Institute of Oceanology, Chinese Academy of Sciences
Article Information
- IBRAHIM G., ELGHAZALY M.
- Molecular characterization of yolk proteins in the female crab Neptunus pelagicus (A. Milne-Edwards, 1861) from the Mediterranean Sea of Alexandria, Egypt
- Journal of Oceanology and Limnology, 38(2): 438-453
- http://dx.doi.org/10.1007/s00343-019-9145-2
Article History
- Received May. 24, 2019
- accepted in principle Jul. 8, 2019
- accepted for publication Jul. 16, 2019
2 Department of Zoology, Faculty of Science, Damnhour University, El Behara 22516, Egypt
Crabs colonize a wide variety of aquatic environments, but some species make almost the entire cycle outside the water. In the tropics, there are marine, freshwater and land crabs (Anger, 1995). The crab grows through molting and can lay thousands of eggs that attach themselves under the abdomen until hatching (Abdu et al., 2002). In the tropics, the vast majority of crab species presents prolonged or continuous reproductive periods throughout the year, with higher incidence in winter and summer (Antunes et al., 2016). Reproductive period in crustaceans is marked by a series of modifications, including differentiation, growth, gametogenesis, and reproductive behavior associated with mating, ovulation, and development of the larvae until juveniles (Cobo and Fransozo, 2005; Becker et al., 2011). Among the brachyuran crabs, there is a perfect relationship between the morphological, pubertal molting and the onset of gametogenesis. Depending on the analysis of morphometric characteristics, (Choy, 1988) concluded that the carapace and abdominal widths for both sexes indicate the stage of gonadal maturity. The colors of the gonads may have different variations according to maturity (Palacios et al., 2003). Ovaries of crab are located dorsally underneath the hepatopancreas and extended on both sides of the cephalothorax. The ovaries continued down towards the cardiac stomach in a posteromedial course. They are attached by a median connection at the level of the medial stomach (Zinski, 2006; Zhijun et al., 2011). The ovaries showed several morphology stages during their annual cycles. The immature ovary resembled a small filament at both distal regions of the oviducts where the seminal receptacles lie. The seminal receptacles were found laterally between the stomach and heart (Vehof et al., 2017). The seminal receptacles varied in size and color depending on the stages of ovarian maturity (Vallina et al., 2014). The ovaries changed from the light, small form of the immature phase to the orange and large of the mature phase (Swiney and Shirley, 2001).
Vitellogenesis is a vital process for the reproduction of crustaceans and oviparous invertebrates (Engelman, 1979; Wallace, 1985; Kung et al., 2004). The complexity of molecules involved in vitellogenesis is not well covered in the available literature. A great controversy stated among authors regarding the origin of vitelloginin (VN), the precursor of VL in different species of invertebrates. Authors propose that there was an exogenous synthesis of yolk precursor protein in the hepatopancreas and in adipose tissue and an endogenous synthesis in the ovary in various species of marine crustaceans (Tseng et al., 2001; Mak et al., 2005; Ibrahim, 2018). Some studies supported the theory that VN entered the ovary through endocytosis (Kung et al., 2004; Girish et al., 2014; Wang et al., 2015). Likewise, molecular studies pointed out that the hepatopancreas was the organ that synthesizes the largest amount of VN in comparison with the ovary (Tsutsui et al., 2004; Puengyam et al., 2013). However, the role of the ovary in the synthesis of VN is not covered in detail at molecular level. Results of modern studies in penaeid shrimp suggest that the presence of multiple genes that encode VN (Zmora et al., 2007). However, it has not yet been possible to explain the mechanisms developed by these molecules from their synthesis until their accumulation in the oocyte (Qiu, 1991). There is a conflict of opinion regarding where vitelloginin is synthesized; in the hepatopancreas or in the adipose tissue or in both tissues and is there a single gene or if the mRNA is expressed in the mentioned tissues equally or if this expression varied during the cycle of ovarian development (Browdy et al., 1990; Mak et al., 2005). In studies of DNA of the ovary and hemolymph in penaeid, Abdu et al. (2002) suggested that the VN of the hemolymph and VL of the ovary were products of the same gene. However, Antunes et al. (2016) concluded that in the green tiger prawn Penaeus semisulcatus there were two genes involved in expression of VN, the VN gene Ⅰ, which was expressed in both ovary and hepatopancreas and the VN gene Ⅱ that only expressed in the hepatopancreas. The VN was a specific protein occurred in the hemolymph of the females and transported to the oocytes where it was incorporated and transformed into VL. This VL was the main protein in the oocytes and was consumed as an important source of nutrition during embryogenesis and larval development (Lee and Puppione, 1988, Chang and Jeng, 1995).
In this study, the authors investigate the molecular characterization of yolk proteins in the female crab Neptunus pelagicus, identify vitelloginin (VN) and vitelline (VL), and protein with PAGE-SDS; study the mRNA that encodes the C-terminal region of VN cDNA; identify the role of the hepatopancreas and hemolymph during vitellogenesis.
2 MATERIAL AND METHOD 2.1 Samples collection and preparationsThe blue crab Neptunus pelagicus were collected from Ras El-Tin beach of the Mediterranean Sea of Alexandria monthly during 2017. Ovaries and oocytes in primary and secondary vitellogenesis were detached from specimens and treated for histological examination (Ibrahim, 2015; Sharifian et al., 2015). Ovaries were fixed in alcoholic Bouin liquid, processed by routine histological method. Ovarian sections of 5–8 μm were stained with Hematoxylin and Eosin, Weigert-Van Gieson stain, and trichrome method (Bisen, 2014). The histological organization of the ovary and the characteristics of follicular development according to growth, vitellogenesis, atresia, and ovulation were commented. The different stages of ovarian maturity were determined under the classification of (Hard, 1942; Rotllant et al., 2007; Ibrahim, 2015; Sharifian et al., 2015). Three stages of oocyte development were chosen for this study. Oocytes were sampled at stage Ⅰ (yellow color), in stage Ⅲ (dark yellow colony) and in stage Ⅴ (orange color). Collection of the hemolymph performed at the base of the pereiopods then mixed (1.10 v/v) with 10% EDTA, 0.10 mmol/L sulfonyl phenyl methyl urea (PMSF), and centrifuged to 2 000 r/min for 15 min to 4℃.
2.2 Identification, purification, and characterization of VN and VLs 2.2.1 Native PAGE Bis-Tris GelsHomogenization of the ovaries and oocytes was carried out in a Potter- Elvehjem glass with a Tris buffer 0.05 mol/L, 0.10 mol/L NaCl, 5 mmol/L EDTA, pH 7.0 (3.1 v/v, cushion/fabric) and a proteases inhibitory cocktail solution (0.3 μmol/L, Bestatin, 1 mmol/L, E-64, 2 mmol/L Aprotinin, 130 μmol/L, AEBSF, EDTA, 14 μmol/L, Leupeptin, 1 μmol/L 0.003%, BioRad®). Preparations were centrifuged to 10 000 r/min by 15 min to 4℃. The supernatant was obtained and stored at -20℃ until later analysis. The content of the soluble extracted proteins in the total samples were quantified following the method of Abdu et al. (2002), using a standard curve of BSA (10–50 μg). Identification and characterization of VLs of the hemolymph, of the ovary and oocytes was done. In order to identify the specific fractions of yolk proteins in primary and secondary vitellogenesis, the polyacrylamide gel electrophoresis (PAGE) with extracts homogenates of hemolymph, ovaries and the oocytes under conditions native polyacrylamide gels going to 6% in presence of shock absorber 1.5 mol/L Tris-glycine (pH 8.7). This technique is used to separate the molecules in a matrix (agarose or polyacrylamide) according to its electrical charge. Polyacrylamide gels measures in gradient 4%–20% were used to determine the molecular mass of the protein fractions corresponding to the VLs. The amount of Trans used in each sample was 1 μg. The collective eluent Corresponding to the peak at which the presence of the VLs of the oocytes extract in the stage Ⅴ (1 mL) in the column of Sepharose CL-2B, was fraction tail in a column of hydroxylapatite for the separation molecules according to their loads. It balanced with shock absorber 2.5 mmol/L PMSF with 0.1 mol/L potassium phosphate (PPB) (pH 7.5). The elution was performed with 0.35 mol/L cushion Potassium Phosphate (PPB) (pH 7.5) in a gradient of conc. input of PPB 0.01 mol/L, 0.10 mol/L, 0.20 mol/L, and 0.35 mol/L at flow of 0.35 mL/min. The eluent was collective in fraction 2.50 mL, and the absorbency was measured at 270 nm. The fractions were conc. entries in tubes Sartorius-Centrisart-C4, and the presence of the purified protein was determined by native PAGE electrophoresis (6%).
