Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LI Yongfu, LI Ruiqian, YI Xiaoyan
- Effects of light quality on growth rates and pigments of Chaetoceros gracilis (Bacillariophyceae)
- Journal of Oceanology and Limnology, 38(3): 795-801
- http://dx.doi.org/10.1007/s00343-019-9171-0
Article History
- Received Jun. 28, 2019
- accepted in principle Aug. 19, 2019
- accepted for publication Sep. 27, 2019
2 Laboratory for Marine Biology and Biotechnology, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China;
3 Nantong Research and Development Center of Marine Science and Technology, Institute of Oceanology, Chinese Academy of Sciences, Nantong 226019, China;
4 School of International Affairs and Public Administration, Ocean University of China, Qingdao 266100, China;
5 Xishuangbanna Tropical Botanical Garden, Chinese Academy of Sciences, Mengla 666303, China
Chaetoceros gracilis (Bacillariophyceae), an important microalgal species from an economic point of view, represents a direct or indirect food source for bivalve mollusks, sea urchins, sea cucumbers, crustaceans and other valuable ocean aquatic animals. Currently, the closed photobioreactor (PBR) has become one of the most used production methods for cultivating C. gracilis (Pérez et al., 2017). One of the critical parameters that dramatically affect the growth of microalgae, regardless of the adopted production method, is light. Indoor PBRs require artificial light sources. In the past, fluorescent lamps and metal halide lamps were used (Yeh and Chung, 2009; Gao et al., 2018). However, in order to effectively increase the biomass accumulation of microalgae, an economical, durable and efficient light source that is easy to assemble and automatically controlled is highly desirable (Wagner et al., 2016). LED light is the best option among the existing light sources, meeting all the above-mentioned requirements. Moreover, it provides the possibility of integrating solar power technology, which would further decrease light costs for microalgal culture (Gordon and Polle, 2007). Recently, the use of LED light in the cultivation of microalgae has greatly increased (Yim et al., 2016; Shu et al., 2018). The flexible chromatic LED strip, in particular, is suitable for PBR as it possesses a good waterproof property and multi-angled bendability (Cui et al., 2016). However, little is so far known about the influence and the efficiency of different monochromatic LED lights on C. gracilis growth. Since light is a crucial factor in the cultivation of this organism, a detailed understanding of how microalgae perform in response to different light sources is needed.
In this work, we analyze the effects of light quality on C. gracilis growth. Different light sources (all with the same quantum flux density) are compared: LED red right (LR), LED blue light (LB), fluorescent lamp white light (FW) and LED white light (LW) as well as combinations of white light with enhanced proportions of monochromatic red or blue light, i.e., LW+LB, LW+LR, FW+LB, and FW+LR. A simplified Lambert-Beer law is used to calculate the average extinction coefficient of microalgae at the specific wavelength and to identify its relationship with the specific growth rate. The obtained results provide a basis to explore the mechanism by which light influences the growth rate of microalgae.
