Institute of Oceanology, Chinese Academy of Sciences
Article Information
- GUO Rui, ZHANG Yingqi, ZHANG Xianyu, ZHANG Qian, CHENG Rui, MD MOSTAFIZUR Rahman, LIU Ying
- Effects of florfenicol exposure on growth, development and antioxidant capacity of flounder Paralichthys olivaceus larvae at different developmental stages
- Journal of Oceanology and Limnology, 38(2): 550-559
- http://dx.doi.org/10.1007/s00343-019-9023-y
Article History
- Received Jan. 24, 2019
- accepted in principle Apr. 24, 2019
- accepted for publication Jul. 2, 2019
2 Liaoning Aquacultural Engineering R&D Center, Dalian 116023, China
Antibiotics have been extensively used in the prevention and treatment of human and animal diseases, and in the feed additives to promote animal growth in husbandry and aquaculture. Due to their pervasive application, antibiotics have been detected in various aquatic environments matrices, with detected concentrations ranging from nanograms per liter to micrograms per liter (Alonso and Camargo, 2009; Biošić et al., 2017). Among many antibiotics, florfenicol (FLO) is one of the antibiotics approved to be used in aquaculture by the Food and Agriculture Organization (FAO) as the replacement of chloramphenicol (Serrano, 2005). FLO may inhibit a variety of aerobic and anaerobic microorganisms and has been commonly used in the treatment of bacterial diseases, such as fish streptococcosis, bacterial cold-water disease, enteric septicemia etc. In 2016, approximately 1 000 tons of FLO were used in agriculture and medicine in China (Zhang et al., 2015), of which most ended up in the water environment. FLO is pseudo-persistently existed in the aquatic environment because of their physicochemical stability and hard photo-degradation under vis-light (λ=300–800 nm). Reportedly, FLO was detected extensively in a range of 8–937.21 ng/L near fish farms, and 29.8–11 103 μg/L in the coastal aquacultural area of Dalian, NE China (Sørensen and Elbæk, 2004; Ye et al., 2008).
The long-term existence of antibiotics in aquatic environment may pose a potential threat to the ecosystem, posing toxic effects on non-target aquatic organisms, resulting in growth and development abnormality, reproductive dysfunction, physiological function instability, and oxidative stress (Botelho et al., 2015). Many previous studies demonstrated that antibiotics could promote the growth and development of fish at a low dose, while it may arouse a safety risk to the animals in a long run, especially during the developmental stage. Sulfamethazine at 0.2, 20, and 2 000 mg/L exposed to zebrafish embryos inhibited the hatching rates and the body length of larvae, and increased the malformation and the heartbeat (Yan et al., 2018).
An acute exposure of FLO was proven toxic to shrimp Penaeus vannamei at different larval stages and juvenile abalone Haliotis discus, i.e., in the LC50 of 64 mg/L (24 h) and 100 mg/L (48 h) for nauplius and mysis larvae, and 163 mg/L (96 h) for juvenile abalone. In addition, FLO could inhibit the immune response and antioxidant defense ability; the phagocytosis and lymphocyte proliferation were suppressed in the blood of FLO-fed rainbow trout for 10 days (Lundén et al., 2002; Caipang et al., 2009).
Aquatic animals in early developmental stages are sensitive to environmental contaminants, especially for a fish in metamorphosis (Hopkins et al., 2006). Japanese flounder, Paralichthys olivaceus, is an important maricultural fish (Zhao et al., 2018), and has three developmental stages in post-embryonic period, i.e., pre-larvae, post-larvae, and juveniles. Dramatic morphological changes appear in 20–30 days after hatching (dah), such as right-eye migration, which is closely related to thyroid homeostasis regulated by hypothalamic-pituitary-thyroid (HPT) axis (Yue et al., 2017).
In this study, to elucidate potential effects of FLO on the early development of the fish, pre-larval and post-larval flounders were used to assess the potential risk of FLO in the aspects of growth and development, motor behavior, and immune resistance, to determine the safe range of FLO application, and to provide a theoretical basis and standard for the scientific and rational use of FLO in marine aquaculture.
2 MATERIAL AND METHOD 2.1 Experimental animalsFertilized eggs of Japanese flounder (P. olivaceus) were obtained from the Fugu Fish Farm, Dalian, China. Eggs were incubated in aquaria (640 mm× 420 mm×355 mm) with sand-filtered seawater (salinity 32.2±0.05, pH 7.2±0.1). During acclimatization, water temperature and photoperiod were maintained at 17±1℃ and 14 h L:10 h D, respectively. Air pump was used to aerate and maintain dissolved oxygen above 6.7±0.05 mg/L.