2.2.2 Protein analyses 2.2.2.1 Polyacrylamide gel electrophoresis with SDSThe analysis of the proteins that make up the crystal of the native VN or VLs was analyzed by means of discontinuous electrophoresis in denaturing gels of polyacrylamide with sodium dodecyl sulphate tricine (SDS-PAGE), according to the technique described by (Abdu et al., 2002). It was used for concentration and resolution gels, 4% acrylamide and 10% respectively. From lyophilized sample containing the complex amino acids crystals of the standard and native protein molecules were prepared and the samples to be loaded in the gel, at a concentration of 2 μg/μL, which were mixed 1:1 with buffer 2.89 mmol/L β-mercaptoethanol, 25 mmol/L TrisHCl pH 6.8, SDS 138 mol/L; glycerol 2.17 mol/L and boiled for 5 min. The electrophoretic run was performed with a Tris-glycine buffer and SDS (glycine 0.38 mol/L, SDS 0.1%, pH 8.8, Tris-Base 0.05 mol/L) at constant voltage, using first 50 V×40 min and then doubled the voltage until the marker dye reached the end of the plate. After the electrophoresis the gel was fixed in a 50% methanol solution and 15% acetic acid for 45 min then Coomassie brilliant blue R-250 was applied, 0.025% (in 40% methanol, 9% acetic acid) for 20 min. Then it proceeded to its discoloration in a solution of 5% ethanol and acetic acid 7.5% until visualizing protein bands.
2.2.2.2 Analysis of REP PCR band patternsThe patterns of REP-PCR bands obtained were identified by their specific migration speeds in the gel. Binary matrices were built (0/1) to be compared with the patterns; the presence of a band was indicated by one (1) and the absence as zero (0). Once the matrix was obtained binary, a matrix of genetic distances used to build a dendrogram with the UPGMA algorithm (grouping method of unweighted pairs with arithmetic significance) (Bambeck, 1996). The cluster analysis was evaluated in the dendrogram generated by the UPGMA algorithm and represented with the dendrogram of similarity of standard (n=10) and native (n=53) vitellogenin or VLs of amino acids, estimated with the REP-PCR band patterns, using the NTSYS program (Garfin, 1990; Jeannot et al., 1999).
2.3 Agarose gel electrophoresisThe amplified products were subjected to separation by the 1-agarose gel electrophoresis method; 1.5% and 2%, prepared in a TBE X buffer solution, containing 0.1% ethidium bromide to visualize the bands using UV illumination with a photodocument, or a transilluminator, electrophoresis was performed at a voltage constant from 80 to 100 V for 1 to 2 h, using solution TBE X buffer as run buffer (Sheer et al., 1990).
2.3.1 PrimersThe genomic and plasmid DNA extracted from each sample were applied as a template in the PCR reaction and were amplified using universal primers for each group of genes. With the genomic DNA the amplification of few genes was observed. The initial degenerate primers were built regarding the conserved amino acid domains TR: A0A2K6GU91_PROCO and TR:A0A2K6FJD7_PROCO of the yolk proteins of the Chinese mitten crab Eriocheir sinensis (GenBank accession Nos. 379532 NCBI379532, DQ23748, AY284351, and UP000233160). An amplicon of 700 bp generated from hepatopancreas cDNA by PCR using the degenerate primers VitpF1 (5′-MGCCGCGGCS SGCGGGCGGC SSGCGGGCGG GCGGCSSCRS YRVGCCSSCC-3′) and VitpR1 (5′-PCCCGCCGGC CSTPVICCCR RSCGSCGCGG GCGKGCCQQK CCCQKQCCC-3′). A 50-RACE fragment was amplified using reverse transcriptionpolymerase chain reaction (RT-PCR) (5′-MGCCGCGGCG GCGSGCGSGC GGGCGGG-CGG GCGGGCGGGC GGGCGGGCSS-3′) and the primer from a 50-RACE hepatopancreas cDNA gene bank, and analyzed (Abdu et al., 2002; Tsutsui et al., 2004).
2.3.2 Determination of the expression site of mRNA from VNOvaries and hepatopancreas samples were extracted from females in secondary vitellogenesis in first maturity phase, of consecutive spawning and ovigerous females. The total DNA was extracted following the methodology of Abdu et al. (2002). They were homogenized with 500 μL of extracted damper (0.6% SDS, 100 mmol/L EDTA pH 8.0, 100 mmol/L NaCl, 10 mmol/L Tris HCl, pH 8.0). They were added to 20 mg/mL proteinase K to the samples. They were left incubating at room temperature for 1 h. They were added to 10 mg/mL RNase and incubated 30 min at room temperature. The samples were centrifuged at 18 000×g for 2 min at lab temperature and passed to a clean tube. The extraction was performed with phenol / chloroform adding a volume equivalent (v/v) of a saturated phenol solution. It was stirred 10 min manually being in a soft way until a complete emulsion was obtained. It was centrifuged at 18 r/min for 2 min at lab temperature. The upper phase was taken. A second extraction was made with saturated phenol, passing the superior part to a clean tube. The two extractions were carried out on phenol/chloroform (v/v) centrifuged to 17 949 r/min for 2 min at lab temperature, passing part of the superior to a clean tube (Poms et al., 2001).
2.3.3 DNA precipitationThe mixture was prepared with 1 volume of samples, 2 volumes of absolute alcohol and 0.1 volume of 3 mol/L sodium acetate (1/10). The samples were left to 20℃ all night. Samples centrifuged and escaped to 17 949 r/min for 15 min lab temperature. The alcohol residue was immediately eliminated with a pipette with taking care not to discard the A button. It was dried 15 min at ambient temperature. It was suspended again on water or TE 1X buffer Tris-HCl for 10 mmol/L, pH 7.4, 1 mmol/L EDTA, pH 8.0) (Avarre et al., 2003).
2.3.4 DNA quantificationA dilution of 1:250 was prepared, and the absorbance was read in spectrophotometer an OD of 260 nm. The formula was applied. OD 260× dilution factor×50=μg DNA/mL PCR. The PCR method was used for the selective amplification of a region chosen within a molecule of DNA. It allowed copying in vitro of DNA and CDNA. The genomic DNA prepared from the ovaries and hemolymph of females in secondary vitellogenesis was amplified by PCR and sequenced. Oligonucleotides were designed. These primers from the cDNA of the VN of the hepatopancreas of females were designed based on in the 3′ region. The amplification was initiated with the pair of oligonucleotides (Vg F-5′GTG CGT CGC CTA CTG GAACA 3′ and Vg R-5′CTT GGC GGA ATA CTC GGA CTG 3′).
The program consisted of 3 steps. denaturation to 94℃ for 2 min, and 35 cycles of 94℃ for 1 min, 45℃ for 1 min and 72℃ for 3 min. The final elongation was made to 72℃ for 10 min. The PCR reaction was performed in a thermal cycler in a volume of 50 μL using 0.50 U of Pfu DNA polymerase), 10× shock absorber Pfu DNA, 20.50 nmol dNTP, 25.50 pmol each primer, 2.5 μL of DMSO and 550 ng of genomic DNA as a mold. The products of PCR were observed by Electrophoresis in 1% agarose gels in TAE 1× buffer (Tris-acetate 0.04 mol/L, 0.002 mol/L EDTA). The band was cut and placed in a tube clean for further purification of DNA with Gene Clean Ⅱ Kit (Hotzel et al., 1999).
2.3.5 Preparation of the cDNA by RT-PCRTotal RNA isolated from hemolymph and hepatoppancreas of females in secondary vitellogenesis at first maturity, consecutive spawning and of ovigerous females were subjected to reverse transcription to synthesize the cDNA. In the tubes of micro-centrifuge of 500 μL the mixture was added. Tube 1; 1–2 μg of RNA, 250 ng of Random Primer (0.5 μL), 2 μL of RT 10× buffer, 2 μL of dNTP's 5 mmol/L, 0.25 μL of RNase inhibitor 10 U/μL, and 2 μL of RT 20 U/μL, gauge with H two Or DEPC to 20 μL. Tube 2 (negative control); 1–2 μg of RNA, 250 ng of Random Primer (0.5 μL), 2 μL of RT buffer 10×, 2 μL of 5 mmol/L dNTP's, 0.25 μL of RNase 10 inhibitor U/μL, gauge with H two Or DEPC to 20 μL. It was incubated 60 min 37℃. The cDNA obtained by RT is amplified by the chain reaction of the polymerase (PCR). Northern Blot method allowed the detection of RNA, which was transferred from the gel to a support solid (usually nylon membranes or nitrocellulose), where it was immobilized (covalently linked). Gel electrophoresis was performed of denatured 0.8% agarose with guanidine thiocyanate, with 30 μg of total ovarian RNA and radioactively (α 32 P) dATP obtained from the genomic DNA of female hepatopancreas in secondary vitellogenesis at the 5′ end, to allow both to join. The labeled probe (1.1 Kb) corresponded to nucleotides 6 574–6 770 of the hepatopancreas VN cDNA sequence. Hybridization was performed overnight at 42℃. The membrane was fixed with UV rays for 3 min. The hybridizing solution was removed and different washes of the membrane were carried out; twice in 10 mL of solution 2×SSC, 0.1% SDS for 10 min each washed. Washed twice in 10 mL of 0.5×SSC solutions, 0.1% SDS for 10 min each wash, and once in 10 mL of 0.1×Sodium Citrate/Sodium Chloride (SSC) solutions, 0.1% SDS for 10 min at room temperature, to phase out e probe attached with low specificity, remaining only double chains with high degree of homology (100%). All chemical reagents used were purchased from BioRad® (Okuno et al., 1999).