2 MATERIAL AND METHOD 2.1 Microalgal strain and culture conditionsChaetoceros gracilis was obtained from the Microalgae Culture Center (MACC), Ocean University of China. The algae were placed in a 500-mL conical flask with 300 mL of F/2 culture medium with 2 times concentrated silicate (Guillard and Ryther, 1962). Nine light qualities were tested, all at the same light quantum flux density (60 μmol photons/(m2·s)), and three biological replicates were performed for each treatment. The light intensity was selected to avoid light saturation of the microalgal cells, so that only the effect of the light sources was measured (Chen et al., 1982). LED light sources in our study, i.e. LW, LR, and LB, are all surface mounted light emitting diodes. Each LED lamp bead emits light, and different light quality lamp beads have a specific light intensity. The luminous intensity of each LW bead measured by using a portable light quantum meter (3415F type, pulse photoelectric sensor; Spectrum Technologies, Inc., USA) is 1.0 μmol photons/(m2·s), and both LR and LB are 0.5 μmol photons/(m2·s). The incident light intensity of microalgal culture under different light qualities was set by controlling the number of light beads for each specific color. We lit 60 white LED beads, 120 red LED beads and 120 blue LED beads, respectively, to create 60 μmol photons/(m2·s) of white, red and blue light. Similarly, 60 red or blue LED beads and 30 white LED beads were used to provide LW+LR or LW+LB. Sixty red beads and 60 blue beads were simultaneously lit to provide LR+LB of 60 μmol photons/(m2·s). As for FW+LR and FW+LB, the 30 μmol photons/(m2·s) fluorescent white light was first constructed and then 30 μmol photons/(m2·s) LR or LB provided by 60 red or blue beads was added. The algae were continuously cultured under sterile conditions for seven days. The starting inoculum was 5×105 cells/mL placed in 500 mL flasks. Temperature was maintained stable at 35℃, cultures were hand shaken every 8 h throughout the experiments and continuous light was provided. This temperature was chosen for this study to accelerate the growth of this alga. According to a previous study, 35℃ was not high enough to induce heat stress of algal cells (Liang et al., 2006). Light qualities, i.e., LW, LB and LR were provided by flexible LED lamp strips (LXHL, Philips Ltd., Holland). The FW was provided by a fluorescent lamp tube (F25T8/TL 950, Philips Ltd., Holland). Light spectra were measured with a plant illumination spectroradiometer (PLA-20, EVERFINE's Quality Measurement Instruments, China) between 350 and 800 nm with 1-nm resolution (Fig. 1).
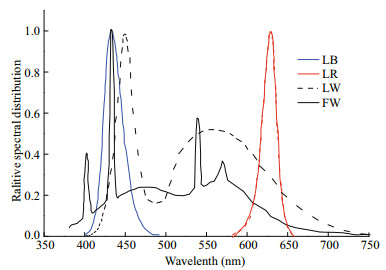
|
| Fig.1 Relative emission spectra of the light qualities used, blue light (LB), red light (LR), white LED light (LW) and white fluorescent light (FW) |
For dry weight (DW) measurements, 10 mL of cultures were filtered on pre-treated GF/C Whatman filter (Whatman, Maidstone, UK). GF/C filters were washed by distilled water and then dried at 50℃. The pre-treated GF/C filters were weighted before collecting cells. DWs were calculated in g/L after the filtrates were re-dried in an oven at 80℃ overnight. The specific growth rate (μ (/d)) during the exponential growth period was obtained according to the method described in Cui et al. (2017). Total chlorophyll (chlorophyll a and c) and carotenoids were extracted as described in (Li et al., 2016) and were quantified using a UV visible spectrophotometer, according to protocols from Ritchie (2008) and Jensen (1978). The method of Li et al. (2016) and a simplified LambertBeer law were used to calculate the average extinction coefficient (α) of C. gracilis suspension under different wavelengths.
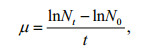
where N0 and Nt represent the cell densities (cells/mL) at the beginning and the end of the exponential phase, and t is the time of the exponential phase in days. In this study, the exponential growth of algae cells finished within two days. Cell numbers in the first two days were used to calculate specific growth rates.

where OD is the absorbance at the indicated wavelength.
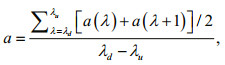
where a represents the average absorbance. λd and λu are the lower-limit wavelength (400 nm for LW and LB, and 570 nm for LR) and the upper-limit wavelength (700 nm for LW, 500 nm for LB, and 680 nm for RL), respectively. a(λ) means the monochromatic light absorbance of each wavelength. The specific extinction coefficient of mixed light quality was expressed by the average value of each monochrome light quality.
SPSS 17.0 was used for the implementation of statistical analyses. Data in the figures represent the mean±SD (n=3) and were subjected to one-way ANOVA and Tukey tests.