2.2 Experimental designThe experiment was executed in two different developmental stages of larvae: the pre-larvae (1 day post-fertilization, dpf) and the post-larvae (20 dpf). Five exposure concentrations of FLO (including environment-related concentrations) were set at 0, 0.01, 0.1, 1, and 10 mg/L, in triplication for each concentration. Sixty healthy P. olivaceus were randomly selected and cultured in 220 mm×150 mm×180 mm glass aquarium containing 3-L FLO (purity >98%, purchased from Shandong Yakang Pharmaceutical Co., Ltd.) solution at corresponding concentrations. Larvae were reared with rotifers (Brachionus rotundiformis) 3 times a day from 3 dpf, ensuring that the density was maintained at 5–6 rotifers/mL in the culture water. After 15 dpf brine shrimp (Artemia nauplii) were added as food and maintained the density above 5–6 shrimps/mL. To keep the constant exposure concentrations, half of the experimental solution in aquaria was renewed daily, and dead individuals were removed every day to maintain water quality.
2.3 Detection of the body length and heart ratesFor each group, 9 larvae were selected randomly at 0, 96, and 168 h after FLO exposure at early and late developmental stages. After anesthetization with MS- 222, morphological changes of larvae in body length and heart rates were observed with a stereo microscope (Nikon, 745T/SMZ1000). No significant deaths were observed in both control and treatment groups.
2.4 Spontaneous behavior analysisThe pre- and post- larvae in FLO for 168 h were used for spontaneous behavior analysis. For each experimental group, 9 larvae were randomly selected, and vertically recorded for 30 min in 100-mL culture dishes. A video tracking system (Noldus, EthoVision XT) was used to record the spontaneous behavior trajectory of P. olivaceus at different development stages. Meanwhile, the swimming distance, movement speed, and activity were analyzed.
2.5 Elisa analysisSuperoxide dismutase (SOD) activity, contents of malondialdehyde (MDA), triiodothyronine (T3), and thyroxine (T4) were detected at 168 h after FLO exposure for both pre- and post- larvae with the commercial kits (Nanjing Jiancheng Bioengineering Institute). As the larvae were too small to collect blood, 30 larvae from each group were stored at -80℃ for the use. Later, the larvae were homogenized with physiological saline in a ratio of weight (g) ׃volume (mL)=1׃9 by ultrasonic cell disrupter system (Scientz, JY92-IIN) on ice. The homogenate was centrifuged at 3 000 r/min for 10 min at 4℃ to obtain supernatant, then it was detected by microplate reader (Tecan, Infinite 200 Pro) as per the instruction. Protein concentrations were determined using bicinchoninic acid (BCA) protein kit. The results are presented on a protein basis. SOD activity was calculated by Eq.1, MDA content by Eq.2. The concentrations of T3 and T4 were calculated according to their standard curve equations.
 (1)
(1) (2)
(2)Data were analyzed with SPSS 18.0 software and expressed as mean±standard deviation (SD) (n=3). All data were checked for normality and homoscedasticity before statistical analysis using Kolmogorov-Smirnov test. Comparisons between the control and treatment groups were analyzed by oneway ANOVA, and post hoc comparisons were performed using the least significant difference (LSD) test (equal variances assumed) or Dunnett's C test (equal variances not assumed). Probabilities values were considered significant when P < 0.05 and extremely significant when P < 0.01.
3 RESULT3.1 Effects of FLO exposure on growth and development of P. olivaceus
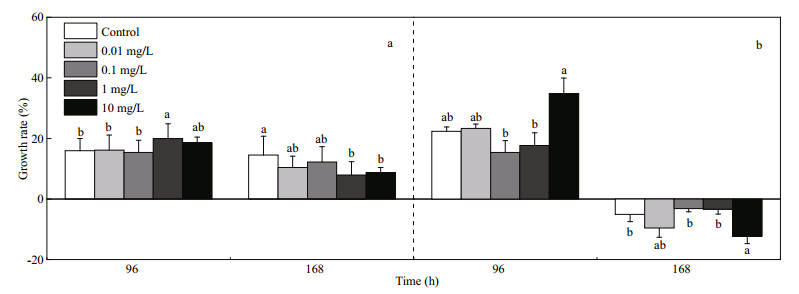
After the hatching of fertilized eggs, the initial average body length of P. olivaceus was 3.0±0.1 mm. For pre-larvae (1 dpf), the body length significantly increased after FLO exposure at different concentrations for 96 h, while it was obviously inhibited after exposure for 168 h. The growth rate in control group (average body length: 3.48±0.21 mm) was significantly higher than 1 mg/L and 10 mg/L FLO groups (P < 0.05) (Fig. 1a). For post-larvae (20 dpf), the initial average body length was 6.8±0.1 mm. The increase of body length varied at different degrees with an increasing dosage of FLO for 96 h, the growth rate of body length in 10 mg/L FLO group was significantly higher than 0.1 mg/L and 1 mg/L FLO groups (P < 0.05), with an increase by 12.4% from the control's. However, it showed a decline in the body length of larvae in FLO exposure for 168 h compared to the groups for 96 h in control and 10 mg/L FLO groups, by 5.05% and 12.5% drop, respectively (P < 0.05).