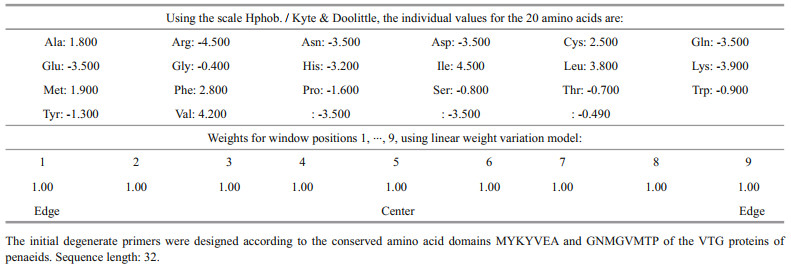
3 RESULT 3.1 ReproductionThe blue crab Neptunus pelagicus exerted a high potential reproductively since this species is always available in huge quantities in fish markets around the year. Females with a weight between 70 and 100 g reached maturity during June until October in the Mediterranean Sea. Ovaries were lobulated and showed different phases of maturation. Primary vitellogenesis consisted of 4 phases. In stage Ⅰ, chromatin stage, the ovary was occupied with oogonia and previtellogenic oocytes. The oogonia were with a mean diameter of 15–25 μm, they were the first cells of the germ line. Generally, they formed nests in the central region of the ovary, these cells had spherical conformation, scarce ooplasm and conspicuous nucleus, with condensed chromatin concentrated in the nucleolar region at the earliest stage and in Stage Ⅱ a perinucleolar chromatin appeared indicating a highly basophilic character (Fig. 1a & b). The ooplasm presented little accumulation of protein, showed poor reaction to the dye, blushing in purple. At this stage, Oogonia were accompanied by a cluster of cubic follicular cells (Fig. 1c). Previtellogenic oocytes possessed a basophia in the ooplasm with oocyte diameter of 30–45 μm. A layer of follicle cells surrounded the oocytes (Fig. 1d). Stage Ⅲ it represented an early perinuclear stage in which the oocyte diameter 70–100 μm. This stage of oocytes was found in the central region of the germinal epithelium. Oval in shape, highly basophilic and ooplasm more developed than the previous stage, little vitellogenic activity was observed. Cubic follicular cells seemed to surround the cells. The nucleus showed more condensed chromatin than the stage previously described (Fig. 1e). In stage Ⅳ, Perinuclear phase, the oocyte (170–290 μm) had a layer of flattened follicle cells and eosinophilic yolk bodies filled the ooplasm (Fig. 1f). The second phase of vitellogenesis was in stage Ⅴ, lipid stage, where deposition of lipoprotein took place (acidophilus character). This depositions proceeded in the vitellogenic oocyte, being more evident in the peripheral region. Pre-cubic follicular cells became slightly flattened and surrounded the whole cell. The core of basophilic character is found in the central region and nucleolus lateralized to the nucleus (Fig. 1g). In stage Ⅵ, vitellogenesis processed, the oocytes had an average diameter of 350–400 μm (Fig. 1h). Stage Ⅶ featured larger volume than the previously described stage, the oocytes being ready to be released and fertilized. They had a polyhedral shape and slightly rounded edges (Fig. 1i). Stage Ⅷ, atretic oocytes, seemed to be matured, but they were not released yet. Without a well-defined format, these cells passed through the process of resorption performed by the ovary. Strongly stained in rose by Hematoxylin and eosin, these oocytes were highly proteinic, surrounded by follicle cells that were also arranged and made up almost the entire ovary. It was possible to observe greater space between the cells, being filled by follicular cells, forming a post ovulatory follicle (Fig. 1j).
|
| Fig.1 Cross sections in ovaries of Neptunus pelagicus a–b. the ovary was occupied with oogonia and previtellogenic oocytes. The oogonia formed nests in the central region of the ovary, with scarce ooplasm and conspicuous nucleus, with condensed perinucleolar chromatin; c. little accumulation of protein in the ooplasm, oogonia were accompanied by a cluster of cubic follicular cells; d. previtellogenic oocytes possessed a basophilic ooplasm and a layer of follicle cells surrounded the oocytes; e. the nucleus showed more condensed chromatin than the previous stage; f. a layer of flattened follicle cells surrounded the oocyte; eosinophilic yolk bodies filled the ooplasm; g. a large deposition of lipoprotein composing the peripheral ooplasm, the pre-cubic follicular cells become slightly flattened and surrounded the whole cell; h. complete vitellogenesis processed; i. the oocyts had a polyhedral shape, in response to cell slightly rounded edges; j. atretic oocytes without well-defined formats, these cells passed through the process of resorption performed by the ovary; k–l. the ovary had, in its composition, non-germinative elements, hemolymph cells, gonadal wall, and seminal receptacles. o: oogonia; po: previtellogenic oocyte; op: ooplasm; n: nucleus; nu: nulcleolus; ct: connective tissue; pc: perinucleolar chromatin; fc: follicular cell; vo: vitellogenic oocyte; yb: yolk bodies; l: lipoprotein; ao: atretic oocyte; hc: hemolymph cell; gw: gonadal wall; sr: seminal receptacle. |
The ovary had in its composition non-germinative elements. They were follicular cells, hemolymph cells, gonadal wall, oviducts, and seminal receptacles (Fig. 1k & l). Follicular cells were found in the entire germinal region of the ovary. They were characterized by little visible cytoplasm and highly basophilic nucleus. The vitellogenic and mature oocytes were surrounded by flattened shape follicle cells. Hemolymph cells filled the stoma and the intensity of their coloring was related to the stage of ovarian development. Gonadal wall of the ovaries were represented with a fibrous connective tissue. Oviduct had a large muscle extension, probably to aid the entry of spermatophores and exit of fertilized eggs.
