Institute of Oceanology, Chinese Academy of Sciences
Article Information
- CAI Haoyuan, WANG Peng, ZHANG Dun
- Smart anticorrosion coating based on stimuli-responsive micro/nanocontainer: a review
- Journal of Oceanology and Limnology, 38(4): 1 045-1 063
- http://dx.doi.org/10.1007/s00343-020-0058-x
Article History
- Received Feb. 16, 2020
- accepted in principle Apr. 16, 2020
- accepted for publication May. 11, 2020
2 University of Chinese Academy of Sciences, Beijing 100049, China;
3 Open Studio for Marine Corrosion and Protection, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao 266237, China;
4 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
Metallic materials involving steel, magnesium, and aluminum, and their alloys are widely used in field of ocean engineering such as marine shipping, navy warship, offshore platform and construction owing to their high specific strength and ductility properties. Facing such harsh marine environment, however, the tendency to marine corrosion is a severe problem shortening the service lifespan of metals and causing great economic losses. It is considered that the cost of corrosion in China is approximately 310 billion USD, accounting for nearly 3.34% of the gross domestic product (Hou et al., 2017). Therefore, corrosion protection has become an issue of prime importance to minimize the financial loss.
Various methods have been proposed to mitigate the corrosion process. For instance, conventional cathodic protection strategy by using an impressed current to induce negative steel polarization, where corrosion process is suppressed (Christodoulou et al., 2010). Among the corrosion protection strategies, organic coating is the most extensively used, and the cost adds up to two thirds of all anticorrosion spending (Hou et al., 2017). A typical coating system is made up of the sequence pretreatment layer, primer and topcoat (Bierwagen et al., 2010). The pretreatment layer is the conversion coating which contacts with the metal directly and provides adhesion between the substrate and primer. It is particularly typical to apply chromate/phosphate conversion coatings on aluminum alloys (Clark et al., 2002). However, there are concerns about the known toxic, carcinogenic and pollutive properties of hexavalent chromium (Twite and Bierwagen, 1998). The primer layer is mainly applied to improve the corrosion protection of the underlying substrates. Due to low cost and easy application, conventional organic polymer paints, including polyurethane (Wei et al., 2013), epoxy (Yang et al., 2012), alkyd (Krishnamoorthy et al., 2014), and polyvinyl butyral (Mahmoudian et al., 2012) are commonly used as primer coatings. The chromate-derived inhibitive pigments are often incorporated into the primer coatings to delay the occurrence of corrosion (Tedim et al., 2010). A topcoat provides an additional barrier against environmental aging and aesthetic functionality (Du et al., 2001). Nowadays, main focus is developing alternative eco-friendly anticorrosion coatings for corrosion protection. Many attempts have been reported, focusing on "green" polymeric coating (Andreeva et al., 2008; Ostrowski et al., 2013), microarc oxidation (MAO) coating (Zeng et al., 2016; Cui et al., 2017), anodized coating (Capelossi et al., 2014), layered double hydroxides (LDHs) coating (Tedim et al., 2014; Luo et al., 2017), and hybrid sol-gel coating (Wang and Bierwagen, 2009; Yasakau et al., 2013). Recently, superhydrophobic coating, inspired from lotus leaf, is considered as a potential strategy for corrosion protection as its extremely hydrophobic nature (Wang et al., 2014; Dou et al., 2018; Zhang et al., 2018a). Specially, superhydrophobic surfaces based on coalescence-induced droplet jumping behavior have attracted increasing attention for atmospheric corrosion protection (Chen et al., 2019).
When coating is in a good situation, it can exert admirable barrier performance against environmental factors including ultraviolet (UV), heat, oxygen, moisture and ions. However, the barrier effect of the coatings can be badly weakened due to the damages generated by environmental and mechanical attacks during service. The formed damages may be localized scratches and delaminations, or more macroscopic cracks. If the damaged coatings cannot be repaired in time, the corrosive medium will penetrate into coating easily through created paths and ultimately causes coating failures. At present, most damaged coatings need artificial restoration and replacement, which are high-cost and troublesome. A coating with selfhealing property is emerging, which acts by inhibiting the corrosion reaction at the coating defects, or restoring the physical barrier effects by defect sealing. The self-healing process of the coating without any external intervention prolongs the service lifespan of the coating, significantly decreasing the maintenance cost (Zhang et al., 2018c).
The self-healing anticorrosion coating can be classified into two categories based on different working mechanisms: (ⅰ) polymer healable materials, such as hexamethylene diisocyanate (Sun et al., 2015, 2018), isocyanate (Yang et al., 2008), linseed oil (Siva and Sathiyanarayanan, 2015), bisphenol A (Yuan et al., 2006; Zhang et al., 2018b), etc. Micro/nanocapsules or hollow nanofibers containing polymer healing agents are incorporated into polymer coatings. When polymer coatings are mechanically damaged, active agents release into the damaged region and lead to the interior bonding via chemical reactions, thus filling the cracks partially and recovering the initial performance (Huang and Yang, 2011; Lee et al., 2015); (ⅱ) corrosion inhibitors, such as 1H-benzotriazole (Serdechnova et al., 2014), 2-mercaptobenzothiazole (Kermannezhad et al., 2016), polyaniline (Gupta et al., 2013), etc. Two technologies have been developed for introducing inhibitors into self-healing coatings, including direct doping inhibitors and encapsulation of these molecules into micro/nanocontainers that are dispersed evenly in the coating (Makhlouf, 2014). The direct doping strategy has problems of continuous elution of the inhibitor and undesired interaction between the matrix and the inhibitor, which destroy barrier effect of the coating. In comparison, incorporation of corrosion inhibitor into smart container can overcome the shortcomings of direct doping. Some advanced work has been performed in the organic polymeric micro/nanocapsules (Tatiya et al., 2013; Cui et al., 2019), inorganic clay based nanocontainers (Snihirova et al., 2010; Zahidah et al., 2017), mesoporous silica nanoparticles (Fu et al., 2013b; Wang et al., 2015), polyelectrolyte multilayer based micro/nanocapsules (Zheludkevich et al., 2007; Andreeva et al., 2010), etc. Such smart containers can quickly respond to various environmental stimuli and release corrosion inhibitors immediately, thus forming a protective film on the corroded metal surface by chemisorption or physisorption process to restrain the corrosion expansion.
Herein, we review the representative works particularly over the past ten years on the development of smart anticorrosion coating based on micro/nanocontainers with different types of stimuliresponsive properties, including pH, electrochemical potential, redox, aggressive corrosive ions, heat, light, magnetic field and mechanical impact (Fig. 1). At last we present a brief perspective on the future research directions and major challenges in this promising field.