|
| Fig.1 Effect of FLO on the growth rate of pre-larvae (a) and post-larvae (b) Body growth rates at 96 h were calculated by (96 h body length-initial body length)×100%. Body length growth rate at 168 h were calculated by (168 h body length-96 h body length)×100%. The means with different letters in different concentrations are significant differences at the 0.05 probability level, and the means with the same letters are no significant differences, et sequentia. |
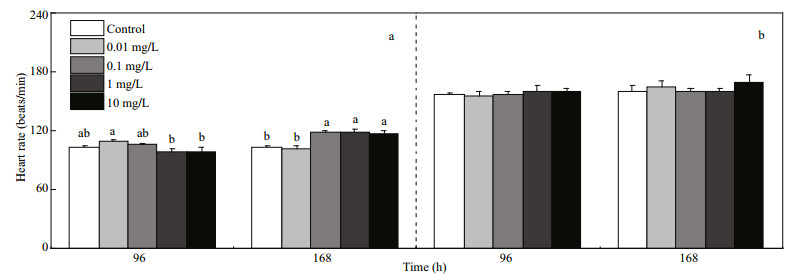
As shown in Fig. 2, the heart rates of larvae increased with the growth of P. olivaceus. The heart rate of post-larvae was 160±6 beats/min, which is significantly higher than that of pre-larvae of control group (107±8 beats/min). Larvae at early developmental stage was very sensitive to FLO. The heart rate of pre-larvae in exposure to FLO at 1 and 10 mg/L for 96 h was lower than that of control group, while at 0.1, 1, and 10 mg/L for 168 h, and the heart rate was statistically increased by 15.5% in 10 mg/L treated group. The post-larvae exposed to FLO for 96 h and 168 h had no significant difference in heart rate between treated and the control groups.
|
| Fig.2 Effects of FLO on the heart rates of pre-larvae (a) and post-larvae (b) |
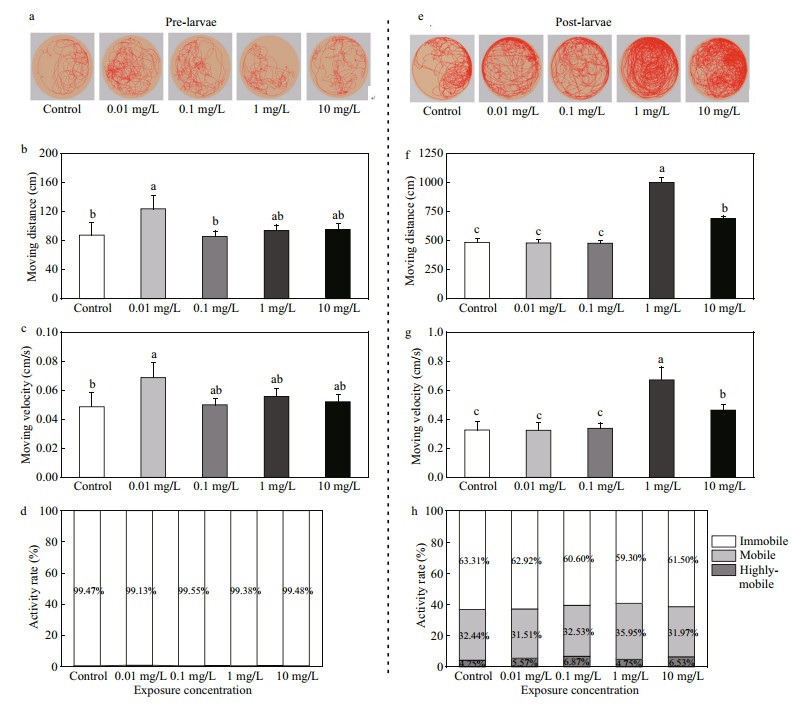
As shown in Fig. 3, the moving distance and moving velocity increased with the growth of larvae. The moving distance and moving velocity of prelarvae and post-larvae in control group increased by 83.04%±6.07% and 85.9%±4.81%, respectively. Prelarvae were sensitive to FLO at low concentrations. The moving distance in the 0.01 mg/L treatment was 41.2% higher than that of control, while no significant difference was observed in other treatment groups (Fig. 3b). Variation in moving velocity was consistent with that in moving distance (Fig. 3c). For the postlarvae, no obvious change was shown in the lowconcentration treatment groups in terms of moving distance and moving velocity, while a significant increase was found in high concentration treatment groups (1 and 10 mg/L) (Fig. 3f, g).