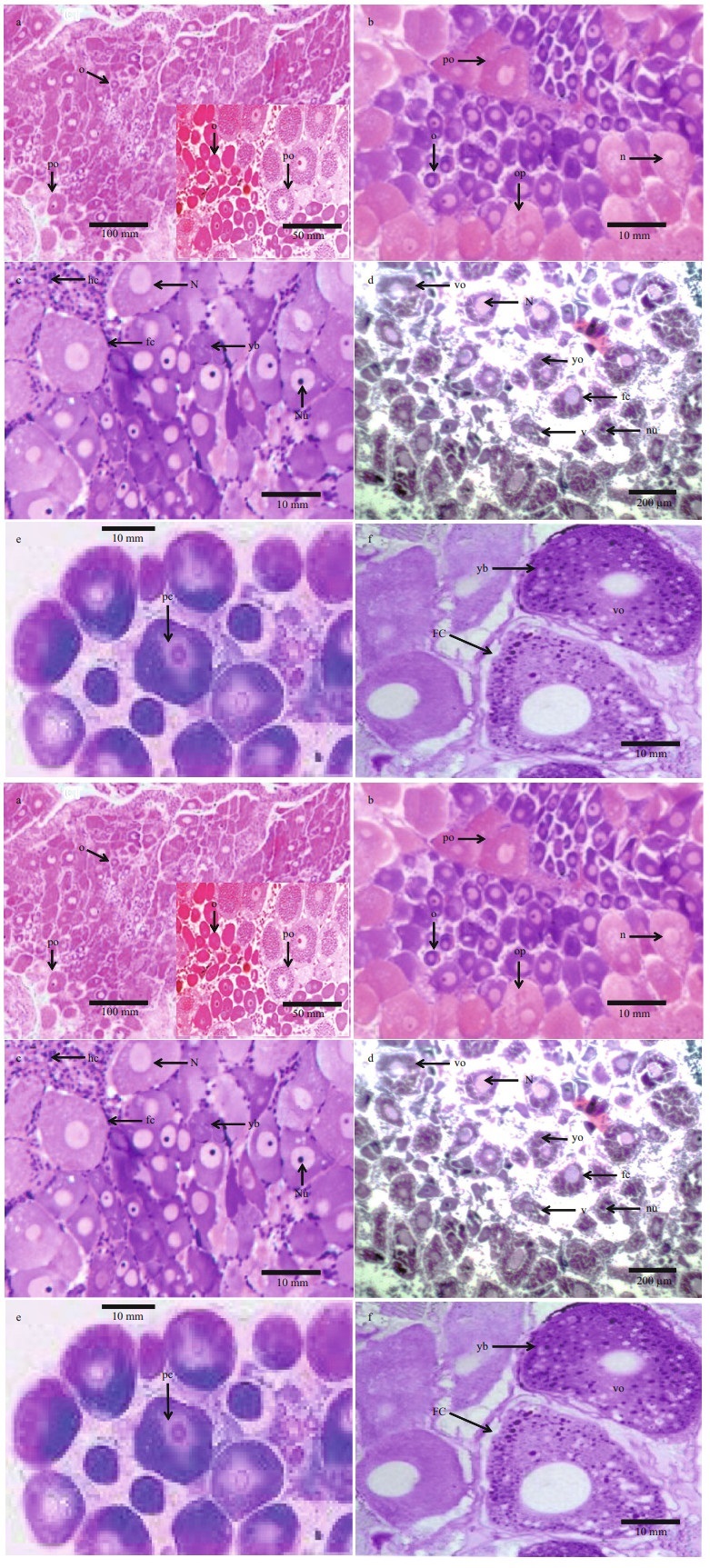
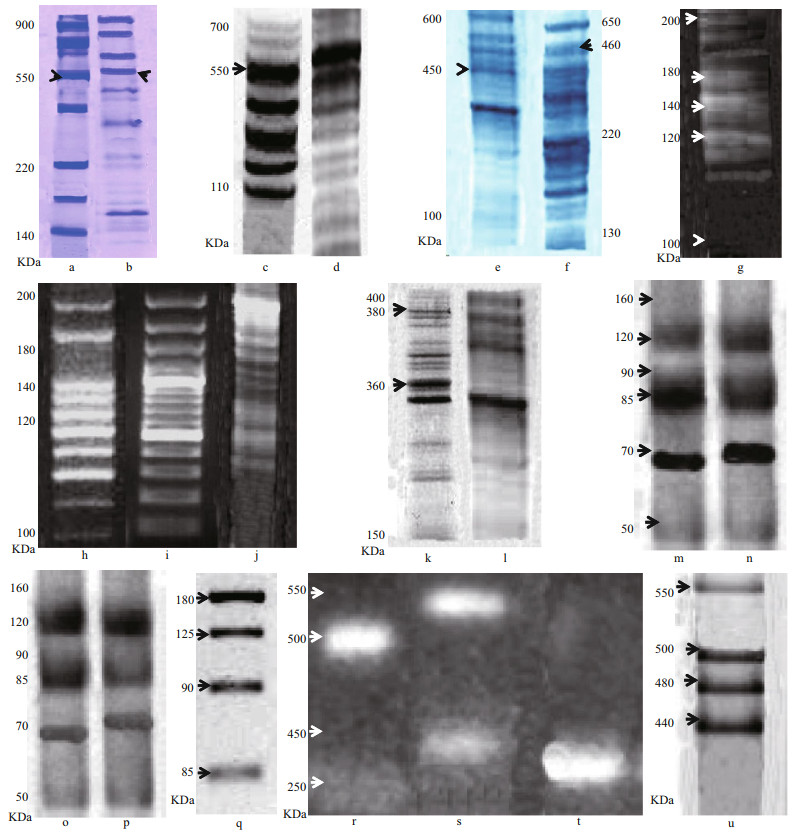
3.3 Vitelloginin and vitelline moleculesLipoprotein fraction was found in the hemolymph of females in secondary vitellogenesis revealed with Coomassie blue stain and Sudan black (Fig. 2a–d). This fraction was not found in the hemolymph of previtellogenic females. The molecular weight of this fraction, obtained by gradient electrophoresis (4%– 20%), was 550 kDa. Two protein fractions were identified (VLI and VLII) in secondary vitellogenic oocytes. These molecules were not present in immature ovary. The electrophoresis performed with extract of stage Ⅰ oocyte, showed two protein fractions presented a molecular weight of 550 kDa (VLI) and 460 kDa (VLII) (Fig. 2e & f). In stage Ⅱ and Ⅲ, oocyte 4 subunits were presented of 180, 195, 140 and 120 kDa in VLI and 2 subunits with molecular weight of 110 and 95 kDa in VLII (Fig. 2g–j). Another two fractions in stage Ⅴ oocyte presented a molecular weight of 380 kDa and 360 kDa (Fig. 2k & l). The lipid and glucidic nature in both tissues was determined. However, the ovary presented only a fraction of glycoprotein. The chromatogram of oocyte extract or in stage Ⅴ showed two peaks (Fig. 3a & b), electrophoretic analysis PAGE (6%) made of the two peaks in presence of the three dyes, revealed two lipoglycoproteins (VLI and VLII) in peak Ⅱ. The fractions corresponding to the peak Ⅱ were separated in a hydroxylapate adsorption column and showed seven peaks. The fractions corresponding to each peak were collected and analyzed with PAGE (6%) (1.6 μg PT) (Fig. 2m–p), confirming by the different dyes the presence of two lipo-glycoproteins in the peak 1 and 4, corroborating with this, the results of Fig. 2a, b, c, and d. The molecular masses the two Vts (VLI and VLII) separated by electrophoresis preparations in the ovary and in three stages of oocyte development in stages Ⅰ, Ⅲ and Ⅴ, analyzed by SDS-PAGE were shown in Fig. 2e & f. The VLI and VLII of the oocytes in stage Ⅴ separated by the Hydroxylapatite column (peak 1 & 4) and analyzed by SDS-PAGE (10 μg of PT) showed four subunits with molecular masses of 120, 90, 85 and 70 kDa in each of the Vt. (Fig. 2m–p). To confirm the subunits of each VL, the Western blot analysis was performed in the presence of the two antivitelline antibodies (Ac1 and Ac2) showing that both were molecules consisted of four major polypeptide subunits withmolecular masses of 180, 125, 90, and 85 kDa in each of the two VLs and seven subunits with similar secondary effects in both VLs (Fig. 2q), confirming in this way the specificity of antivithelin antibodies. The chromatogram of the extract of fractionated ovary in a column SepharoseCl-2B showed three peaks. The fractions corresponding to the peaks were collected and analyzed with PAGE-native (6%), identifying by different colors before the Vts in the peak C. The fractions corresponding to peak C of the SepharoseCL-2B column, were subsequently separated into a co-ion exchange lumens (DEAE-Sepharose-CL-6B), where the presence of different peaks.
|
| Fig.2 Illustrating vitelloginin and vitelline molecules of yolk globules a–d. PAGE-native electrophoresis of lipoprotein fraction found in the hemolymph of females in secondary vitellogenesis. This fraction was not found in the hemolymph of previtellogenic oocyte. The molecular weight of this fraction was 550 kDa; e–f. PAGE electrophoresis showing two protein fractions in secondary vitellogenesis. These molecules were absent in previtelogénesis. In stage Ⅰ oocyte two protein fractions presented a molecular weight of 550 kDa (VLI) and 460 kDa (VLII); g–j. PAGE electrophoresis in stage Ⅱ and Ⅲ oocyte, 4 subunits presented of 180, 195, 140 and 120 kDa in VLI and 2 subunits with molecular weight of 110 kDa and 95 kDa in VLII. Another two fractions in stage Ⅴ oocyte presented a molecular weight of 380 kDa and 360 kDa; k–l. the ovary presented only a fraction of glycoprotein. The chromatogram of oocyte extract or in stage Ⅴ showed two peaks. The fractions corresponding to each peak were analyzed with PAGE (6%) (1.6 μg PT), confirming the presence of two lipo-glycoproteins in the peak 6; m–p. SDS-PAGE, the VLI and VLII of the oocytes in stage Ⅴ showed four subunits with molecular masses of 120, 90, 85, and 70 kDa in each of the Vts. Western blot analysis showed that both were molecules composed of four major polypeptide subunits (molecular masses of 180, 125, 90 and 85 kDa in each of the two VLs and seven subunits with similar secondary effects in both VLs. The chromatogram of the extract of fractionated ovary in a column Sepharose-Cl-2B showed three peaks. q. the fragment of 1.1 Kb obtained from genomic DNA amplified by PCR was used as a probe to hybridize in the Northern blot analysis. This fragment was aligned with the sec nucleotide influence of Vl; r–t. the hybridization signal obtained by the Northern blot was found simultaneously in the hepatopancreas and in the ovary in females in secondary vitellogenesis of the first maturity. The result of the RT-PCR analysis showed that the mRNA that encodes the C-terminal region of the VN cDNA was present in the hepatopancreas and in the ovary in secondary vitellogenesis of the first maturity; u. the ovarian mRNA was expressed only in the early maturity, not expressed even in females with consecutive spawning or in ovigerous females. |
|
| Fig.3 Chromatogram of oocyte extract or in stage Ⅴ showed two peaks (a, b); fragment of 1.1 Kb obtained from genomic DNA amplified by PCR was used as a probe to hybridize in the Northern blot analysis (c) |
The fractions corresponding to the AG peaks in the ion exchange chromatography (DEAE-SepharoseCL-6B), were collected and analyzed with PAGE (6%), confirming with staining of Coomassie blue the presence of the two VL molecules in fractions E, F, and G. The fractions corresponding to peaks AG of the primers column of ion exchange (DEAESepharose-CL-6B) were separated in another column of ion exchange with buffer (B) 500 mmol/L (Table 1). This partial sequence was localized within the amino acid sequence deduced and comparable to the DNA of the VN of Chinese mitten crab Eriocheir sinensis (Chinese mitten crab) in the classification of lipid binding protein in the gene bank. According to PFAM Domain Annotation, the protein model the gene was Q5EC70 Q5EC70_ERISI Unreviewed; 159 AA. Vitellogenin {ECO:0000313|EMBL:AAX07169.1}; 1LSH; Flags: Fragment; Eriocheir sinensis (Chinese mitten crab). It was also found in Australian red claw crayfish Cherax quadricarinatus (Australian red claw crayfish) in lipid-protein interactions in lipovitellin, Method: X-RAY; diffraction resolution: 1.9 Å; R-Value Free: 0.255; R-Value Work: 0.193; wwPDB Validation 3D Report Full Report (PubMed: 12135361).