|
| Fig.1 Self-healing mechanisms of the smart coating with incorporated stimuli-responsive micro/nanocontainer |
As previously reported, when carbon steel suffered from the attack of corrosive media, the dissolution of Fe and subsequent hydrolysis of Fe2+ or Fe3+ result in the reduction of pH value in the micro-anodic area, while the oxygen reduction leads to the increase of pH value in the micro-cathodic area. Hence, the fluctuation of pH values nearby corrosive microzones can be considered as a typical feature and is often used as stimulus to control the release of corrosion inhibitors (Fu et al., 2013b).
Polyelectrolyte molecules with sensitive components can be applied to fabricate containers, which can wisely release inhibitors in response to pH variations at the coating defects. The increase/decrease in the local pH can deprotonate/protonate the polymer molecules for improved swelling (Tavandashti et al., 2016a, b; Cai et al., 2019b) or accelerate their hydrolytic degradation (Chen et al., 2014), which both result in the enhanced inhibitor release. A typical example of the method is the composite nanocontainers consisted of nanoparticle core and polyelectrolyte multilayer film. The coreshell structure can be conveniently fabricated using layer-by-layer (LBL) technique involving the stepwise deposition of oppositely charged polyelectrolytes on the nanoparticle surface (Skorb et al., 2009a). Corrosion inhibitors can be loaded into the nanoparticles or serve as the components of the polyelectrolyte multilayers (Singh et al., 2016; Feng et al., 2017). When the local pH values fluctuate due to the anodic and cathodic reactions at the site of coating damage, the polyelectrolyte conformation is changed and the shell is opened to release the encapsulated inhibitors. With the inhibition of corrosion process and subsequent recovery of pH, the shell is closed to store the inhibitors. For example, poly(methacrylic acid) (PMAA) chains keep a shrunken conformation in acidic condition and extremely swell in basic environment owing to the ionization of COOH groups to COO–, leading to large negative charges, strong electrostatic interaction, and a high degree of hydration (Wei et al., 2017). Taking advantage of the pH responsiveness of PMAA, Li et al. (2013) synthesized silica nanotubes coated with PMAA to achieve pH-responsive controlled release of the loaded inhibitor benzotriazole (BTA).
In addition, the net charge on inhibitors themselves can also be affected by the pH value and matches the change rule of the zeta potential of containers. Considering the pH-dependent electrostatic interactions between hollow mesoporous zirconia nanospheres (HMZSs) and inhibitor L-Carnosine (L-CAR), the intelligent nanocontainers prepared by directly binding L-CAR to HMZSs and achieved acid/alkalineresponsive release (Fig. 2). At pH 7.0, L-CAR molecules carry weak positive charges and will be adsorbed inside the interior cavity of HMZSs by electrostatic attractions. As the pH increases, the deprotonation of the amino group will compel the negative charged L-CAR molecules to release from HMZSs due to the strong electrostatic repulsion. In acidic circumstances, the electrostatic attraction between L-CAR molecules and HMZSs reduces. Additionally, with the continuous accumulation of positive charges, L-CAR molecules cannot pack well inside the cavity of HMZSs due to the intermolecular electrostatic repulsion. A similar research was reported by Haddadi et al., who prepared carbon hollow spheres (CHSs) with encapsulated 2-mercaptobenzimidazole (MBI) inhibitor (Haddadi et al., 2018). The remarkable release of MBI was realized in alkaline solution owing to deprotonation of MBI molecules, which allowing smart protection of mild steel when exposed to corrosive media.
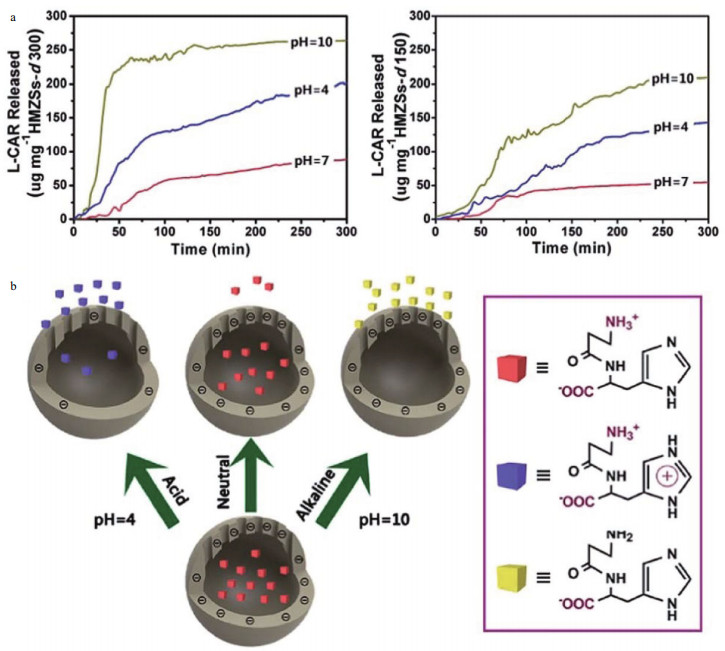
|
| Fig.2 The released concentration of L-CAR in different pH condition (a), and schematic mechanism for the pH-responsive release of L-CAR from L-CAR loaded HMZSs (b) Reprinted with permission (Wang et al., 2015). Copyright 2015, Royal Society of Chemistry. |
The development of supramolecular chemistry has broadened a new vision to construct smart containers by installing supramolecular nanovalves. In particular, the rotaxane or pseudorotaxane nanoswitches on the mesoporous silica nanoparticles (MSNs) transform the "close" to "open" state owing to the removable macrocycles encircling the linear stalks as pH variation (Chen and Fu, 2012; Ding et al., 2017a). For example, to obtain the acid/alkaline dual stimuliresponsive MSNs, Fu et al. (2013a) modified the MSNs with axle molecules containing a hexylammonium unit and a ferrocenecarboxylic acid bonded via amide linkages, the cucurbit[7]uril (CB[7]) macrocycle valves moving through the axle that controlled the release of inhibitor with the pH change. Ding et al. (2017b) constructed an alkali/Mg2+ dual-responsive system in which MSNs were utilized as nanocontainers to load an inhibitor 2-hydroxy-4-methoxy-acetophenone (HMAP), and supramolecular nanovalve containing water-soluble pillar[5]-arenes (WP[5]) host and functional guest stalk with two recognition stations, hexanediamine (HDA) station and alkyl pyridine (APy) station, were used to realize controlled release of HMAP (Fig. 3). In neutral solution, the water-soluble pillar[5]-arenes (WP[5]) macrocycles thread onto the HDA recognition sites by C-H·π interaction and the electrostatic attraction, blocking the pores to prevent inhibitor leakage. With increase of pH value, the deprotonation of ammonium groups in HDA recognition site makes the initial electrostatic attraction disappear, thus the water-soluble pillar[5]-arenes (WP[5]) macrocycle transmit to APy station, leading to the alkalinedependent release. The assembled nanocontainers were dispersed in a sol-gel coating on magnesium alloy, which indicated a excellent self-healing performance due to release of inhibitor activated by alkali stimuli in the corrosive micro-cathodic zone.