|
| Fig.3 Effects of FLO on the motor behavior of pre-larvae and post-larvae a. moving traces of pre-larvae; b. moving distance of pre-larvae; c. moving velocity of pre-larvae; d. activity of pre-larvae; e. moving traces of post-larvae; f. moving distance of post-larvae; g. moving velocity of post-larvae; h: activity of post-larvae. Refer to the note of Fig. 1 for the meaning of different letters. |
The activity of fish mobility is measured based on the extent of change in fish outline to indicate the frequency of change in the swimming posture. The activity was recognized as being highly mobile, mobile, and immobile according to the percentage changes of pixels between current and previous. The changes over than 60% was regarded as being highly mobile, and less than 20% was defined as being immobile, and the rest, being mobile. As shown in Fig. 3d, the percentage of immobile activity of prelarvae was 99.47%, 99.13%, 99.55%, 99.38%, and 99.48% in the control, 0.01, 0.1, 1, and 10 mg/L groups, respectively. The percentage of immobile activity in treatment groups was significantly higher than that of high mobile and mobile activity, and that of mobile activity in 0.01 mg/L group was higher than that of other groups. With the growth of P. olivaceus, average moving distance and moving velocity increased. For post-larvae (Fig. 3h), the percentage of mobile activity was 32.44%, 31.51%, 32.53%, 35.95%, and 31.97% in the control, 0.01, 0.1, 1, and 10 mg/L groups, respectively. The moving distance and moving velocity of larvae were significantly increased in 1 and 10 mg/L groups.
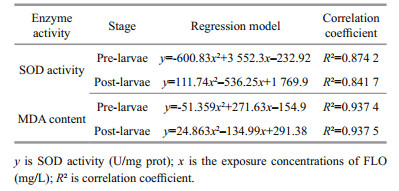
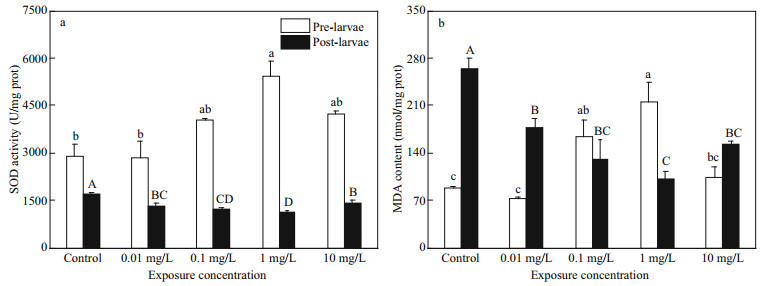
3.4 Effects of FLO exposure on antioxidant enzyme activity and MDA level of P. olivaceusActivities of SOD and MDA contents varied in both pre- and post-larvae. As shown in Fig. 4, the SOD content in pre-larvae was significantly higher than that in post-larvae, and significantly increased after FLO exposure in a dose-dependent manner (P < 0.01), with a slight decrease in 10 mg/L group. However, SOD activities were significantly inhibited in all the treatment groups of post-larvae (P < 0.01) as FLO dosage increased, and higher in 10 mg/L group than that of 1 mg/L group.
|
| Fig.4 Effects of FLO on the SOD activity and MDA content of pre-larvae (a) and post-larvae (b) The lowercase letters indicate significant difference at P < 0.05 among control and the FLO concentration groups of pre-larvae. The capital letters indicate significant difference at P < 0.05 among control and the FLO concentration groups of post-larvae. |
The MDA contents of pre-larvae in treatment groups at all FLO concentrations were accumulated. The increasing trend slightly decreased in 10 mg/L group. On the contrary, FLO posed significant repression on MDA contents in post-larvae, with the lowest point in 1 mg/L group, which was 62.1% lower than that of control group.
The MDA content in pre-larvae showed an opposite trend to that of post-larvae in response to FLO exposure, being consistent with the SOD activities, for which we built quadratic polynomials among the SOD activity and MDA content in FLO treated preand post-larvae, and the correlations are significant (Table 1).