The result of the partial sequence of amino acids of the 120 subunit kDa obtained in the VL one of the ovary by electrophorasis denaturing SDS-PAGE (7.5%). 1.1 Kb PCR product was obtained from the genomic DNA used as a template. This was comparable to the nucleotide sequence of the 3′ region of the VN cDNA of blue crab Callinectes sapidus (blue crab). The gene was M4GKW8 M4GKW8_ CALSI Unreviewed; 2 564 AA. Vitellin {ECO:0000313|EMBL:AEI59132.1}; blue crab Callinectes sapidus; 4D4Z; PubMed: 12135361.
The fragment of 1.1 Kb obtained from genomic DNA amplified by PCR was used as a probe to hybridize in the Northern blot analysis (Fig. 3c). This fragment was aligned with the sec nucleotide influence of Vl. It was comparable to cDNAof Methanocaldococcus jannaschii (strain ATCC 43067/ DSM 2661 (AF306784), obtaining an identity of 96% in DNA-Binding Protein: Template PDB ID: 1ku9A; Sequence identity: 26%; Target region: 8–120; classification: DNA binding protein; Method: X-RAY diffraction; Resolution: 2.8 Å; R-Value Free: 0.280; R-Value Work: 0.241; wwPDB Validation 3D Report Full Report (PubMed: 306784).
The hybridization signal obtained by the Northern blot was detected in the hepatopancreas and in the ovary in females in secondary vitellogenesis of the first maturity. This analysis showed that the probe hybridized with an approximate fragment 8 kb of mRNA in both tissues (Fig. 2p). The result of the RT-PCR analysis showed that the mRNA that encodes the C-terminal region of the VN cDNA was found in the ovary in secondary vitellogenesis and in the hepatopancreas of the first maturity (Fig. 2r–t). The bands revealed in approx. in 850 bp in both tissues. The same analysis was done with samples of ovary and hepatopancreas of females in secondary vitellogenesis of the first maturation, of consecutive spawns of vigorous females, showing the expression of the mRNA of the hepatopancreas in the three different stages of development, however, the ovarian mRNA was expressed only in the early maturity, not expressed even in females with consecutive spawning or in ovigerous females, as shown in (Fig. 2u).
4 DISCUSSIONThroughout the development of female brachyurans, the coloration of the ovary is altered; this modification can be used in the macroscopic classification of the maturational stages of the species (Ando and Makioka, 1999). Females classified based on the mature and exhausted gonadal stage presented the same staining pattern in the outer structures. This classification system was applied to the reproductive aspects of the swamp ghost crab Ucides cordatus in mangroves (Flávia et al., 2011); the Japanese blue crab Portunus trituberculatus (Hamasaki et al., 2006) and the giant freshwater prawn Macrobrachium rosenbergii (Okuno et al., 2002). In recent years, it has been observed that macroscopy and microscopy are methodologies quite functional and wellpublicized for the determination of the reproductive aspects of crustaceans, especially for species of economic importance living in marine environments and estuaries. Several studies have used macroscopic analysis (Hard, 1942; Ando and Makioka, 1999; Rotllant et al., 2007; Flávia et al., 2011). In the view of (Lautenschlager et al., 2010), the macroscopic classification of the gonads, in color and size, is closely related to the development and organization of cells that make up the ovary.
Most brachyurans are gonochoric with internal fertilization. The fertilized eggs remain attached to the female pleopods. The abdomen is then taken off from cephalothorax and provides a protective space for oviposition (de Souza et al., 2013; Ewers-Saucedo et al., 2015). The eggs are brooded for a time that varies by species and then hatch at the protozoate or zoea; there is a variable number of stages zoea according to the species (de Souza and Silva, 2009; Cobo and Fransozo, 2005). After the last zoea stage, the crab goes through a last larval stage, also pelagic, the megalope; its morphology is intermediate between the zoea form and the crab shape. After a certain period, this larva migrates towards the substrate where it performs its last larval molt, which leads to the 1st crab stage (Ewers-Saucedo et al., 2015). This study concluded that the translucent ovary in immature female is reduced to a filamentous structure difficult to observe with the naked eye. In mature female, the two anterior horns of the ovary extend into the cephalic region along the lateral-frontal expansions of hepatopancreas. The two posterior horns fit on both sides of the cardiac plexus. These four lobes unite behind the gastric cavity forming a central bridge. Ovaries pass to the ventral position in the cardiac region. Authors added that the two short oviducts, to which are annexed the two spermatheca open to the outside by the vulvae located on the sternum of the sixth thoracic segment. The different phases of oocyte development were commented in this study. In CS, gonads of a mature female present, from the periphery to the center of the ovary a germinal zone, of mesodermic origin, place of oogenesis of primary oocytes. In a more internal intermediate zone there are pre-vitellogenesis and secondary oocytes accompanied by a few follicular cells. The establishment of this envelop characterize the primary folliculogenesis. The formation of the vitellus divided into two distinct phases referred to as primary and secondary vitellogeneses. These finding were described as "yolk 1" and "yolk 2" in hermit crab Coenobita clypeatus; the fresh-water crab Candidiopotamon rathbunae and in the southeastern Alaskan Dungeness crab Cancer magister (Komm and Hinsch, 1987; Liu and Li, 2000; Swiney and Shirley, 2001). The phase "yolk 1" seemed, according to these authors, to correspond to an accumulation of yolk of endogenous origin characterizing the establishment of the primary vitellogenesis of the present study. The second phase, "yolk 2", corresponds to the installation of secondary vitellogenesis and yolk deposition of exogenous origin. This second step would also be accompanied by further establishment of nutritive materials and drainage by intense cytochemical pinocytosis (Mak et al., 2005). vitellogenin was highlighted in many publications dealt with the contribution from the hepatopancreasspecific vitellogenin gene (MeVg2) in the sand shrimp Metapenaeus ensis (Kung et al., 2004); the hepatopancreas-specific expression and farnesoic acid stimulation of vitellogenin gene expression in the red crab Charybdis feriatus (Mak et al., 2005) and the vitellogenin cDNA of Australian red claw crayfish Cherax quadricarinatus encoded a lipoprotein with calcium binding ability, and its expression is induced following the removal of the androgenic gland in a sexually plastic system (Abdu et al., 2002; Luo et al., 2008). The synthesis of vitellogenin would be controlled by an ovarian hormone (Okuno et al., 1999). The entry of this nutritive protein of exogenous origin by pinocytosis is conditioned by the architectural modification of the surface of the oocyte itself and modified by secondary folliculogenesis (Sal Moyano et al., 2010). This last phase allows the establishment of a permanent follicular tissue around each primary oocyte that will be released during the laying.
The results obtained in this study showed that in phases Ⅰ and Ⅴ oocytes and in ovarian tissues of secondary vitelogenesis the presence of two vitelline fractions in each of the tissues. These observations agreed with the criteria used in the identification of VLs in crustaceans. The nature of lipo-glico-protein molecules of the yolk globules; the proteins in the ovaries were associated with ovarian maturity, since these molecules were absent in previtellogenic ovaries. The electrophoregram of the oocytes presented two low proteins with electrophoretic mobility under native condition. These indicated that they were VLs because they were the most abundant materials in the oocyte. Authors analyzed the possible functions of Proteins, lipids and carbohydrates in the mature oocyte and concluded that proteins were the most abundant elements used as a metabolic fuel, but the lipids were usually the main pro-energetics (first of all from the combustion of triglycerides) (Lee and Puppione, 1988; Sheer et al., 1990; Chang and Jeng, 1995; Jeannot et al., 1999). In this study, the molecular weight obtained in the two VLs of the ovary by electrophoresis in native gradients conditions (4%– 20%) was 480 kDa (VLI) and 450 kDa (VLII two). This finding agreed with the results obtained by (Yang et al., 2000, Okuno et al., 2002) in the giant freshwater prawn M. rosenbergii, who identified three VLs with molecular weight 780, 450 and 220 kDa; while in other Crustacea one or two VLs were identified in ovaries of vitellogenic females with molecular weight that ranged from 300 to 530 kDa, depending on the species and the methodology used (Wallace, 1985; Tufail and Takeda, 2002; Zinski, 2006; Yu et al., 2007; Zmora et al., 2007).