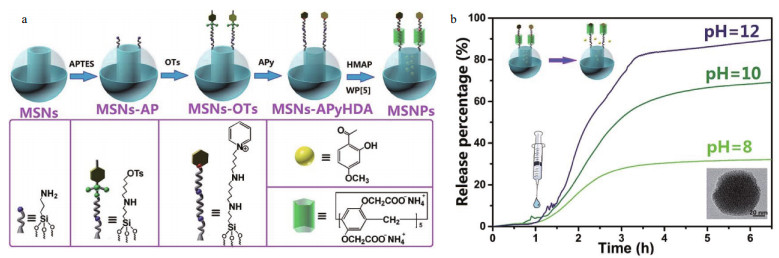
|
| Fig.3 Schematic preparation of MSNs (a) and alkali-triggered release profiles of HMAP from MSNs (b) Reprinted with permission (Ding et al., 2016). Copyright 2016, Royal Society of Chemistry. |
Recently, natural materials have attracted much attention as pH-controlled release gatekeepers owing to their low-cost, non-toxic and biodegradable properties. For instance, novel nanocontainers (MSNs-BTA@TAC) with pH-controlled release function were developed by capsulizing corrosion inhibitor BTA in MSNs, which employing tannic acid-Fe(Ⅲ) complexes (TAC) as responsive gatekeepers (Qian et al., 2019a). The electrochemical impedance of blank coating demonstrated the downtrend during 20 days. For coating incorporated with MSNs-BTA@TAC, the impedance modulus at 0.01 Hz enhanced from 4.7×104 Ω·cm2 to 1.8×105 Ω·cm2. The self-healing performance of the coating resulted from the released BTA and the detached tannic acid (TA) from the surface of MSNs. Inspired from the adhesive nature of catechols and amines in mussel adhesive proteins, polydopamine (PDA) coated MSNs have been reported as scaffolds for pH-sensitive drug release (Chang et al., 2016; Zeng et al., 2017). Qian et al. (2019b) fabricated a novel acid-responsive release system by using PDA as the gatekeeper for MSNs loaded with a corrosion inhibitor BTA (Fig. 4). The nanoparticles were embedded into a water-based alkyd coating on mild steel. The PDA shell could control the release of corrosion inhibitors and chelate with corrosion products to form protective complex films. These studies will inspire other researchers to develop natural gatekeepers with price advantage and ecofriendly characteristics.
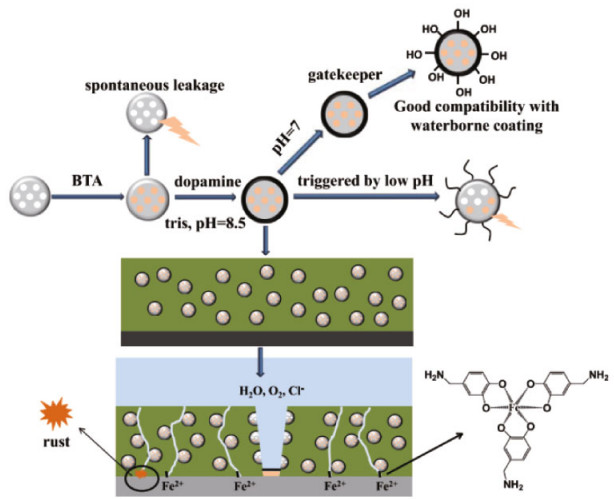
|
| Fig.4 Self-healing mechanism of mussel-inspired coatings Reprinted with permission (Qian et al., 2019b). Copyright 2019, American Chemical Society. |
Although the fluctuation of pH as trigger for sensing the metallic corrosion has been widely investigated. The most reliable stimulus is the change of the electrochemical potential, because it possesses features of in situ generation, uniform distribution, strong reducibility, and always decreases when corrosion occurs. The decrease in the local corrosion potential after the localized corrosion onset was used as a trigger for initiating self-healing actions (Tran et al., 2015; Zhao et al., 2015), which gave new inspiration. For example, Ding et al. (2017b) successfully synthesized the corrosion potentialresponsive smart nanocontainers (CP-SNCs) that were consisted of magnetic vehicles (Fe3O4@mSiO2) and gatekeepers (bipyridinium ⊂ water-soluble pillar[5]-arenes (BIPY ⊂ WP[5]) supramolecular assemblies) and disulfide linkers. When the corrosion potential of the magnesium alloy is exerted, corrosion inhibitor 8-hydroxyquinoline (8-HQ) was released immediately due to the breaking of disulfide linkages and subsequent removal of the nanovalves. Then a corrosion potential stimulus-feedback anticorrosion coating (CP-SFAC) was fabricated on the magnesium alloy by embedding CP-SNCs into the hybrid sol-gel coating. CP-SFAC showed not only a superior anticorrosion performance immersed in NaCl solution but also an obviously rapid self-healing functionality (Fig. 5).
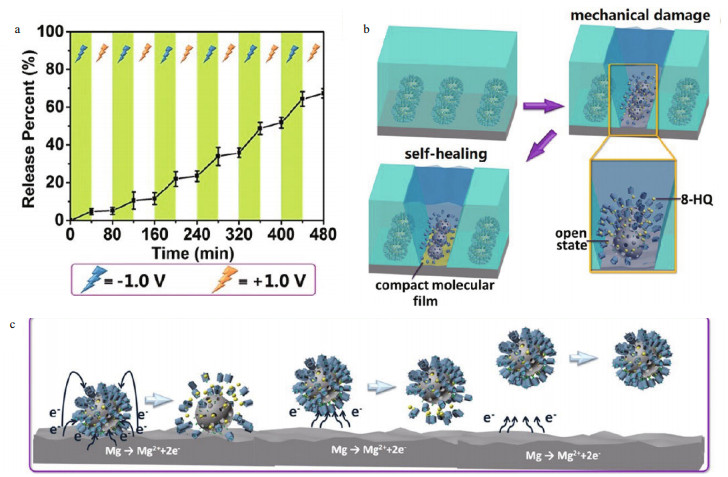
|
| Fig.5 Release profile of 8-HQ under alternating stimulation voltage of -1.0 or +1.0 V (a), corrosion protection mechanism of CP-SFAC (b), and corrosion potential-driven release mechanism of 8-HQ from CP-SNCs (c) Reprinted with permission (Ding et al., 2017b). Copyright 2017, American Chemical Society. |
The conducting polymer (CP) can store active negative ions as counter-charge to the oxidized polymer backbone, which can be released with the onset of corrosion and subsequent reduction of CP (Kowalski et al., 2010). Vimalanandan et al. (2013) prepared a redox-responsive CP-based nanocapsule system that a redox-sensitive polyaniline (PANI) was utilized as the shell material and the corrosion inhibitor 3-nitrosalicylic acid (3-NisA) in the core, respectively. To circumvent the possibility of a Fermilevel misalignment, the nanocapsule was modified with gold nanoparticles (AuNPs) to avoid direct contact between the metal and CP, which insures a steady electronic contact (Fig. 6). When no potential was applied (simulating intact coating), the PANI shells acted as a gatekeeper to prevent the release of 3-NisA from the core. The reduction of PANI (applying low potential to simulate the onset of corrosion) led to an increase of the permeability of the shell and a release of inhibitor. On the other hand, reoxidation (applying high potential to simulate the passivation of metal) caused a decrease in permeability and further release was blocked.
