, 22(5): 1 057-1 075.
DOI:10.1111/j.1420-9101.2009.01716.x |
Larson R J. 1980. Competition, habitat selection, and the bathymetric segregation of two rockfish (Sebastes)species. Ecological Monographs, 50(2): 221-239.
DOI:10.2307/1942480 |
Leavy T R, Bonner T H. 2009. Relationships among swimming ability, current velocity association, and morphology for freshwater lotic fishes. North American Journal of Fisheries Management, 29(1): 72-83.
DOI:10.1577/M07-040.1 |
Leclercq E, Taylor J F, Migaud H. 2010. Morphological skin colour changes in teleosts. Fish and Fisheries, 11(2): 159-193.
|
Lenarz W H, Echeverria T W. 1991. Sexual dimorphism in Sebastes. In: Boehlert G W, Yamada J eds. Rockfishes of the Genus Sebastes: Their Reproduction and Early Life History. Springer, Dordrecht.p.71-80.
|
Lessios H A, Cunningham C W. 1990. Gametic incompatibility between species of the sea urchin Echinometra on the two sides of the Isthmus of Panama. Evolution, 44(4): 933-941.
DOI:10.1111/j.1558-5646.1990.tb03815.x |
Lessios H A, Kessing B D, Robertson D R. 1998. Massive gene flow across the world's most potent marine biogeographic barrier. Proceedings of the Royal Society B:Biological Sciences, 265(1396): 583-588.
DOI:10.1098/rspb.1998.0334 |
Lessios H A, Robertson D R. 2006. Crossing the impassable:genetic connections in 20 reef fishes across the eastern Pacific barrier. Proceedings of the Royal Society B:Biological Sciences, 273(1598): 2 201-2 208.
DOI:10.1098/rspb.2006.3543 |
Li C, Wang L, Qin L Z, Zhang X M. 2014. Comparison study of four species of coral-reef teleosts (Scorpaeniformes)with photoreceptor cells and the angle of minimum resolution. Journal of Fisheries of China, 38(3): 400-409.
(in Chinese with English abstract) |
Love M S, Carr M H, Haldorson L J. 1991. The ecology of substrate-associated juveniles of the genus Sebastes. Environmental Biology of Fishes, 30(1-2): 225-243.
DOI:10.1007/BF02296891 |
Love M S, Morris P, McCrae M, Collins R. 1990. Life history aspects of 19 rockfish species (Scorpaenidae: Sebastes)from the Southern California Bight. NOAA Technical Report NMFS-87, U.S. Department of Commerce, Washington. 38p.
|
Love M S, Westphal W V. 1981. Growth, reproduction, and food habits of olive rockfish, Sebastes serranoides, off central California. Fishery Bulletin, 79(3): 533-545.
|
Love M S, Yoklavich M, Thorsteinson L. 2002. The Rockfishes of the Northeast Pacific. University of California Press, Berkeley.
|
Ma D Y, Hou Y M. 2013. Basic Status Quo of Offshore Marine Environment and Resources in Shandong Province. China Ocean Press, Beijing. (in Chinese)
|
Maan M E, Sefc K M. 2013. Colour variation in cichlid fish:developmental mechanisms, selective pressures and evolutionary consequences. Seminars in Cell & Developmental Biology, 24(6-7): 516-528.
|
Macedonia J M, Brandt Y, Clark D L. 2002. Sexual dichromatism and differential conspicuousness in two populations of the common collared lizard (Crotaphytus collaris) from Utah and New Mexico, USA. Biological Journal of the Linnean Society, 77(1): 67-85.
DOI:10.1046/j.1095-8312.2002.00092.x |
Malmquist H J. 1992. Phenotype-specific feeding behaviour of two arctic charr Salvelinus alpinus morphs. Oecologia, 92(3): 354-361.
DOI:10.1007/BF00317461 |
Markevich A I. 1988. Nature of territories and homing in the eastern sea-perch, Sebastes taczanowski. Journal of Ichthyology, 28: 161-163.
|
Massare J A. 1994. Swimming capabilities of Mesozoic marine reptiles: a review. In: Maddock L, Bone Q, Rayner J. M.V eds. Mechanics and Physiology of Animal Swimming.Cambridge University Press, Cambridge. p.133-149.
|
Mills K L, Laidig T, Ralston S, Sydeman W J. 2007. Diets of top predators indicate pelagic juvenile rockfish (Sebastes spp.) abundance in the California Current System. Fisheries Oceanography, 16(3): 273-283.