The results of SD electrophoresis S-PAGE of the VLs in the ovary and oocytes showed the dynamics of molecules through vitellogenesis. The differences between the molecular masses s of the VLs in the ovaries and oocytes in this study indicated, that the VLs of the ovary were transformed proteolytically in new products, which, in turn, can join other free amino acid molecules already present in the oocyte. Authors concluded that the VLs join other molecules in the oocyte forming molecular masses of greater size of those found in the ovary (Demeusy, 1962; Wallace, 1985). In the giant freshwater prawn M. rosenbergii three Vts were identified, one of them presented two subunits 104 and 90 kDa polypeptides (Okuno et al., 2002). On the other hand, the purification of the Vts of ovary by chromatography in ion exchange column (DEAE-Sepharose-CL-6B), fractionated by SDS-PAGE showed 4 subunits of Vts (80, 95, 120 and 180 kDa), confirming the results obtained with the electrophoresis of ovarian preparations. Similar results were found in Australian red claw crayfish Cherax quadricarinatus and the green tiger prawn P. semisulcatus (Browdy et al., 1990; Abdu et al., 2002) who found subunits ranged from 78; 103; 150, and 177 kDa in pre-ovulatory ovaries and ovaries in secondary vitellogenesis. It is important to mention that the partial sequence of amino acids of the 75 kDa subunit of Vt one present in eggs in stage Ⅴ, is a part of the deducted sequence of amino acids from the cDNA of the VL of hepatopancreas of vitellogenic females (Abdu et al., 2002). Different and controversial studies have tried to clarify the site of synthesis of VN in crustaceans, and this synthesis varies depending on the species, stage of vitellogenesis and period of molting (Browdy et al., 1990; Abdu et al., 2002; Kung et al., 2004). The presence of mRNA suggests the expression of the precursor gene of yolk, and is the most reliable criterion to detect and quantify the synthesis of VN. It is the first study reported in two different analyzes, RT-PCR and Northern blot that showed the expression of mRNA detected simultaneously in the ovary and in the hepatopancreas of females in vitellogenesis (Abdu et al., 2002).
The results obtained in the analysis Northern blot, indicated the presence of mRNA in the ovary and in the hepatopancreas of females in secondary vitellogenesis. The analysis by RT-PCR showed the expression of mRNA in both tissues. These results are consistent between the VN of hepatopancreas and hemolymph with the Vts of the ovary and oocytes (Avarre et al., 2003). The discrepancy between our results and those obtained by (Abdu et al., 2002; Supriya et al., 2017) worked with Australian red claw crayfish Cherax quadricarinatus and the female fiddler crab, Uca triangularis respectively may be due to maturation status of females. This author found expression of VN only in the hepatopancreas of females in secondary vitellogenesis, while in our research, the expression of VN was found in different stages of maturity. Females in secondary vitellogenesis, females in secondary vitellogenesis with consecutive spawning and in the ovary, detecting mRNA expression that encodes VN in the hepatopancreas in the three different stages of ripening. Therefore, there is a possibility that only during in secondary vitellogenesis the ovary participates in the synthesis of the VN, while the hepatopancreas synthesize it first in the vitellogenic cycle. These results showed that not only the hepatopancreas synthesizes VN, also the ovary plays a very important role in the production of the main yolk protein. Yin and Clive (1992) and Peerapong and Prapaporn (2013) pointed out that the levels in the expression of mRNA in the hepatopancreas and in the ovary of the banana shrimp Fenneropenaeus merguiensis are variables during ovarian development. Other investigations reported the expression of the mRNA of the VN in both tissues (Yu et al., 2007; Guan et al., 2016). There was a controversial observation that claimed that the hepatopancreas but not ovary is the site of vitellogenin synthesis in female fresh water crab Oziothelphusa senex senex (Girish et al., 2014). In this study, the size of the mRNA of VN obtained from the hepatopancreas and the ovary by analyzing the Northern blot, it was approximately 7 kb in both tissues. These results agreed with the structure of the cDNA of the VN (7 944 nt) of C. quadricarinatus reported by Abdu et al. (2002). Vasquez-Boucard et al. (1986), Opplt (1999), and Puengyam et al. (2013) found through the Northern blot analysis, about the same size of the VN mRNA in the hepatopancreas and in the ovary in vitellogenic females of the banana prawn Fenneropenaeus merguiensis and the Kuruma shrimp Penaeus japonicus, indicating that the synthesis of VN is done in both tissues according to ovarian maturation. For its part, Avarre et al. (2003) worked with Penaeus semisulcatus, they detected by Northern blot analysis a transcript of VN of approx. 7.8 kb both in the ovary as in the hepatopancreas of vitellogenic females but not in the heart, muscle, or male hepatopancreas.
The differences found in the deduced amino acid sequences obtained in this study, allows suggesting the presence of more than one gene involved in the synthesis of VN, because the sequence of the ovary, however that presents a percentage of identity greater than 50% compared with the sequence deducted amino acid residues of the hepatopancreas. The results obtained by Abdu et al. (2002) concluded that these affect the region 3' of the gene and not the entire gene, so the presence of more of a gene in the synthesis of the VN in the freshwater lobster C. quadricarinatus. Despite this, there were several studies carried out in other crustaceans that suggest the presence of various genes encode VN. Guan et al. (2016) suggested the presence of multiple genes that encode to VN synthesis in the red swamp crayfish Procambarus clarkii. Likewise, Kung et al. (2004) working with the mud prawn Metapenaeus ensis pointed out the existence of at least two genes that encode VN.
5 CONCLUSIONThe formation of the yolk protein is divided into two distinct phases referred to as primary and secondary vitellogeneses. In phases Ⅰ and Ⅴ oocytes and in ovarian tissues of secondary vitellogenesis, two vitelline fractions were identified. The electrophoregram of the oocytes presented two low proteins with electrophoretic mobility under native condition. The molecular weight obtained in the two VLs of the ovary by electrophoresis in native gradients conditions (4%–20%) was 480 kDa (VLI) and 450 kDa (VLII two). The results of SD electrophoresis S-PAGE of the VLs in the ovary and oocytes showed the dynamics of molecules through vitellogenesis. The differences between the molecular masses of the VLs in the ovaries and oocytes in this study indicated, that the VLs of the ovary were transformed proteolytically in new products, which, in turn, can join other free amino acid molecules already present in the oocyte. The hybridization signal obtained by the Northern blot was found simultaneously in the hepatopancreas and in the ovary in females in secondary vitellogenesis. This study proved that vitelloginin is synthesized in the hepatopancreas during the vitellogenic cycle and transported through the hemolymph to enter the ovary through pinocytosis. Other vitelloginin is synthesized directly inside the ovary during secondary vitellogeneses. These vitelloginin molecules were matured to vitelline molecules inside the ovary.
6 DATA AVAILABILITY STATEMENTThe datasets generated and/or analyzed during the current study are available in the Thompson and Banaszak, 2002 repository (http.//www.expasy.org). It was found in the yolk proteins of the Chinese mitten crab Eriocheir sinensis (GenBank accession Nos. 379532 NCBI379532, DQ23748, AY284351, and UP000233160) and Cherax quadricarinatus (Australian red claw crayfish) in lipid-protein interactions in lipovitellin, 3D Report Full Report (PubMed: 12135361). Data that support the nucleotide sequence of this study have been deposited in the VN cDNA of Callinectes sapidus (blue crab). The gene was M4GKW8 M4GKW8_CALSI Unreviewed; 2 564 AA. Vitellin {ECO:0000313|EMBL: AEI59132.1} and blue crab Callinectes sapidus; 4D4Z; PubMed: 12135361. The fragment of 1.1 Kb obtained from genomic DNA in this study are available in cDNA of Methanocaldococcus jannaschii (strain ATCC 43067/DSM 2661 (AF306784), obtaining an identity of 96% in DNA-Binding Protein (https://sourceforge.net/projects/sibsim4/): Method: X-RAY diffraction; Resolution: 2.8 Å; R-Value Free: 0.280; R-Value Work: 0.241; wwPDB Validation 3D Report Full Report (PubMed: 306784).