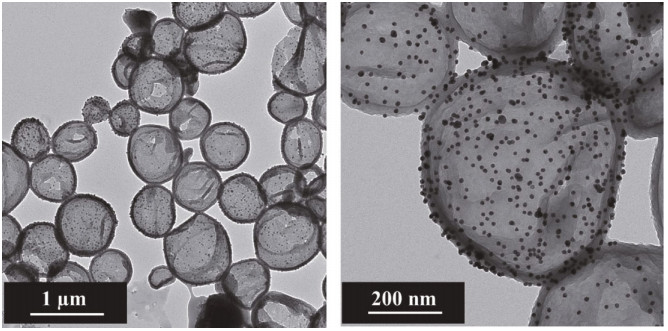
|
| Fig.6 Transmission electron microscopy (TEM) images of PANI capsules decorated with AuNPs Reprinted with permission (Vimalanandan et al., 2013). Copyright 2013, John Wiley and Sons. |
Similar approach was developed to design nanocontainers that allowed the release of two corrosion inhibitors with different release mechanisms (Zhao et al., 2015). The first inhibitor, 2-mercaptobenzothiazole (MBT), is covalently linked to the shell of the nanocapsules. The second healing agent, polydimethylsiloxane diglycidyl ether, is packaged in the inner of the nanocapsules. The release of the active molecules is induced by reduction of the polymeric shell and the release efficiency depends on the property of the healing agent and the structure of the shell. In addition, the MBT released from the nanocontainer shell which allows the release of the packaged healing agent and ultimately leads to a cascade release of the two inhibitors. The multifunctionalization of the containers is a new research route, however, a lot of effort have to be done in this field.
The macrocycle molecules are also utilized as a supramolecular switch of smart nanocontainers to control the release of corrosion inhibitors. According to the report from Fu et al.(2013a, b), redoxresponsive MSNs with supramolecular nanovalves were embedded into a sol-gel coating deposited on top of another Ce(Ⅳ)-doped sol-gel coating (Wang et al., 2017). At the damaged area of coating, the leached Ce(Ⅳ) switched on the supramolecular nanovalves and then the loaded inhibitor p-coumaric acid (CA) was released by redox reaction. Moreover, Ce(Ⅳ) was reduced to Ce(Ⅲ), which also inhibited the corrosion process.
2.3 Aggressive corrosive ions-triggered micro/nanocontainersApart from the changes of pH and electrochemical potential around corrosive micro-regions, aggressive species can penetrate coatings through defects and initiate surface corrosion. Aggressive corrosive ion is also regarded as a stimulus to smart nanocontainers, which can rapidly give the feedback to release entrapped corrosion inhibitors to depress corrosion spread. For instance, steel tends to corrosion caused by chloride ions (Cl−), especially in marine environments. Consequently, it is necessary to design smart containers which can be sensitive to Cl−. A feasible approach is to design responsive capsules shell containing metal ions (such as Ag+, Pb2+). When the intelligent responsive capsules contact with Cl−, the metal ions can be extracted out and precipitated with Cl− to decompose the capsules. Xiong et al. (2015) reported a novel silver alginate hydrogelbased microcapsule that disintegrated on contact with Cl−, and therefore released the active functional molecules.
The anion-exchange clays are more effective to control the release behavior of corrosion inhibitors in the coating. For anion-exchanging clays, the nanoclay layers are charged positively, only negatively charged inhibitors can be intercalated into the interlayers to neutralize the negative charges. The release of the corrosion inhibitors from anion-exchanging nanoclaybased nanocontainers is induced by ion-exchange in face of aggressive ions (Cl−, SO42-), which is efficient for autonomic corrosion protection. The layered double hydroxides (LDHs), also seen as anionic clays or hydrotalcite-like compounds. The most common LDHs are represented by the general formula [M1−x2+Mx3+(OH)2]Ax/nn−·mH2O, where the cations M2+ (Mg2+, Zn2+, Fe2+, Co2+, Cu2+ and others) and M3+ (Al3+, Cr3+, Fe3+, Ga3+ and others) occupy the octahedral holes in a brucite-like layer and the anion An− is located in the hydrated interlayer galleries (Williams and O'Hare, 2006). To date, there are a large number of studies on the corrosion protection property of the LDHs (Buchheit et al., 2003; Kendig and Hon, 2005; Zheludkevich et al., 2010; Serdechnova et al., 2014). In particular, various inhibitors such as 2-mercaptobenzothiazolate (Poznyak et al., 2009), quinaldic acid (Poznyak et al., 2009), succinic acid (Hang et al., 2012), fatty acid (Chen et al., 2013), vanadate (Mahajanam and Buchheit, 2008) and oxalate (Williams and McMurray, 2004) were loaded in the LDHs galleries and their active corrosion protection performances were investigated. Poznyak et al. (2009) synthesized Zn-Al and Mg-Al LDHs loaded with quinaldate and 2-mercaptobenzothiazolate via anion-exchange method. They found that the nanocontainers released sequentially the inhibitors through an exchange mechanism (release of an inhibitor and entrapment of aggressive Cl−), resulting in self-healing behavior. Dong et al. (2014) explored the active protection property of the epoxy coating containing 2-mercaptobenzothiazole doped Zn-Al-LDHs. The corrosion inhibitors are released via ion exchange mechanism nearby the scratched area of the coating, which provides corrosion protection on demand (Fig. 7a). As an example of LDHs, Fig. 7b & c) presents the morphology and structure of Zn-Al-NO3- and Zn-Al-PO43- LDHs.