DOI:10.1111/j.1365-2419.2007.00429.x |
Mora C, Sale P F. 2002. Are populations of coral reef fish open or closed?. Trends in Ecology & Evolution, 17(9): 422-428.
|
Motomura H, Ishikawa S. 2013. Fish Collection Building and Procedures Manual. The Kagoshima University Museum, Kyoto.
|
Nanami A. 2007. Juvenile swimming performance of three fish species on an exposed sandy beach in Japan. Journal of Experimental Marine Biology and Ecology, 348(1-2): 1-10.
DOI:10.1016/j.jembe.2007.02.016 |
Narum S R, Buonaccorsi V P, Kimbrell C A, Vetter R D. 2004. Genetic divergence between gopher rockfish (Sebastes carnatus) and black and yellow rockfish (Sebastes chrysomelas). Copeia, 2004(4): 926-931.
DOI:10.1643/CG-02-061R2 |
Nelson J S, Grande TC, Wilson M V H. 2016. Fishes of the World.5th edn. John Wiley & Sons, Hoboken.
|
Orr J W, Brown M A, Baker D C. 2000. Guide to rockfishes(Scorpaenidae) of the genera Sebastes, Sebastolobus, and Adelosebastes of the Northeast Pacific Ocean. 2nd edn.NOAA Tech. Memo. NMFS-AFSC-117, NMFS, Seattle, WA. 47p.
|
Orr M R, Smith T B. 1998. Ecology and speciation. Trends in Ecology & Evolution, 13(12): 502-506.
|
Oshima N, Yokozeki A. 1999. Direct control of pigment aggregation and dispersion in tilapia erythrophores by light. Zoological Science, 16(1): 51-54.
|
Palumbi S R. 1994. Genetic divergence, reproductive isolation, and marine speciation. Annual Review of Ecology and Systematics, 25: 547-572.
DOI:10.1146/annurev.es.25.110194.002555 |
Pearson D E, Hightower J E. 1990. Spatial and Temporal Variability in Growth of Widow Rockfish (Sebastes entomelas). NOAA Tech. Memo. NMFS-SWFSC-167, NOAA, Washington. 45p.
|
Peres M B, Vooren C M. 1991. Sexual development, reproductive cycle, and fecundity of the school shark Galeorhinus galeus off southern Brazil. Fishery Bulletin, 89(4): 655-667.
|
Polloni P, Haedrich R, Rowe G, Hovey Clifford C. 1979. The size-depth relationship in deep ocean animals. Internationale Revue der gesamten Hydrobiologie und Hydrographie, 64(1): 39-46.
DOI:10.1002/iroh.19790640103 |
Puebla O. 2009. Ecological speciation in marine v. freshwater fishes. Journal of Fish Biology, 75(5): 960-996.
DOI:10.1111/j.1095-8649.2009.02358.x |
Rayner J M V. 1985. Linear relations in biomechanics:the statistics of scaling functions. Journal of Zoology, 206(3): 415-439.
|
Ringler N H. 1983. Variation in foraging tactics of fishes. In: Noakes D L G, Lindquist D G, Helfman G S, Ward J Aeds.Predators and Prey in Fishes. Springer, Dordrecht. p.159-171.
|
Roberts D A. 1979. Food Habits as an Ecological Partitioning Mechanism in the Nearshore Rockfishes (Sebastes) of Carmel Bay, California. San Francisco State University, San Francisco.
|
Rocha L A, Robertson D R, Roman J, Bowen B W. 2005. Ecological speciation in tropical reef fishes. Proceedings of the Royal Society B:Biological Sciences, 272(1563): 573-579.
DOI:10.1098/2004.3005 |
Roques S, Sévigny J M, Bernatchez L. 2001. Evidence for broadscale introgressive hybridization between two redfish (genus Sebastes) in the North-west Atlantic:a rare marine example. Molecular Ecology, 10(1): 149-165.
DOI:10.1046/j.1365-294X.2001.01195.x |
Rundle H D, Nosil P. 2005. Ecological speciation. Ecology Letters, 8(3): 336-352.