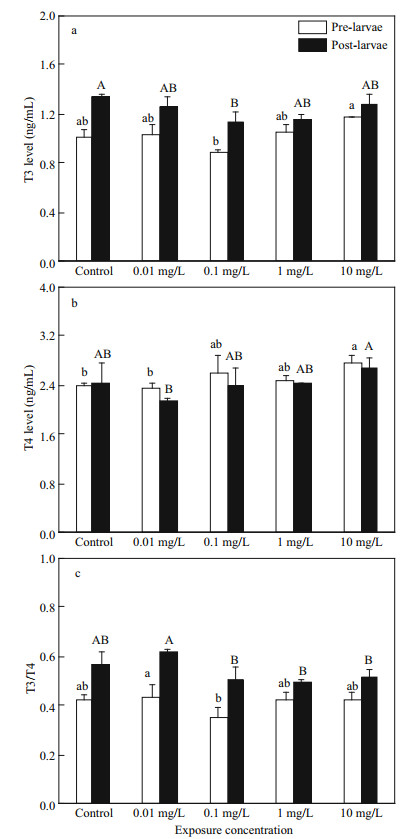
Thyroid hormone T3 and T4 in pre- and postlarvae showed high induction after exposure to FLO (Fig. 5a, b). For T3 level, it was significantly increased in pre-larvae of 10 mg/L group compared with 0.1 mg/L group (P < 0.05), and no statistical changes in other groups. The T4 level in pre-larvae of 10 mg/L group was significantly higher than control and 0.01 mg/L groups (P < 0.05). As shown in Fig. 5c, T3/ T4 ratios in 0.01 mg/L groups was significantly higher than those of the other groups (P < 0.05).
|
| Fig.5 Effects of FLO on the level of T3 and T4 in P. olivaceus larvae at different developmental stages a. T3 level; b. T4 level; c. the ratio of T3/T4. The lowercase letters indicate significant difference at P < 0.05 among control and the FLO concentration groups of pre-larvae. The capital letters indicate significant difference at P < 0.05 among control and the FLO concentration groups of post-larvae. |
It remains doubtful whether antibiotics can be added into fish feed as a growth promoter. Reda et al. (2013) reported that the weight gain of tilapia significantly increased with FLO-added feeds (5 mg/kg BW/d) for 12 weeks. Zhang et al. (2015) described phenotypes of developmental delay of zebrafish embryos, including hatching delay, shorter body length, and uninflated swim bladder upon exposure to tetracycline. In this study, 1 and 20 dpf flounder larvae were exposed to different FLO concentrations. FLO exposure for 96 h to pre-larvae promoted significantly the body length; however, as the exposure period lasted, the increase of growth rate slowed down.
In early stage of a larva, yolk sac is absorbed completely, develops from endogenous to exogenous nutrition mode, and begins to meet the external environment and becomes more sensitive to outside pollutants. In the flounder, the post-larvae stage (20 dpf) is the time of intensive changes, including the formation of coronal dorsal fins, shifting of right eye, and the upturning of end notochord (Brewster, 1987). In our study, the growth rate of body length in post-larvae showed a downward trend, which should be related to the upward tilting of notochord and the formation of the tail bone.
During the growth and development of the flounder, the heart rates of larvae increased gradually, indicating the progress of fish growth. The heart rates in postlarvae of the control group increased significantly compared with those of the pre-larvae in this study. In addition, the heart rates of pre-larvae increased in a dose-dependent manner after FLO exposure for 168 h, which might be related to the stress response and dysfunctional heart function of larvae induced by the long-term FLO exposure. It has been demonstrated that the heart rate of zebrafish embryos was induced by sulfamethazine at the concentration of 0.2, 20, and 2 000 μg/L for 72 h, which caused arrhythmia and heart dysfunction of fish (Yan et al., 2018). To our knowledge, the mechanism by which FLO affecting the heart rate is not clear yet. It supposed that antibiotics could act as gamma-aminobutyric acid (GABA) agonists for inducing acute neurotoxicity to fish under the high-dose exposure (Dorman, 2000), or it could act on the cardiac-adrenergic receptors as epinephrine, which resulted in the increase of the heart rate in larval zebrafish (Milan, 2003; Steele et al., 2011).
4.2 Effects of FLO exposure on spontaneous behavior of P. olivaceusAnimal behavior is a sensitive indicator in response to the changes of external environment, and it is an overall reflection of variations at molecular, biochemical, and physiological levels after being exposed to environmental contaminants. We aimed to analyze the spontaneous movement of larvae by visual tracking software. Flounder larvae in the early life stage showed relative low movement. Distance, velocity, and mobility of swimming changed in an overturned U trend in FLO exposure, which is consistent with previous study on zebrafish behavior by β-diketone antibiotics exposure. FLO exposure at low concentrations may stimulate the motor nerves of fish, while high concentrations could do the opposite (Fent and Meier, 1992). The fish behavior is similar to the change in body length, indicating that FLO at high concentrations may impair the nervous system and affect the growth and development of larvae. A high concentration of FLO significantly promoted the motility of post-larvae but pre-larvae, which might be related to sensitivity upon the developmental stage as post-larvae were more tolerant to FLO than pre-larvae. As Lin et al. (2014) reported, spontaneous swimming was weakened significantly with sulfonamide concentration increase, because sulfonamides may act as GABA (gamma-aminobutyric acid) agonists, causing acute neurotoxical effects and damaging the coordination ability.