3 RESULT AND DISCUSSION 3.1 Influence of light quality on C. gracilis growthAfter being pre-cultured under fluorescent white light, C. gracilis cells were transferred and cultured using nine light qualities. For every light treatment, at the initial stage (within 2 days after starting of the culture), C. gracilis grew rapidly, and then the biomass accumulation rate decreased until it reached a plateau stage (Fig. 2). In the samples treated with the monochromatic light, the growth rates of C. gracilis under LB and LR were 1.24 and 0.59/d, respectively (Fig. 3). In the case of combined light qualities, the growth rate (μ) related to LED white light was LW+LB>LW≈LW+LR, while in the case of fluorescent white light the μ was FW+LB>FW>FW+LR. Under the exposure of LR+LB, μ value was 0.96/d, ranging between LB and LR. Notably, the μ values under LB (1.24/d), FW+LB (1.31/d) and LW+LB (1.18/d) showed no significant difference (P>0.05), indicating that the blue light played a dominant role among FW+LB and LW+LB.
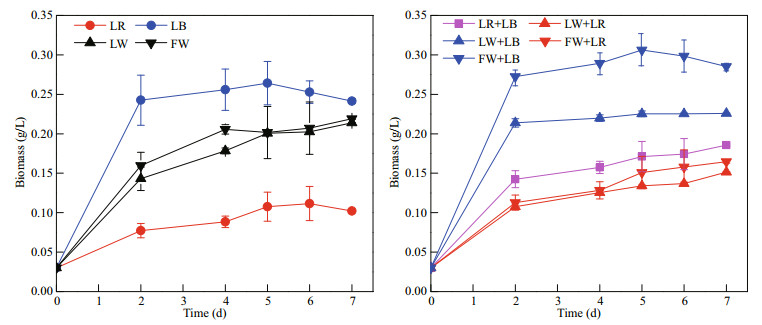
|
| Fig.2 Mean biomass production of C. gracilis under different light qualities (n=3) |

|
| Fig.3 Specific growth rates of C. gracilis cultivated for 2 d under different light qualities (n=3) |
The effect of light quality on the growth of microalgae has been reported in many studies. It was previously shown that the maximum biomass accumulation of Spirulina platensis was reached at 3 000 μmol photons/(m2·s) under LR radiation; however, this alga showed no obvious differences below the light intensity of 300 μmol photons/(m2·s) under red, white, yellow, green and blue lights of LED lamp (Wang et al., 2007). Tang et al. (2011) demonstrated that red LED light and white fluorescent light were all suitable for the growth of Dunaliella tertiolecta. Conversely, the optimal light condition for Chlorella vulgaris growth was shown to be red light (Yan et al., 2013); in addition, germplasm diversities of microalgae are important parameters to consider when determining the most suitable light quality (Aidar et al., 1994). For another marine diatom, Haslea ostrearia, low blue light favored growth (Mouget et al., 2005). Yoshioka et al. (2012) also reported the effects of three light qualities, i.e. white, blue, and red provided by LED panels, on the growth and fatty acid composition of Isochrysis galbana and showed that the highest biomass production and lipid content was obtained under blue light. Marchetti et al. (2013) further compared the effects of blue light and white light derived from fluorescent tubes, on the biochemical composition and photosynthetic rate of Isochrysis sp. (T-iso) CCAP 927/14. Cell concentration and productivity did not vary significantly under the different light conditions at a steady state, whereas the metabolism of carbohydrates and proteins were especially regulated by blue light. For C. gracilis, our study illustrates that blue light facilitates biomass accumulation when the light intensity is 60 μmol photons/(m2·s) and regardless of the combination with other light spectra (FW+LB or LW+LB). In view of a practical application, LB seems to be the most suitable light source to be used in a closed photobioreactor.