Abdu U, Davis C, Khalaila I, Sagi A. 2002. The vitellogenin cDNA of Cherax quadricarinatus encodes a lipoprotein with calcium binding ability, and its expression is induced following the removal of the androgenic gland in a sexually plastic system. General and Comparative Endocrinology, 127(3): 263-272.
DOI:10.1016/S0016-6480(02)00053-9 |
Ando H, Makioka T. 1999. Structure of the ovary and mode of oogenesis in a freshwater crab Potamon dehaani. Journal of Morphology, 239(1): 107-114.
DOI:10.1002/(SICI)1097-4687(199901)239:1<107::AID-JMOR8>3.0.CO;2-J |
Anger K. 1995. The conquest of freshwater and land by marine crabs:adaptations in life history patterns and larval bioenergetics. Journal of Experimental Marine Biology and Ecology, 193(1-2): 119-145.
DOI:10.1016/0022-0981(95)00114-X |
Antunes M, Zara FJ, López-Greco LS, Negreiros-Fransozo ML. 2016. Morphological analysis of the female reproductive system of Stenorhynchus seticornis(Brachyura:Inachoididae) and comparisons with other Majoidea. Invertebrate Biology, 135(2): 75-86.
DOI:10.1111/ivb.12118 |
Avarre J C, Michelis R, Tietz A, Lubzens E. 2003. Relationship between vitellogenin and vitellin in a marine shrimp(Penaeus semisulcatus) and molecular characterization of vitellogenin complementary DNAs. Biology of Reproduction, 69(1): 355-364.
DOI:10.1095/biolreprod.102.011627 |
Bambeck G S. 1996. Electrophoresis separation gel and method for preparing an electrophoresis separation gel.US Patent No. 5589104.
|
Becker C, Brandis D, Storch V. 2011. Morphology of the female reproductive system of European pea crabs(Crustacea, Decapoda, Brachyura, Pinnotheridae). Journal of Morphology, 272(1): 12-26.
DOI:10.1002/jmor.10884 |
Bisen P S. 2014. Microbial staining. In: Bisen P S ed. Microbes in Practice. IK International, New Delhi, p.139-155.
|
Browdy C L, Fainzilber M, Tom M, Loya Y, Lubzens E. 1990. Vitellin synthesis in relation to oogenesis in in vitroincubated ovaries of Penaeus semisulcatus (Crustacea, Decapoda, Penaeidae). Journal of Experimental Zoology, 255(2): 205-215.
DOI:10.1002/jez.1402550209 |
Chang C F, Jeng S R. 1995. Isolation and characterization of the female-specific protein (vitellogenin) in mature female hemolymph of the prawn Penaeus chinensis. Comparative Biochemistry and Physiology Part B:Biochemistry and Molecular Biology, 112(2): 257-263.
DOI:10.1016/0305-0491(95)00059-3 |
Choy S. 1988. Reproductive biology of Liocarcinus puber and L. holsatus (Decapoda, Brachyura, Portunidae) from the Gower Peninsula, South Wales. Marine Ecology, 9(3): 227-241.
DOI:10.1111/j.1439-0485.1988.tb00330.x |
Cobo V J, Fransozo A. 2005. Physiological maturity and relationships of growth and reproduction in the red mangrove crab Goniopsis cruentata (Latreille)(Brachyura, Grapsidae) on the coast of São Paulo, Brazil. Revista Brasileira de Zoologia, 22(1): 219-223.
DOI:10.1590/S0101-81752005000100027 |
de Souza L P, Silva J R F. 2009. Morphology of the female reproductive system of the red-clawed mangrove tree crab (Goniopsis cruentata Latreille, 1803). Scientia Marina, 73(3): 527-539.
DOI:10.3989/scimar.2009.73n3527 |
de Souza L P, Silva J R F, Araujo A M, Camargo-Mathias M I. 2013. Morphology of the female genital ducts of the blue land crab Cardisoma guanhumi (Crustacea:Brachyura:Gecarcinidae). Acta Zoologica, 94(3): 300-307.
DOI:10.1111/j.1463-6395.2011.00556.x |
Demeusy N. 1962. Rôle de la glande de mue dans l'évolution ovarience du crabe Carcinus maenas Linné. Cahiers de Biologie Marine, 3: 37-56.
|
Engelman F. 1979. Insect vitellogenin:identification, biosynthesis, and role in vitellogenesis. Advances in Insect Physiology, 14: 49-108.
DOI:10.1016/S0065-2806(08)60051-X |
Ewers-Saucedo C, Hayer S, Brandis D. 2015. Functional morphology of the copulatory system of box crabs with long second gonopods (Calappidae, Eubrachyura, Decapoda, Crustacea). Journal of Morphology, 276(1): 77-89.
DOI:10.1002/jmor.20322 |
Flávia S D, Tania M S C, Assunta M P. 2011. Morphology and histology of the female reproductive system of the mangrove land crab Ucides cordatus (Linnaeus, 1763)(Brachyura. Ocypodidae). Nauplius, 19: 145-153.
DOI:10.1590/S0104-64972011000200006 |
Garfin D E. 1990. One-dimensional gel electrophoresis. Methods in Enzymology, 182: 425-441.
DOI:10.1016/0076-6879(90)82035-Z |
Girish B P, Swetha C H, Reddy S P. 2014. Hepatopancreas but not ovary is the site of vitellogenin synthesis in female fresh water crab, Oziothelphusa senex senex. Biochemical and Biophysical Research Communications, 447(2): 323-327.
DOI:10.1016/j.bbrc.2014.03.148 |
Guan Z B, Yin J, Chen K, Shui Y, Cai Y J, Liao X R. 2016. The hepatopancreas and ovary are the sites of vitellogenin synthesis in female red swamp crayfish (Procambarus clarkii (Girard, 1852)) (Decapoda:Astacoidea:Cambaridae). Journal of Crustacean Biology, 36(5): 637-641.
DOI:10.1163/1937240X-00002459 |
Hamasaki K, Fukunaga K, Kitada S. 2006. Batch fecundity of the swimming crab Portunus trituberculatus (Brachyura:Portunidae). Aquaculture, 253(3-4): 359-365.
|
Hard W L. 1942. Ovarian Growth and Ovulation in the Mature Blue Crab, Callinectes sapidus Rathbun. Chesapeake Biological Laboratory, Solomons Island, MD. p.1-17.
|
Hotzel H, Müller W, Sachse K. 1999. Recovery and characterization of residual DNA from beer as a prerequisite for the detection of genetically modified ingredients. European Food Research and Technology, 209(3-4): 192-196.
DOI:10.1007/s002170050478 |
Ibrahim G A. 2015. Oogenesis in the cancer crab Parthenope longimanus. Journal of Advances in Biology, 6(3): 1 036-1 065.
|
Ibrahim G A. 2018. Oogenesis of the carpet sea squirt Didemnum vestitum (Kott, 2004) (Ascidiacea-Aplousobranchia) in the Arabian Gulf-ultrastructural profile. International Journal of Oceans and Oceanography, 12(2): 173-190.
|
Jeannot M A, Zheng J, Li L. 1999. Observation of sodium gelinduced protein modifications in dodecyl sulfate polyacrilamide gel electrophoresis and its implications for accurate molecular weight determination of gel-separated proteins by matrix-assisted laser desorption ionisation time-of-flight mass spectrometry. Journal of the American Society for Mass Spectrometry, 10(6): 512-520.
DOI:10.1016/S1044-0305(99)00022-7 |
Komm B S, Hinsch G W. 1987. Oogenesis in the terrestrial hermit crab, Coenobita clypeatus (Decapoda, Anomura):Ⅱ. Vitellogenesis. Journal of Morphology, 192(3): 269-277.
DOI:10.1002/jmor.1051920309 |
Kung S Y, Chan S M, Hui J H L, Tsang W S, Mak A, He J G. 2004. Vitellogenesis in the sand shrimp, Metapenaeus ensis:the contribution from the hepatopancreas-specific vitellogenin gene (MeVg2). Biology of Reproduction, 71(3): 863-870.
DOI:10.1095/biolreprod.103.022905 |
Lautenschlager A D, Brandis D, Storch V. 2010. Morphology and function of the reproductive system of representatives of the genus Uca. Journal of Morphology, 271(11): 1 281-1 299.
DOI:10.1002/jmor.10869 |
Lee R F, Puppione D L. 1988. Lipoproteins Ⅰ and Ⅱ from the hemolymph of the blue crab Callinectes sapidus:lipoprotein Ⅱ associated with vitellogenesis. Journal of Experimental Zoology, 248(3): 278-289.
DOI:10.1002/jez.1402480306 |
Liu H C, Li C W. 2000. Reproduction in the fresh-water crab Candidiopotamon rathbunae (Brachyura:Potamidae) in Taiwan. Journal of Crustacean Biology, 20(1): 89-99.