4.3 Effects of FLO exposure on antioxidant enzyme activity and MDA content of P. olivaceusIt had been reported that FLO has immunotoxicity to aquatic animals, especially during the early developmental stages. SOD is an enzyme that can scavenge superoxide anion radicals (O2-) by catalyzing free radicals, which plays a critical role in balancing between oxidation and antioxidant (Shen et al., 2014). We found that pre-larvae in a low concentration of FLO may cause excessive production of O2-. To maintain the dynamic balance of SOD activity and O2- content in larvae, SOD activity increased. However, with the increasing of FLO concentration, SOD is not enough to scavenge the excessive O2-, which may impair the antioxidant capability of the larvae. SOD activity in post-larvae but pre-larvae, was significantly decreased, suggesting that SOD may not the main actor in defensing oxidative stress that induced by FLO during the late developmental stage. As larvae grew, antioxidant defense system is gradually matured. Additional to antioxidant enzymes SOD etc., non-enzymatic small-molecule antioxidants were also involved in antioxidant defense system to protect the fish. These substances include vitamin C, vitamin E, and GSH (glutathione) etc. Studies have reported that vitamin E is an effective biological antioxidant that reacts with O2- directly, which inhibit lipid peroxidation in a tissue (Huang and Huang, 2004). In addition, GSH is a water-soluble antioxidant found in the cytosol and mitochondria. It also plays an important role in scavenging free radicals and active oxygen species (Yang et al., 2011).
MDA is a byproduct of membrane lipid peroxidation and is frequently used as a marker of cell membrane damage caused by oxidative stress (Mendes et al., 2009). Consistent with the changes of SOD activity, FLO increased the lipid peroxidation. As the exposure concentration of FLO increased, the degree of free radical attacking larvae was significantly aggravated. It has been demonstrated that MDA contents were kept at extremely low level under no environmental stress, while it increased after exposing to the external contaminant, which lead to reactive oxygen species accumulation and membrane lipid peroxidation (Khan et al., 2018). FLO showed a dose-dependent decrease in SOD activity and MDA content in postlarvae but pre-larvae, which may be related to the higher tolerance in the late stage of larvae. Research in the antioxidant activity and MDA level in adult and embryonic zebrafish showed that the adult are more tolerant to sulfonamide than the embryonic (Yan et al., 2018).
4.4 Effects of FLO on thyroid hormones T3 and T4 of P. olivaceusThyroid hormone includes mainly thyroxine (T4) and triiodothyronine (T3); they play an essential role in physiological processes such as growth and development, reproductive breeding, and energy metabolism (Politis et al., 2018). Miwa and Inui (1987) proposed that dramatic morphological changes of metamorphosis are associated with thyroid hormones levels, and T4 stimulated the metamorphosis of Japanese flounder larvae. After the hatching, the levels of T3 and T4 stayed at relatively low levels, and gradually increased during the metamorphosis (De Jesus et al., 1991). Moreover, Yue et al. (2017) found that 100 μg/L semicarbazide exposure shortened the time by 2 days for flounder larvae to complete metamorphosis as compared to the control group. T4 is the main thyroid hormone produced by hypothalamic-pituitary-thyroid (HPT) axis; T3 is transformed by T4 via three deiodinases and exerts biological activity by binding thyroid hormone receptors. In our study, the post-larvae at 20 dpf exposed to FLO for 7 d was in an important moment of metamorphosis, the ratio of T3/T4 was significantly higher than that of the pre-larvae. In addition, we found that the T3/T4 ratio went down with the FLO dosage increase in both pre- and post- larvae, suggesting that FLO may inhibit the conversion from T4 to T3. However, the ratio of T3/T4 in the 0.01 mg/L exposed group did not change, indicating that FLO may promote the growth and development of larvae at a moderate concentration. It is consistent with the result of short-term hexabromocyclododecane exposure on the levels of T3 and T4 in juvenile crimson snapper (Chen et al., 2016).
5 CONCLUSIONThe short-term FLO exposure promoted the growth and development of the pre-larvae of P. olivaceus; however, the long-term one could slow down the body length increase.
Paralichthys olivaceus larvae showed a different degree of tolerance to FLO in different developmental stages. Post-larvae is more tolerant to external pollutants than pre-larvae, and the spontaneous behavior of pre-larvae can be stimulated by FLO of low concentration. However, for post-larvae, no significant effect was observed in the spontaneous behavior in a low FLO concentration, while in a high concentration, the behavior was promoted.
The antioxidant activity of pre-larvae declined in high FLO concentration, while the excessive O2- was hard to eliminate. With the aggregation of free radical attacking, the levels of SOD and MDA were raised.