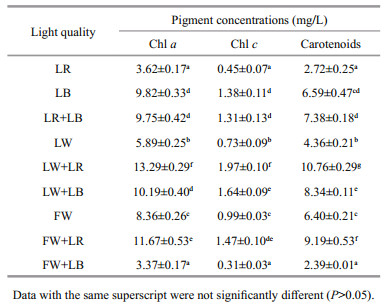
Although chl a is commonly used as a measure of pigment content, other pigments such as chl c and carotenoids can contribute significantly to light absorption (Macintyre et al., 2002). These accessory pigments may also contribute to light harvesting or to the dissipation of excitation energy. When the incident light spectrum changes, microalgae are able to adjust photosynthetic organs in order to obtain the maximum light use efficiency and the concentration of additional photosynthetic pigments is normally observed (MacIntyre et al., 2002). It can be observed that LBcultured C. gracilis showed the highest concentrations of chl a, chl c, and carotenoids (9.82, 1.38, 6.59 mg/L, respectively), under monochromatic light and white light (LW, FW). However, LW+LR and FW+LR induced the highest concentration of carotenoids for all light qualities, either monochromatic or trichromic light. Except for FW+LB, the increase of the proportion of monochromatic light quality (both LR and LB) in the white light induced an increase in the pigment concentration (Table 1). This work is not yet able to explain this point. However, it has been reported that green light yielded the best chlorophyll-a production in Gloeothece membranacea, compared to violet, orange, and red light (Mohsenpour et al., 2012). Green light, an important component of white light, may also play an important role in regulating the accumulation of pigments in C. gracilis, although this effect may not be as obvious as LB or LR. This may be the reason why increasing the proportion of LR or LB in white light can significantly increase the content of pigments.
|
Under photosynthetically active radiation (PAR, 400–700 nm), C. gracilis suspension was scanned to obtain the absorption spectrogram (Fig. 4a). Two absorption peaks of chlorophyll at 433 nm and 675 nm were observed without any other visible peaks. Our results were consistent with the observation of Moberg et al. (2002) and indicated that algal cells can absorb more blue light photons under the same photosynthetic quantum flux.

|
| Fig.4 Absorption spectrum (a) and the relation between the specific growth rates and mean extinction coefficients (b) of C. gracilis under photosynthetically active radiation |
Although large chromatic effects do not occur in some microalgae under light-limited conditions, some studies found that light quality actually regulates physiological and biochemical processes of microalgae (i.e. growth, photosynthetic pigment synthesis and photosynthesis parameter) (Mouget et al., 2004, 2005). Since such differences cannot be simply explained by germplasm differences of microalgae, underlying mechanisms, that remain unknown, are likely to be involved.
Generally, light affects microalgae through three pathways: (i) photon energy; (ii) distribution of light energy on the surface of microalgae and the penetration inside cells; (iii) capture and use of light energy. In the first case, when microalgae use PAR for photosynthesis, the light energy is absorbed by quantization. The photon energy is directly proportional to electromagnetic radiation frequency (υ) and inversely proportional to wavelength (λ). Under the same photon flux density, the photon in the short wavelength area (approaching the visible bluepurple band) has more energy for photosynthesis than the one in the long wavelength area (approaching the red band) (Prézelin et al., 1991). The second process is connected to the penetrability of light in water and the apparent absorption of photons by microalgae. When the color spectrum with a narrow wavelength band overlaps with the absorption spectrum, the light utilization rate shows a rising trend (Chen et al., 2011). In the third pathway, the absorption of light energy in the cell depends on the protein complex of photosynthetic and light-harvesting pigments. The light energy is transferred between pigment molecules toward the two light reaction centers (PSI and PSII on the eukaryotic thylakoid membrane) to complete the final photosynthesis process. Extinction coefficient reflects the absorption magnitude of light energy from the spectrum. Our results showed a significant positive correlation calculated with the Pearson's correlation test between the specific growth rate and each light quality (Fig. 4b). Thus, the light absorption property of microalgal