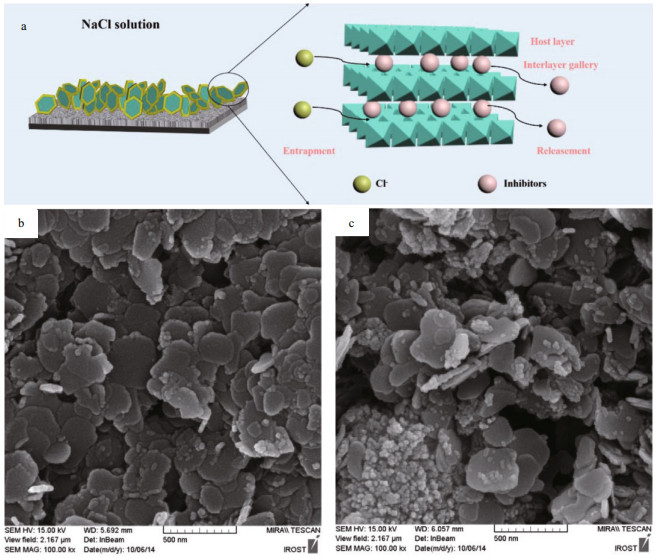
|
| Fig.7 Schematic illustration of the corrosion protection mechanism of LDHs (a); SEM micrographs of Zn-Al-NO3- (b) and Zn-Al-PO43- LDHs (c) a. reprinted with permission (Wang et al., 2020). Copyright 2020, Elsevier; b & c. reprinted with permission (Alibakhshi et al., 2017). Copyright 2017, Elsevier. |
In the meantime, metallic materials have been increasingly suffered from polluted environment of sulfide ions (S2−), which stimulating and accelerating the dissolution of metal ions from a virgin metal surface by formation of stable sulfides (Eklund, 1974). Zheng et al. (2013) fabricated a MSNs-based S2−-responsive release system using Cu-BTA complexes blocked the openings of the mesopores, which BTA was loaded. The spontaneous leakage of inhibitor was completely avoided and the responsive release began when S2− concentration was higher than 0.02 mmol/L. The hybrid coating containing the smart nanoparticles exhibited S2− feedback-responsive selfhealing performance. Very recently, our group reported a novel metal-phenolic supramolecular film, which can control release of loaded antibacterial agents when S2− concentration is higher than 10 mmol/L or in sulfate reducing bacteria (SRB) environment with a long incubation period (Cai et al., 2020). The smart film keeps a longtime antibacterial performance due to its S2−-responsive character, which enhances the microbiological corrosion resistance.
2.4 Thermo-responsive micro/nanocontainersAs one of the most common stimuli, heat has been widely utilized to trigger self-healing effects. As emerging intelligent materials, shape memory polymers (SMPs) can restore their initial shapes from temporary deformations owing to entropically driven processes induced by external heat stimulus (Behl et al., 2010; Xie, 2011). Recently, thermo-responsive SMPs have been applied to construct new types of self-healing coatings for the corrosion protection. For instance, a great number of studies have been reported on the application of polyurethane (PU) SMPs as selfhealing coatings containing poly(ε-caprolactone) (PCL) soft segments (Jorcin et al., 2010; González-García et al., 2011a, b; Lutz et al., 2014). These researches showed that the cracks can be narrowed to a great extent by shape memory effect above 60℃. However, complete recovery of the barrier properties kept challenging, owing to the absence of healing agents that could fill and glue the crevices. An advancement in the field was carried out by Li and co-workers who proposed a "close-then-heal" idea and designed SMPs-based composites with selfhealing ability (Li and Nettles, 2010; Nji and Li, 2012). This concept stems from the self-healing process of skin wounds, which involving ⅰ) crack closure via the shape memory property, and ⅱ) crack sealing to renew original function. Luo and Mather (2013) reported similar work that a two-step process was prepared via electrospinning PCL microfibers on surface of metal and then infiltrating and curing epoxy within the scaffolds. With increase of temperature from 25℃ to 80℃, the shape memory effect of epoxy was motivated to close cracks and the melt of PCL microfibers flowed into crevices and filled the defects. After cooling, PCL healed the cracks and recovered the barrier property of the coating. An obvious advantage of the strategy is that the closed cracks with the aid of shape memory effect can reduce the dosage of healing agents, thus making complete restorations of comparatively large coating damages.
Recently, the BTA inhibitors were incorporated into a superhydrophobic epoxy-based SMP coating fabricated on carbon steel to simulate the surface microstructure of lotus leaves (Qian et al., 2017). The thermo-sensitive shape memory effect can not only close the coating scratches but also recover the superhydrophobic surface morphology. The released BTA at the damage areas insured timely corrosion resistance ahead of the utilization of heat. In return, the SMP enhanced the inhibition efficiency of BTA by decreasing the size of the coating scratches. In consideration of a practical situation which the damaged coating may be exposed to corrosive media before triggering the SMP-based self-healing, the effects of pre-existing corrosion products in the scratched sites was also studied. The results exhibited that the BTA-doped coating had better crack-closed capacity and adhesion strength than the BTA-free coating in the presence of pre-existing corrosion products. Remarkably, the sunlight illumination can also induce shape memory effect of the coating which improves the surface temperature from nearly 30℃ to over 50℃, indicating that the coatings possess nicer practical application prospect. However, the selfhealing capacity of SMP is restricted at the outdoors without the help of the exogenous heat of an external energy supply (Jorcin et al., 2010; Wang et al., 2016; Cai et al., 2018).
One possible solution to this issue is introducing a handful of photothermal fillers, such as carbon black, carbon nanotubes, aniline black or graphene, which can transform the light energy into the heat energy required for the shape memory effect (Chen et al., 2016; Fang et al., 2017a). Very recently, Fan et al. (2019) fabricated shape-memory polyurethane (SMPU) high-performance self-healing coating that incorporated with two different kinds of microcapsules and applied to corrosion protection of metals. A successful release of the inhibitor Alodine and heat generating agent (HGA) from the damaged microcapsules was achieved. The HGA is composed of malleable iron, carbon powder and vermiculite. The shape memory effect of the polyurethanes was realized via the reaction between the HGA from the HGA-microcapsules and oxygen. The passivation film was generated at the bottom of the scratch due to released inhibitor from the damaged Alodinemicrocapsules. Compared with reference samples, the corrosion resistance evaluations demonstrated that the combination of the Alodine conversion coating and the recovery polyurethane can be an effective means to fabricate self-healing coatings with induction heating (Fig. 8).