Therefore, an appropriate amount of FLO could promote the growth and development of the larvae, the spontaneous behavior, and the antioxidant capacity. However, in higher concentrations and longer exposure times, these items could be inhibited. The pre-larvae (1 dpf) were more sensitive to FLO residues in the environment than post-larvae (20 dpf). A long-term FLO exposure even at an environmental related level could damage the growth and immune resistance of the fish. It is not suitable to prevent disease as a drug for long-terms. Controlling the use and emission of FLO and other antibiotics shall be emphasized.
6 DATA AVAILABILITY STATEMENTAll date generated and/or analyzed during this study are available from the corresponding author upon reasonable request.
Alonso A, Camargo J A. 2009. Effects of pulse duration and post-exposure period on the nitrite toxicity to a freshwater amphipod. Ecotoxicology and Environmental Safety, 72(7): 2 005-2 008.
DOI:10.1016/j.ecoenv.2009.06.008 |
Biošić M, Mitrevski M, Babić S. 2017. Environmental behavior of sulfadiazine, sulfamethazine, and their metabolites. Environmental Science and Pollution Research, 24(10): 9 802-9 812.
DOI:10.1007/s11356-017-8639-8 |
Botelho R G, Christofoletti C A, Correia J E, Ansoar Y, Olinda R A, Tornisielo V L. 2015. Genotoxic responses of juvenile tilapia (Oreochromis niloticus) exposed to florfenicol and oxytetracycline. Chemosphere, 132: 206-212.
DOI:10.1016/j.chemosphere.2015.02.053 |
Brewster B. 1987. Eye migration and cranial development during flatfish metamorphosis:a reappraisal (Teleostei:Pleuronectiformes). Journal of Fish Biology, 31(6): 805-833.
DOI:10.1111/j.1095-8649.1987.tb05281.x |
Caipang C M A, Lazado C C, Brinchmann M F, Berg I, Kiron V. 2009. In vivo modulation of immune response and antioxidant defense in Atlantic cod, Gadus morhua following oral administration of oxolinic acid and florfenicol. Comparative Biochemistry and Physiology Part C:Toxicology & Pharmacology, 150(4): 459-464.
|
Chen H G, Sun L W, Zhang B L, et al. 2016. Effects of shortterm exposure to hexabromocyclododecane (HBCD) on T3 and T4 of liver and brain in juvenile crimson snapper(Lutjanus erythopterus). Journal of Agro-Environment Science, 35(7): 1 257-1 263.
(in Chinese with English abstract) |
De Jesus E G, Hirano T, Inui Y. 1991. Changes in cortisol and thyroid hormone concentrations during early development and metamorphosis in the Japanese flounder, Paralichthys olivaceus. General and Comparative Endocrinology, 82(3): 369-376.
|
Dorman D C. 2000. An integrative approach to neurotoxicology. Toxicologic Pathology, 28(1): 37-42.
|
Fent K, Meier W. 1992. Tributyltin-induced effects on early life stages of Minnows phoxinus phoxinus. Archives of Environmental Contamination and Toxicology, 22(4): 428-438.
|
Hopkins W A, Durant S E, Staub B P, Rowe C L, Jackson B P. 2006. Reproduction, embryonic development, and maternal transfer of contaminants in the amphibian Gastrophryne carolinensis. Environmental Health Perspectives, 114(5): 661-666.
DOI:10.1289/ehp.8457 |
Huang C H, Huang S L. 2004. Effect of dietary vitamin E on growth, tissue lipid peroxidation, and liver glutathione level of juvenile hybrid tilapia, Oreochromis niloticus×Oaureus, fed oxidized oil. Aquaculture, 237(1-4): 381-389.
|
Khan M F, Wang G D. 2018. Environmental agents, oxidative stress and autoimmunity. Current Opinion in Toxicology, 7: 22-27.
DOI:10.1016/j.cotox.2017.10.012 |
Lin T, Yu S L, Chen Y Q, Chen W. 2014. Integrated biomarker responses in zebrafish exposed to sulfonamides. Environmental Toxicology and Pharmacology, 38(2): 444-452.
DOI:10.1016/j.etap.2014.07.020 |
Lundén T, Lilius E M, Bylund G. 2002. Respiratory burst activity of rainbow trout (Oncorhynchus mykiss)phagocytes is modulated by antimicrobial drugs. Aquaculture, 207(3-4): 203-212.
DOI:10.1016/S0044-8486(01)00760-8 |
Mendes R, Cardoso C, Pestana C. 2009. Measurement of malondialdehyde in fish:a comparison study between HPLC methods and the traditional spectrophotometric test. Food Chemistry, 112(4): 1 038-1 045.
|
Milan D J, Peterson T A, Ruskin J N, Peterson R T, MacRae C A. 2003. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation, 107(10): 1 355-1 358.