cells played, in part, an important role in regulating cell growth. It is known that the quantum energy of photons at 680 nm is needed for the photolysis of water. Additional energy of lower wavelength photons, e.g. blue, is dissipated as heat (Barber, 2009). This brings forward the question why blue light is the optimum light quality to promote the growth of algae cells. It can be speculated that the constituents of the cells, e.g., proteins, lipids, pigments, and carbohydrates, have their own optical properties that reduce the energy of photons and decrease the light energy reaching the PSII reaction center (Lehmuskero et al., 2018). Future studies are needed to investigate this topic in more detail. Our results also indicated that C. gracilis grown under blue light had higher chl a, chl c, and carotenoid concentrations than that under red light. This phenomenon suggested that LB enhanced the light absorption capacity by increasing the pigment levels, and ultimately improved biomass accumulation. Correspondingly, blue light induces a higher pigmentation content, resulting in more efficient energy absorption and utilization when the equivalent photons, far below the saturated light intensity, are provided. It has been reported that in the diatom H. ostrearia, the most obvious effect of blue light is an enhancement of marennine production, which could reflect the involvement of nitrogen metabolism in the marennine synthesis pathway (Mouget et al., 2005). Interestingly, it has been confirmed that blue light stimulated the carotenogenesis pathway in some fungi by regulating the gene expression, e.g., Monascus (Wang et al., 2012), Fusarium (Avalos et al., 2017) and Neurospora (Polaino et al., 2017). Information about the response of the genes encoding the pigment biosynthesis in the microalgae is still lacking. It is a very interesting subject needing to be investigated further.
4 CONCLUSIONA blue light LED strip was a suitable light source for cultivating Chaetoceros gracilis. Chlorophyll and carotenoid contents under blue light was significantly higher than those under red light. Increasing of the proportion of blue or red light in the white light induced an increase of the chl a, chl c, and carotenoid concentrations. The correlation between the extinction coefficient at different wavelengths and the specific growth rate was positive, suggesting that the light absorption property of microalgal cells played an important role in regulating cell growth.
5 DATA AVAILABILITY STATEMENTFull information developed from this study is available from the corresponding author upon reasonable request.
6 ACKNOWLEDGMENTThe authors thank Dr. MENG Fanping of the Ocean University of China for providing LED light sources. We thank Dr. LI Xian, Key Laboratory of Experimental Marine Biology, Chinese Academy of Sciences, Institute of Oceanology, for her kind help in measuring the light spectra. The authors also thank Dr. John van der Meer of the Pan-American Marine Biotechnology Association for his assistance with proofreading.
Aidar E, Gianesella-Galvão S M F, Sigaud T C S, Asano C S, Liang T H, Rezende K R V, Oishi M K, Aranha F J, Milani G M, Sandes M A L. 1994. Effects of light quality on growth, biochemical composition and photo synthetic production in Cyclotella caspia Grunow and Tetraselmis gracilis (Kylin) Butcher. Journal of Experimental Marine Biology and Ecology, 180(2): 175-187.
DOI:10.1016/0022-0981(94)90065-5 |
Avalos J, Pardo-Medina J, Parra-Rivero O, Ruger-Herreros M, Rodríguez-Ortiz R, Hornero-Méndez D, Limón M C. 2017. Carotenoid biosynthesis in Fusarium. Journal of Fungi, 3(3): 39.
DOI:10.3390/jof3030039 |
Barber J. 2009. Photosynthetic energy conversion:Natural and artificial. Chemical Society Reviews, 38(1): 185-196.
DOI:10.1039/B802262N |
Chen C Y, Yeh K L, Aisyah R, Lee D J, Chang J S. 2011. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production:a critical review. Bioresource Technology, 102(1): 71-81.
DOI:10.1016/j.biortech.2010.06.159 |
Chen S F, Tan G Y, Pan Y Y. 1982. The effect of temperature, light intensity and photoperiod on the growth of Chaetoceros sp. Marine Sciences, (2): 38-40.
(in Chinese with English abstract) |
Cui H W, Meng F P, Li Y F, Wang Y J, Duan W Y. 2016. Effects of artificial light source and light quality on the growth of two species of microalgae. Science Discovery, 4(2): 129-136.