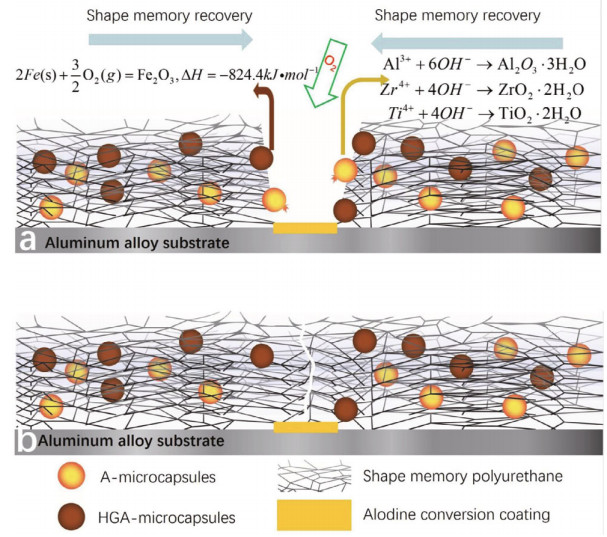
|
| Fig.8 Self-healing mechanism: the Alodine forms a passivation film on the scratched substrate and the released heat via the exothermic reaction is applied to the shape-memory recovery of polyurethane Reprinted with permission (Fan et al., 2019). Copyright 2019, Elsevier. |
Meanwhile, intelligent nanovehicles can be designed by using stimuli-responsive polymers that are sensitive to changes of temperature. Li et al. (2013) prepared silica/polymer double-walled hybrid nanocontainers containing a hollow cavity, a porous silica inner shell, and a stimuli-responsive polymeric outer shell. The method makes it possible to design smart containers with a controllable release behavior by tuning the polymeric outer shells. For instance, the nanocontainers can be thermo-responsive by installing a poly(N-isopropylacrylamide-co-methylenebisacrylamide) (PNIPAM) outer shell. The PNIPAM outer shell tends to swell at 25℃, while the PNIPAM outer shell is shrink at 50℃, which sealing the BTA molecules in the cavity to prevent diffusion out of the shell.
2.5 Light-sensitive micro/nanocontainersLight energy is widely used in many fields (Cai et al., 2019a; Feng et al., 2020; Yang et al., 2020). Particularly, the light-responsive self-healing system by UV light or visible light is interesting (Habault et al., 2013). Compared to other stimulation, such as pH or temperature, the response to light illumination can be operated remotely, accurately and predictably. It is worth mentioning that the periodic irradiation of sunlight can assure the timely recovery of unexpected cracks or other defects without further check.
There are two major categories of light-sensitive self-healing polymer systems: (ⅰ) dynamic polymers (Kuroki et al., 2013; Ying et al., 2014); (ⅱ) polymer composites with micro/nanocontainers loaded with healing agents (Park and Braun, 2010). The dynamic polymers mainly concern various self-healing reactions. Up to now, UV-induced self-healing polymers have been developed according to reversible photo-crosslinking reactions, such as the cycloaddition of cinnamoyl (Song and Chung, 2013), coumarin (Zhao et al., 2016) or anthracene (Fang et al., 2017b) derivatives, and dynamic covalent bonds, such as disulfides (Michal et al., 2013), allyl sulfides (Meng et al., 2016), alkoxyamines (Telitel et al., 2014) and trithiocarbonates (Amamoto et al., 2011). In comparison, the composite strategy is extremely attractive because the initial chemical structures of the polymers do not need to change and it can heal largesize defects. Particularly, the polymer composite healing method utilizes composites incorporated with active capsules and catalyst/photoinitiator. To prepare such light-triggered self-healing composites, Gao et al. (2015) reported poly(urea-formaldehyde)/titania photo-absorbing hybrid microcapsules loaded with epoxy-based healing agents. Due to the presence of the titania nanoparticles, the capsules can absorb UV light with a wavelength of 330 nm. The photocatalytic performance induced by the photo-absorbing titania nanoparticles accelerates the solidification or healing of the epoxy-based healing agent. It should be noted that multicycle healing process can be realized by the light-responsive self-healing system. Song et al. (2013) capsulated a mixture of healing agent methacryloxypropyl-terminated polydimethylsiloxane (MAT-PDMS) and photoinitiator benzoin isobutyl ether (BIE) into urea-formaldehyde polymer microcapsules. The microcapsules are introduced into a coating matrix to obtain the extrinsic photoinduced self-healing system. When the self-healing coating is damaged, the healing agents are easily released from broken microcapsules, filling the damaged area and then solidifying by the photo-crosslinking reaction induced by UV light or sunlight in the presence of BIE.
Apart from polymer composites with microcapsules, the application of light stimulus has been developed through other self-healing mechanisms, such as controlling the release of corrosion inhibitors from nanocontainers. For example, silver nanoparticles were decorated on the polyelectrolyte multilayers of mesoporous titania and silica as photo-absorption sites, to remotely control the release of loaded inhibitors with high precision in time and space, consequently leading to the end of corrosion process (Skorb et al., 2009b, c). Recently, Chen et al. (2015) fabricated a novel and facile photo-responsive release system for corrosion protection via implanting the azobenzene moieties into the nanochannels of HMSs (Fig. 9). Upon UV radiation, isomerization of azobenzene impeller from thermodynamically stable trans isomer to metastable cis isomer makes the nanopores open, simultaneously releasing inhibitor molecules out of the nanoparticles. Therefore, the corrosion inhibitors will be transferred from the nanocontainers to coating damaged areas, which preventing diffusion of anticorrosion agents and protecting the underlying substrate from severe damage. At present, the development of lightresponsive micro/nanocontainers embedded in passive coatings for effective self-healing anticorrosion protection still remains a great challenge.
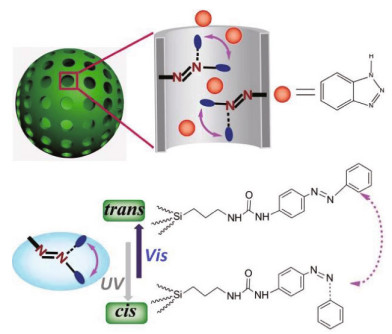
|
| Fig.9 Schematic of reversible release system using trans-cis photo-isomerization of azobenzene molecules grafted in the mesopores of HMSs Reprinted with permission (Chen et al., 2015). Copyright 2015, Royal Society of Chemistry. |
More rarer external trigger used for micro/nanocontainers in self-healing coatings is magnetic field. Conventional self-healing coatings, which often depend on inhibitor passivation containing selfhealing functional additives like active micro/nanocapsules, spatially dispersed in the coatings. The released inhibitors can react with exposed metal and form a passivation film, which not only inhibits the further oxidation of metal, but also suppresses the corrosion of metallic material. However, the corrosion inhibitors randomly disperse throughout self-healing coatings. When mechanical scratch on the self-healing coating occurs, there may not be enough corrosion inhibitors nearby the micro cracks to form a passivation layer to hinder metal corrosion. Inspired from the functionality of the targeted drug delivery (Liu et al., 2011; Kaur et al., 2014), self-healing micro/nanocapsules are designed encapsulated with magnetic materials, which can be aggregated on the surface of the metal with the assist of an external magnetic field.
Wang et al. (2018) prepared the novel magnetic poly(urea-formaldehyde) microcapsules embedded with inhibitors BTA and magnetic multi-wall carbon nanotubes (MWCNTs). The magnetic MWCNTs played a part in the formation process of self-healing magnetic gradient (SMG) coating. The magnetic microcapsules can be easily and quickly migrated in the solution of epoxy resin coating using an external magnetic field (Fig. 10a & b). The SMG coating not only shortened the migration distance of the released BTA, but also accelerated the formation of a passivation layer, and thus the corrosion process of the metal was inhibited (Fig. 10c).