DOI:10.1161/01.CIR.0000061912.88753.87 |
Miwa S, Inui Y. 1987. Effects of various doses of thyroxine and triiodothyronine on the metamorphosis of flounder(Paralichthys olivaceus). General and Comparative Endocrinology, 67(3): 356-363.
|
Politis S N, Servili A, Mazurais D, Zambonino-Infante J L, Miest J J, Tomkiewicz J, Butts I A E. 2018. Temperature induced variation in gene expression of thyroid hormone receptors and deiodinases of European eel (Anguilla anguilla) larvae. General and Comparative Endocrinology, 259: 54-65.
DOI:10.1016/j.ygcen.2017.11.003 |
Reda R M, Ibrahim R E, Ahmed E N G, El-Bouhy Z M. 2013. Effect of oxytetracycline and florfenicol as growth promoters on the health status of cultured Oreochromis niloticus. The Egyptian Journal of Aquatic Research, 39(4): 241-248.
DOI:10.1016/j.ejar.2013.12.001 |
Serrano P H. 2005. Responsible Use of Antibiotics in Aquaculture. Food and Agriculture Organization of the United Nations, Rome. p.1-50.
|
Shen H Y, Wang L X, Yang J D, Gao J X, Jiao X H, Liu J W. 2014. Effect of streptomycin wastewater on SOD activity and MDA content in muscles of zebra fish. Journal of Hebei University of Science and Technology, 35(3): 303-307.
(in Chinese with English abstract) |
Sørensen L K, Elbæk T H. 2004. Simultaneous determination of trimethoprim, sulfadiazine, florfenicol and oxolinic acid in surface water by liquid chromatography tandem mass spectrometry. Chromatographia, 60(5-6): 287-291.
|
Steele S L, Yang X D, Debiais-Thibaud M, Schwerte T, Pelster B, Ekker M, Tiberi M, Perry S F. 2011. In vivo and in vitro assessment of cardiac β-adrenergic receptors in larval zebrafish (Danio rerio). Journal of Experimental Biology, 214: 1 445-1 457.
DOI:10.1242/jeb.052803 |
Yan Z Y, Yang Q L, Jiang W L, Lu J L, Xiang Z R, Guo R X, Chen J Q. 2018. Integrated toxic evaluation of sulfamethazine on zebrafish:including two lifespan stages(embryo-larval and adult) and three exposure periods(exposure, post-exposure and re-exposure). Chemosphere, 195: 784-792.
DOI:10.1016/j.chemosphere.2017.12.119 |
Yang W, Sun H J, Xiang F H, Yang Z, Chen Y F. 2011. Response of juvenile crucian carp (Carassius auratus) to long-term ammonia exposure:feeding, growth, and antioxidant defenses. Journal of Freshwater Ecology, 26(4): 563-570.
|
Ye S, Hu J T, Zong H M, Na G S, Yao Z W, Wang J Y, Ma D Y. 2008. Determination of florfenicol residues in aquiculture seawater by High Performance liquid chromatographytandem mass spectrometry. Journal of Shenyang Agricultural University, 39(3): 368-370.
(in Chinese with English abstract) |
Yue Z H, Yu M, Zhang X N, Dong Y F, Tian H, Wang W, Ru S G. 2017. Semicarbazide-induced thyroid disruption in Japanese flounder (Paralichthys olivaceus) and its potential mechanisms. Ecotoxicology and Environmental Safety, 140: 131-140.
DOI:10.1016/j.ecoenv.2017.02.043 |
Zhang Q, Cheng J P, Xin Q. 2015a. Effects of tetracycline on developmental toxicity and molecular responses in zebrafish (Danio rerio) embryos. Ecotoxicology, 24(4): 707-719.
DOI:10.1007/s10646-015-1417-9 |
Zhang Q Q, Ying G G, Pan C G, Liu Y S, Zhao J L. 2015b. Comprehensive evaluation of antibiotics emission and fate in the river basins of China:source analysis, multimedia modeling, and linkage to bacterial resistance. Environmental Science & Technology, 49(11): 6 772-6 782.
|
Zhao H T, Du X X, Zhang K, Liu Y Z, Wang Y J, Liu J X, He Y, Wang X B, Zhang Q Q. 2018. Weighted correlation network analysis (WGCNA) of Japanese flounder(Paralichthys olivaceus) embryo transcriptome provides crucial gene sets for understanding haploid syndrome and rescue by diploidization. Journal of Ocean University of China, 17(6): 1 441-1 450.
DOI:10.1007/s11802-018-3656-x |
 2020, Vol. 38
2020, Vol. 38