DOI:10.1111/j.1461-0248.2004.00715.x |
Ruzzante D E, Walde S J, Cussac V E, Macchi P J, Alonso M F, Battini M. 2003. Resource polymorphism in a Patagonian fish Percichthys trucha (Percichthyidae):phenotypic evidence for interlake pattern variation. Biological Journal of the Linnean Society, 78(4): 497-515.
DOI:10.1046/j.0024-4066.2002.00159.x |
Ruzzante D E, Walde S J, Macchi P J, Alonso M, Barriga J P. 2011. Phylogeography and phenotypic diversification in the Patagonian fish Percichthys trucha:the roles of Quaternary glacial cycles and natural selection. Biological Journal of the Linnean Society, 103(2): 514-529.
DOI:10.1111/j.1095-8312.2011.01682.x |
Schluter D. 2001. Ecology and the origin of species. Trends in Ecology & Evolution, 16(7): 372-380.
|
Seehausen O, Schluter D. 2004. Male-male competition and nuptial-colour displacement as a diversifying force in Lake Victoria cichlid fishes. Proceedings of the Royal Society B:Biological Sciences, 271(1546): 1 345-1 353.
DOI:10.1098/rspb.2004.2737 |
Shanks A L. 2009. Pelagic larval duration and dispersal distance revisited. The Biological Bulletin, 216(3): 373-385.
DOI:10.1086/BBLv216n3p373 |
Sharpe D M T, Räsänen K, Berner D, Hendry A P. 2008. Genetic and environmental contributions to the morphology of lake and stream stickleback:implications for gene flow and reproductive isolation. Evolutionary Ecology Research, 10: 849-866.
|
Shinomiya A, Ezaki O. 1991. Mating habits of the rockfish Sebastes inermis. Environmental Biology of Fishes, 30(1-2): 15-22.
DOI:10.1007/BF02296872 |
Six L D, Horton H F. 1977. Analysis of age determination methods for yellowtail rockfish, canary rockfish, and black rockfish off Oregon. Fishery Bulletin, 75(2): 405-414.
|
Sköld H N, Amundsen T, Svensson P A, Mayer I, Bjelvenmark J, Forsgren E. 2008. Hormonal regulation of female nuptial coloration in a fish. Hormones and Behavior, 54(4): 549-556.
DOI:10.1016/j.yhbeh.2008.05.018 |
Stevens M, Párraga C A, Cuthill I C, Partridge J C, Troscianko T S. 2007. Using digital photography to study animal coloration. Biological Journal of the Linnean Society, 90(2): 211-237.
DOI:10.1111/j.1095-8312.2007.00725.x |
Tang Q S, Ye M Z. 1990. Exploitation and Protection of Fishery Resources in Shandong Coastal Waters. China Agriculture Press, Beijing. (in Chinese)
|
Tuset V M, Otero-Ferrer J L, Gómez-Zurita J, Venerus L A, Stransky C, Imondi R, Orlov A M, Ye Z, Santschi L, Afanasiev P K, Zhuang L, Farré M, Love M S, Lombarte A. 2016. Otolith shape lends support to the sensory drive hypothesis in rockfishes. Journal of Evolutionary Biology, 29(10): 2 083-2 097.
DOI:10.1111/jeb.12932 |
Wañkowski J W J. 1979. Morphological limitations, prey size selectivity, and growth response of juvenile Atlantic salmon, Salmo salar. Journal of Fish Biology, 14(1): 89-100.
|
Yasir I, Qin J G. 2009. Effect of light intensity on color performance of false clownfish, Amphiprion ocellaris Cuvier. Journal of the World Aquaculture Society, 40(3): 337-350.
DOI:10.1111/j.1749-7345.2009.00254.x |
Yu H J, Im Y J, Jo H S, Lee S J, Kim JK. 2015. Morphological development of eggs, larvae, and juvenile of Sebastes koreanus (Scorpaeniformes:Scorpaenidae) from the Yellow Sea. Ichthyological Research, 62(4): 439-449.
DOI:10.1007/s10228-015-0457-8 |
Yu H J, Kim J K. 2014. New record of Sebastes nudus and redescription of Sebastes pachycephalus (Pisces:Scorpaenidae) from Korea. Fisheries and Aquatic Sciences, 17(1): 129-136.
DOI:10.5657/FAS.2014.0129 |
 2020, Vol. 38
2020, Vol. 38