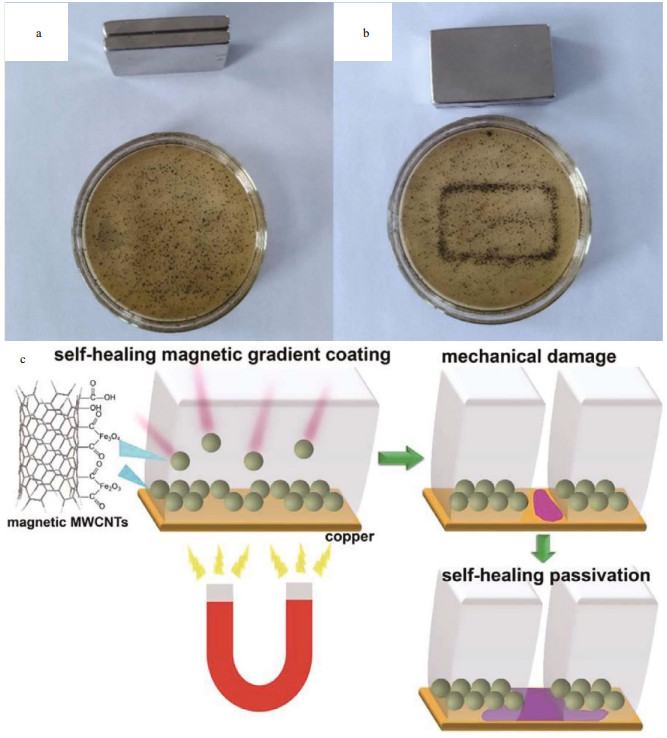
|
| Fig.10 Magnetic microcapsules in the epoxy resin solution (a), aggregated microcapsules induced by a magnet (b), and selfhealing mechanism of the scratched crack of the SMG coating (c) Reprinted with permission (Wang et al., 2018). Copyright 2018, Elsevier. |
Mechanical impact was the first external stimulus confirmed for container-based self-healing coatings by the group of White (White et al., 2001). They encapsulated healing agent (dicyclopentadiene) into polymer microcapsules embedded in an epoxy matrix containing Grubbs' catalyst. When a microcrack forms and starts propagating in this composite, it also penetrates through the capsules. This leads to the liquid dicyclopentadiene to be released, which subsequently moistens and infiltrates the crack. Therefore, the dicyclopentadiene gets in touch with the Grubbs' catalyst embedded in the crack banks, triggering a ring-opening metathesis polymerization process. The resulting polymerization of the healing agent leads to the crack healing and recovery of the barrier properties of the coating. Simultaneous encapsulation of two types of capsules containing different active agents (Keller et al., 2007) or capsules filled with reactive epoxy resin (Caruso et al., 2008) or oil (He and Shi, 2009) was also reported.
In recent years, the development of anti-corrosion coatings with multiple self-healing mechanisms arose great interest. For instance, corrosion inhibitors and polymerizable healing agents can be packaged together or separately into microcapsules (Szabó et al., 2015; Mahmoudian et al., 2018). Leal et al. (2018) fabricated microcapsules containing linseed oil that BTA was entrapped between polyelectrolytes layers assembled outside the microcapsules by Layer-by-Layer (LBL) assembly. These microcapsules were embedded into epoxy resin and coated on a carbon steel substrate. Due to the release of the linseed oil by mechanical stimulus and the controlled release of BTA by pH stimulus, the coating presented effective corrosion protection (Fig. 11).
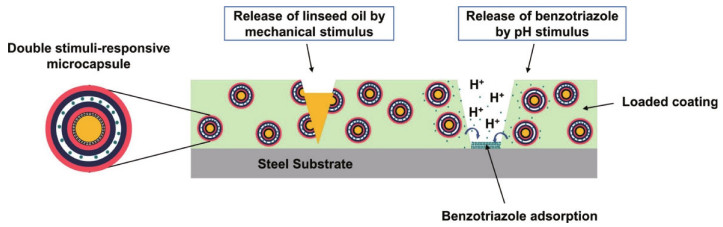
|
| Fig.11 Schematic illustration of the internal structure of the microcapsules and the self-healing mechanisms of the coating Reprinted with permission (Leal et al., 2018). Copyright 2018, Elsevier. |
This review summarizes the recent studies in various micro/nanocontainers encapsulated with diverse functional agents for intelligent anticorrosion coatings for metal protection. The design and preparation of polymeric or inorganic containers with versatile functionalities provide insights for developing a new generation of smart coatings with self-healing property in response to different stimuli such as pH, electrochemical potential, redox, aggressive corrosive ions, heat, light, magnetic field and mechanical impact. In spite of the remarkable progresses have been obtained, many challenges in both science and technology of this field remained and will give directions for future research. Future studies in this field will focus on the following goals: (Ⅰ) developing multifunctional micro/nanocontainers that can load several functional agents and respond to different triggering impacts, (Ⅱ) developing novel shell components and decreasing the container size, meanwhile, maintaining high loading capacity, and (Ⅲ) developing simple, cost-effective, eco-friendly, and reproducible preparation methods. Meeting these important challenges may require cooperative endeavors from researchers in the fields of corrosion science, material science, electrochemistry and chemical engineering to make extraordinary progress.
4 DATA AVAILABILITY STATEMENTData sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
5 ACKNOWLEDGMENTThe authors thank anonymous reviewers of the journal for their careful scrutiny and constructive comments which improved the manuscript.
Alibakhshi E, Ghasemi E, Mahdavian M, Ramezanzadeh B. 2017. A comparative study on corrosion inhibitive effect of nitrate and phosphate intercalated Zn-Al-layered double hydroxides (LDHs) nanocontainers incorporated into a hybrid silane layer and their effect on cathodic delamination of epoxy topcoat. Corrosion Science, 115: 159-174.
DOI:10.1016/j.corsci.2016.12.001 |
Amamoto Y, Kamada J, Otsuka H, Takahara A, Matyjaszewski K. 2011. Repeatable photoinduced self-healing of covalently cross-linked polymers through reshuffling of trithiocarbonate units. Angewandte Chemie International Edition, 50(7): 1 660-1 663.
DOI:10.1002/anie.201003888 |
Andreeva D V, Fix D, Mohwald H, Shchukin D G. 2008. Buffering polyelectrolyte multilayers for active corrosion protection. Journal of Materials Chemistry, 18(15): 1 738-1 740.
DOI:10.1039/b801314d |
Andreeva D V, Skorb E V, Shchukin D G. 2010. Layer-bylayer polyelectrolyte/inhibitor nanostructures for metal corrosion protection. ACS Applied Materials & Interfaces, 2(7): 1 954-1 962.
DOI:10.1021/am1002712 |
Behl M, Razzaq M Y, Lendlein A. 2010. Multifunctional shape-memory polymers. Advanced Materials, 22(31): 3 388-3 410.