DOI:10.11648/j.sd.20160402.22 |
Cui H W, Meng F P, Li F, Wang Y J. 2017. Application of sodium erythorbate to promote the growth of Chlorella vulgaris. Journal of Applied Phycology, 29(3): 1135-1144.
DOI:10.1007/s10811-016-1021-2 |
Gao X B, Kong B, Vigil R D. 2018. Simulation of algal photobioreactors:recent developments and challenges. Biotechnology Letters, 40(9-10): 1311-1327.
DOI:10.1007/s10529-018-2595-3 |
Gordon J M, Polle J E W. 2007. Ultrahigh bioproductivity from algae. Applied Microbiology and Biotechnology, 76(5): 969-975.
DOI:10.1007/s00253-007-1102-x |
Guillard R R L, Ryther J H. 1962. Studies of marine planktonic diatoms:I.Cyclotella nana hustedt, and Detonula confervacea (Cleve) Gran. Canadian Journal of Microbiology, 8(2): 229-239.
|
Jensen A. 1978. Chlorophylls and carotenoids. In: Hellebust J, Craigie J S, eds. Handbook of Phycological Methods; Physiological and Biochemical Methods. Cambridge University Press, Cambridge, UK. p.61-64.
|
Lehmuskero A, Chauton M S, Boström T. 2018. Light and photosynthetic microalgae:a review of cellular- and molecular-scale optical processes. Progress in Oceanography, 168: 43-56.
DOI:10.1016/j.pocean.2018.09.002 |
Li Y F, Liu J G, Zhang L T, Pang T. 2016. Changes of photosynthetic performances in mature thalli of the red alga Gelidium amansii (gelidiaceae) exposed to different salinities. Marine Biology Research, 12(6): 631-639.
DOI:10.1080/17451000.2016.1177654 |
Liang Y, Liang L X, Yin C L, Cao C H. 2006. Effects of high temperature stress on the chlorophyll fluorescence kinetics of Phaeodactylum tricornutum and Chaetoceros gracilis. Periodical of Ocean University of China, 36(3): 427-433.
(in Chinese with English abstract) |
MacIntyre H L, Kana T M, Anning T, Geider R J. 2002. Photoacclimation of photosynthesis irradiance response curves and photosynthetic pigments in microalgae and cyanobacteria. Journal of Phycology, 38(1): 17-38.
DOI:10.1046/j.1529-8817.2002.00094.x |
Marchetti J, Bougaran G, Jauffrais T, Lefebvre S, Rouxel C, Saint-Jean B, Lukomska E, Robert R, Cadoret J P. 2013. Effects of blue light on the biochemical composition and photosynthetic activity of Isochrysis sp.(T-iso). Journal of Applied Phycology, 25(1): 109-119.
DOI:10.1007/s10811-012-9844-y |
Moberg L, Karlberg B, Sørensen K, Källqvist T. 2002. Assessment of phytoplankton class abundance using absorption spectra and chemometrics. Talanta, 56(1): 153-160.
DOI:10.1016/S0039-9140(01)00555-0 |
Mohsenpour S F, Richards B, Willoughby N. 2012. Spectral conversion of light for enhanced microalgae growth rates and photosynthetic pigment production. Bioresource Technology, 125: 75-81.
|
Mouget J L, Rosa P, Tremblin G. 2004. Acclimation of Haslea ostrearia to light of different spectral qualitiesconfirmation of 'chromatic adaptation' in diatoms. Journal of Photochemistry and Photobiology B:Biology, 75(1-2): 1-11.
DOI:10.1016/j.jphotobiol.2004.04.002 |
Mouget J L, Rosa P, Vachoux C, Tremblin G. 2005. Enhancement of marennine production by blue light in the diatom Haslea ostrearia. Journal of Applied Phycology, 17(5): 437-445.