DOI:10.1163/20021975-99990019 |
Luo W, Zhao Y L, Zhou Z L, An C G, Ma Q. 2008. Digestive enzyme activity and mRNA level of trypsin in embryonic redclaw crayfish, Cherax quadricarnatus. Chinese Journal of Oceanology and Limnology, 26(1): 62-68.
DOI:10.1007/s00343-008-0062-z |
Mak A S C, Choi C L, Tiu S H K, Hui J H L, He J G, Tobe S S, Chan S M. 2005. Vitellogenesis in the red crab Charybdis feriatus:hepatopancreas-specific expression and farnesoic acid stimulation of vitellogenin gene expression. Molecular Reproduction and Development, 70(3): 288-300.
DOI:10.1002/mrd.20213 |
Okuno A, Hasegawa Y, Ohira T, Katakura Y, Nagasawa H. 1999. Characterization and cDNA cloning of androgenic gland hormone of the terrestrial isopod Armadillidium vulgare. Biochemical and Biophysical Research Communications, 264(2): 419-423.
DOI:10.1006/bbrc.1999.1522 |
Okuno A, Yang W J, Jayasanka V, Saido-Sakanaka H, Huong D T T, Jasmani S, Atmomarsono M, Subramoniam T, Tsutsui N, Ohira T, Kawazoe I, Aida K, Wilder M N. 2002. Deduced primary structure of vitellogenin in the giant freshwater prawn, Macrobrachium rosenbergii, and yolk processing during ovarian maturation. Journal of Experimental Zoology, 292(5): 417-429.
DOI:10.1002/jez.10083 |
Opplt J J. 1999. Discontinuous and nonsequential polymeric gel systems for separation of macromolecules. US Patent, No. 5968332.
|
Palacios E, Racotta I S, Vallalejo M. 2003. Assessment of ovarian development and its relation to mating in wild and pond-reared Litopenaeus vannamei shrimp in a commercial hatchery. Journal of the World Aquaculture Society, 34(4): 466-477.
DOI:10.1111/j.1749-7345.2003.tb00085.x |
Poms R, Glössl J, Foissy H. 2001. Increased sensitivity for detection of specific target DNA in milk by concentration in milk fat. European Food Research Technology, 213(4-5): 361-365.
DOI:10.1007/s002170100383 |
Puengyam P, Tsukimura B, Utarabhand P. 2013. Molecular characterization of hepatopancreas vitellogenin and its expression during the ovarian development by in situ hybridization in the banana shrimp Fenneropenaeus merguiensis. Journal of Crustacean Biology, 33(2): 265-274.
DOI:10.1163/1937240X-00002116 |
Qiu C. 1991. The cytochemistry of oocytes of Chinese shrimp Penaeus orientalis. Chinese Journal of Oceanology and Limnology, 9(2): 106-114.
DOI:10.1007/BF02850669 |
Rotllant G, González-Gurriarán E, Fernández L, Benhalima K, Ribes E. 2007. Ovarian maturation of the multi-spawning spider crab Maja brachydactyla (Decapoda:Majidae)with special reference to yolk formation. , Marine Biology, 152(2): 383-394.
DOI:10.1007/s00227-007-0688-y |
Sal Moyano M P, Gavio M A, Cuartas E I. 2010. Morphology and function of the reproductive tract of the spider crab Libinia spinosa (Crustacea, Brachyura, Majoidea):pattern of sperm storage. Helgoland Marine Research, 64(3): 213-221.
DOI:10.1007/s10152-009-0180-9 |
Sharifian S, Kamrani E, Safaie M, Sharifian S. 2015. Oogenesis and ovarian development in the freshwater Crab Sodhiana iranica (Decapoda:Gecarcinuaidae) from the south of Iran. Tissue and Cell, 47(2): 213-220.
DOI:10.1016/j.tice.2014.11.006 |
Sheer D G, Yamane D K, Hawke D H, Yuan P M. 1990. The use of micropreparative electrophoresis of protein/peptide isolations for primary structure determinations. Peptide Research, 3(2): 97-104.
|
Supriya N T, Sudha K, Krishnakumar V, Anilkumar G. 2017. Molt and reproduction enhancement together with hemolymph ecdysteroid elevation under eyestalk ablation in the female fiddler crab, Uca triangularis (Brachyura:Decapoda). Chinese Journal of Oceanology and Limnology, 35(3): 645-657.
DOI:10.1007/s00343-017-5337-9 |
Swiney K M, Shirley T C. 2001. Gonad development of southeastern Alaskan dungeness crab, Cancer magister, under laboratory conditions. Journal of Crustacean Biology, 21(4): 897-904.
DOI:10.1163/20021975-99990181 |
Tseng D Y, Chen Y N, Kou G H, Lo C F, Kuo C M. 2001. Hepatopancreas is the extraovarian site of vitellogenin synthesis in black tiger shrimp, Penaeus monodon. Comparative Biochemistry and Physiology Part A:Molecular & Integrative Physiology, 129(4): 909-917.
DOI:10.1016/S1095-6433(01)00355-5 |
Tsutsui N, Saido-Sakanaka H, Yang W J, Jayasankar V, Jasmani S, Okuno A, Ohira T, Okumura T, Aida K, Wilder M N. 2004. Molecular characterization of a cDNA encoding vitellogenin in the coonstriped shrimp, Pandalus hypsinotus and site of vitellogenin mRNA expression. Journal of Experimental Zoology Part A:Comparative Experimental Biology, 301(10): 802-814.
DOI:10.1002/jez.a.53 |
Tufail M, Takeda M. 2002. Vitellogenin of the cockroach, Leucophaea maderae:nucleotide sequence, structure and analysis of processing in the fat body and oocytes. Insect Biochemistry and Molecular Biology, 32(11): 1 469-1 476.
DOI:10.1016/S0965-1748(02)00067-X |
Vallina M, Sal Moyano M P, Cuartas E I, Gavio M A. 2014. Reproductive system and size maturity of the paddle crab Ovalipes trimaculatus (Brachyura:Portunidae) along the Argentine coast. Journal of Crustacean Biology, 34(3): 357-366.
DOI:10.1163/1937240X-00002239 |
Vasquez-Boucard C, Ceccaldi H J, Benyamin Y, Roustan C. 1986. Identification, purification, et caractérisation de la lipovitelline chez un crustacé décapode Natantia Penaeus japonicus (Bate). Journal of Experimental Marine Biology and Ecology, 97(1): 37-50.
DOI:10.1016/0022-0981(86)90066-3 |
Vehof J, Scholtz G, Becker C. 2017. Morphology of the female reproductive system of three dorippid crabs (Crustacea:Decapoda:Brachyura:Dorippidae) and the role of accessory cuticle structures associated with seminal receptacles. Invertebrate Biology, 136(3): 271-289.
DOI:10.1111/ivb.12181 |
Wallace R A. 1985. Vitellogenesis and oocyte growth in nonmammalian vertebrates. In: Browder L W ed.Oogenesis. Developmental Biology (A Comprehensive Synthesis). Springer, Boston, MA, 1: 127-177, https://doi.org/10.1007/978-1-4615-6814-8_3.
|
Wang Y Y, Sun H S, Wang Y J, Yan D C, Wang L. 2015. Cytochemical characterization of yolk granule acid phosphatase during early development of the oyster Crassostrea gigas (Thunberg). Chinese Journal of Oceanology and Limnology, 33(2): 339-346.
DOI:10.1007/s00343-015-3297-5 |
Yang W J, Ohira T, Tsutsui N, Subramoniam T, Huong D T T, Aida K, Wilder M N. 2000. Determination of amino acid sequence and site of mRNA expression of four vitellins in the giant freshwater prawn, Macrobrachium rosenbergii. Journal of Experimental Zoology, 287(6): 413-422.
DOI:10.1002/1097-010X(20001101)287:6<413::AID-JEZ2>3.0.CO;2-V |
Yin M C, Craik J C A. 1992. Biochemical changes during development of eggs and yolk-sac larvae of herring and plaice. Chinese Journal of Oceanology and Limnology, 10(4): 347-358.
DOI:10.1007/BF02843836 |
Yu Z Y, Wu X G, Chang G L, Cheng Y X, Liu Z J, Yang X Z. 2007. Changes in the main biochemical composition in ovaries and hepatopancreas of Chinese mitten crab, Eriocheir sinensis (H. Milne-Edwards) during the second ovarian development. Acta Hydrobiologica Sinica, 31(6): 799-806.
(in Chinese with English abstract) |
Zinski S. 2006. The Blue Crab Archives. Available at https://www.bluecrab.info/taxonomy.html.
|
Zmora N, Trant J, Chan S M, Chung J S. 2007. Vitellogenin and its messenger RNA during ovarian development in the female blue crab, Callinectes sapidus:gene expression, synthesis, transport, and cleavage. Biology of Reproduction, 77(1): 138-146.
DOI:10.1095/biolreprod.106.055483 |
 2020, Vol. 38
2020, Vol. 38