DOI:10.1007/s10811-005-0561-7 |
Pérez L, Salgueiro J L, González J, Parralejo A I, Maceiras R, Cancela Á. 2017. Scaled up from indoor to outdoor cultures of Chaetoceros gracilis and Skeletonema costatum microalgae for biomass and oil production. Biochemical Engineering Journal, 127: 180-187.
DOI:10.1016/j.bej.2017.08.016 |
Polaino S, Villalobos-Escobedo J M, Shakya V P S, MirallesDurán A, Chaudhary S, Sanz C, Shahriari M, Luque E M, Eslava A P, Corrochano L M, Herrera-Estrella A, Idnurm A. 2017. A Ras GTPase associated protein is involved in the phototropic and circadian photobiology responses in fungi. Scientific Reports, 7: 44790.
DOI:10.1038/srep44790 |
Prézelin B B, Tilzer M M, Schofield O, Haese C. 1991. The control of the production process of phytoplankton by the physical structure of the aquatic environment with special reference to its optical properties. Aquatic Sciences, 53(2-3): 136-186.
|
Ritchie R J. 2008. Universal chlorophyll equations for estimating chlorophylls a, b, c, and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol, or ethanol solvents. Photosynthetica, 46(1): 115-126.
|
Shu C H, Tseng K, Jaiswal R. 2018. Effects of light intensity and wavelength on the production of lactobionic acid from whey by Pseudomonas taetrolens in batch cultures. Journal of Chemical Technology & Biotechnology, 93(6): 1595-1600.
|
Tang H, Abunasser N, Garcia M E D, Chen M, Ng K Y S, Salley S O. 2011. Potential of microalgae oil from Dunaliella tertiolecta as a feedstock for biodiesel. Applied Energy, 88(10): 3324-3330.
DOI:10.1016/j.apenergy.2010.09.013 |
Wagner I, Steinweg C, Posten C. 2016. Mono- and dichromatic LED illumination leads to enhanced growth and energy conversion for high-efficiency cultivation of microalgae for application in space. Biotechnology Journal, 11(8): 1060-1071.
DOI:10.1002/biot.201500357 |
Wang C L, Yang H, Chen M H, Wang Y R, Li F J, Luo C, Zhao S Y, He D. 2012. Real-time quantitative analysis of the influence of blue light on citrinin biosynthetic gene cluster expression in Monascus. Biotechnology Letters, 34(9): 1745-1748.
DOI:10.1007/s10529-012-0962-z |
Wang C Y, Fu C C, Liu Y C. 2007. Effects of using lightemitting diodes on the cultivation of Spirulina platensis. Biochemical Engineering Journal, 37(1): 21-25.
DOI:10.1016/j.bej.2007.03.004 |
Yan C, Luo X Z, Zheng Z. 2013. Effects of various LED light qualities and light intensity supply strategies on purification of slurry from anaerobic digestion process by Chlorella vulgaris. International Biodeterioration & Biodegradation, 79(2): 81-87.
|
Yeh N, Chung J P. 2009. High-brightness LEDs-Energy efficient lighting sources and their potential in indoor plant cultivation. Renewable and Sustainable Energy Reviews, 13(8): 2175-2180.
DOI:10.1016/j.rser.2009.01.027 |
Yim S K, Ki D W, Doo H S, Kim H, Kwon T H. 2016. Internally illuminated photobioreactor using a novel type of lightemitting diode (LED) bar for cultivation of Arthrospira platensis. Biotechnology and Bioprocess Engineering, 21(6): 767-776.
DOI:10.1007/s12257-016-0428-6 |
Yoshioka M, Yago T, Yoshie-Stark Y, Arakawa H, Morinaga T. 2012. Effect of high frequency of intermittent light on the growth and fatty acid profile of Isochrysis galbana. Aquaculture, 338-341: 111-117.
DOI:10.1016/j.aquaculture.2012.01.005 |
 2020, Vol. 38
2020, Vol. 38