DOI:10.1002/adma.200904447 |
Bierwagen G, Brown R, Battocchi D, Hayes S. 2010. Active metal-based corrosion protective coating systems for aircraft requiring no-chromate pretreatment. Progress in Organic Coatings, 68(1-2): 48-61.
DOI:10.1016/j.porgcoat.2009.10.031 |
Buchheit R G, Guan H, Mahajanam S, Wong F. 2003. Active corrosion protection and corrosion sensing in chromate-free organic coatings. Progress in Organic Coatings, 47(3-4): 174-182.
DOI:10.1016/j.porgcoat.2003.08.003 |
Cai H Y, Sun L, Wang Y M, Song T W, Bao M T, Yang X L. 2019a. Unprecedented efficient degradation of phenanthrene in water by intimately coupling novel ternary composite Mn3O4/MnO2-Ag3PO4 and functional bacteria under visible light irradiation. Chemical Engineering Journal, 369: 1 078-1 092.
DOI:10.1016/j.cej.2019.03.143 |
Cai H Y, Wang P, Chen X T, Wang Y, Zhang D. 2020. Sulfide ions-induced release of biocides from a metal-phenolic supramolecular film fabricated on aluminum for inhibition of microbially influenced corrosion. Corrosion Science, 167: 108 534.
DOI:10.1016/j.corsci.2020.108534 |
Cai H Y, Wang P, Zhang D. 2019b. pH-responsive linkagesenabled layer-by-layer assembled antibacterial and antiadhesive multilayer films with polyelectrolyte nanocapsules as biocide delivery vehicles. Journal of Drug Delivery Science and Technology, 54: 101 251.
DOI:10.1016/j.jddst.2019.101251 |
Cai T T, Huo S G, Wang T, Sun W X, Tong Z. 2018. Selfhealable tough supramolecular hydrogels crosslinked by poly-cyclodextrin through host-guest interaction. Carbohydrate Polymers, 193: 54-61.
DOI:10.1016/j.carbpol.2018.03.039 |
Capelossi V R, Poelman M, Recloux I, Hernandez R P B, de Melo H G, Olivier M G. 2014. Corrosion protection of clad 2024 aluminum alloy anodized in tartaric-sulfuric acid bath and protected with hybrid sol-gel coating. Electrochimica Acta, 124: 69-79.
DOI:10.1016/j.electacta.2013.09.004 |
Caruso M M, Blaiszik B J, White S R, Sottos N R, Moore J S. 2008. Full recovery of fracture toughness using a nontoxic solvent-based self-healing system. Advanced Functional Materials, 18(13): 1 898-1 904.
DOI:10.1002/adfm.200800300 |
Chang D F, Gao Y F, Wang L J, Liu G, Chen Y H, Wang T, Tao W, Mei L, Huang L Q, Zeng X W. 2016. Polydopaminebased surface modification of mesoporous silica nanoparticles as pH-sensitive drug delivery vehicles for cancer therapy. Journal of Colloid and Interface Science, 463: 279-287.
DOI:10.1016/j.jcis.2015.11.001 |
Chen J M, Fang L, Xu Z Z, Lu C H. 2016. Self-healing epoxy coatings curing with varied ratios of diamine and monoamine triggered via near-infrared light. Progress in Organic Coatings, 101: 543-552.
DOI:10.1016/j.porgcoat.2016.09.020 |
Chen J Y, Chen X B, Li J L, Tang B, Birbilis N, Wang X G. 2014. Electrosprayed PLGA smart containers for active anti-corrosion coating on magnesium alloy AMlite. Journal of Materials Chemistry A, 2(16): 5 738-5 743.
DOI:10.1039/c3ta14999d |
Chen J, Song Y W, Shan D Y, Han E H. 2013. Modifications of the hydrotalcite film on AZ31 Mg alloy by phytic acid: the effects on morphology, composition and corrosion resistance. Corrosion Science, 74: 130-138.
DOI:10.1016/j.corsci.2013.04.034 |
Chen T, Chen R P, Jin Z, Liu J. 2015. Engineering hollow mesoporous silica nanocontainers with molecular switches for continuous self-healing anticorrosion coating. Journal of Materials Chemistry A, 3(18): 9 510-9 516.
DOI:10.1039/c5ta01188d |
Chen T, Fu J J. 2012. An intelligent anticorrosion coating based on pH-responsive supramolecular nanocontainers. Nanotechnology, 23(50): 505 705.
DOI:10.1088/0957-4484/23/50/505705 |
Chen X T, Wang P, Zhang D. 2019. Designing a superhydrophobic surface for enhanced atmospheric corrosion resistance based on coalescence-induced droplet jumping behavior. ACS Applied Materials & Interfaces, 11(41): 38 276-38 284.
DOI:10.1021/acsami.9b11415 |
Christodoulou C, Glass G, Webb J, Austin S, Goodier C. 2010. Assessing the long term benefits of impressed current cathodic protection. Corrosion Science, 52(8): 2 671-2 679.
DOI:10.1016/j.corsci.2010.04.018 |
Clark W J, Ramsey J D, McCreery R L, McCreery R L, Frankel G S. 2002. A galvanic corrosion approach to investigating chromate effects on aluminum alloy 2024-T3. Journal of the Electrochemical Society, 149(5): B179-B185B.
DOI:10.1149/1.1469031 |
Cui J, Li X Q, Pei Z Q, Pei Y S. 2019. A long-term stable and environmental friendly self-healing coating with polyaniline/sodium alginate microcapsule structure for corrosion protection of water-delivery pipelines. Chemical Engineering Journal, 358: 379-388.
DOI:10.1016/j.cej.2018.10.062 |
Cui L Y, Gao S D, Li P P, Zeng R C, Zhang F, Li S Q, Han E H. 2017. Corrosion resistance of a self-healing micro-arc oxidation/polymethyltrimethoxysilane composite coating on magnesium alloy AZ31. Corrosion Science, 118: 84-95.
DOI:10.1016/j.corsci.2017.01.025 |
Ding C D, Liu Y, Wang M D, Wang T, Fu J J. 2016. Selfhealing, superhydrophobic coating based on mechanized silica nanoparticles for reliable protection of magnesium alloys. Journal of Materials Chemistry A, 4(21): 8 041-8 052.
DOI:10.1039/c6ta02575g |
Ding C D, Xu J H, Tong L, Gong G C, Jiang W, Fu J J. 2017a. Design and fabrication of a novel stimulus-feedback anticorrosion coating featured by rapid self-healing functionality for the protection of magnesium alloy. ACS Applied Materials & Interfaces, 9(24): 21 034-21 047.
DOI:10.1021/acsami.7b06347 |
Ding C D, Xu J H, Tong L, Gong G C, Jiang W, Fu J J. 2017b. Design and fabrication of a novel stimulus-feedback anticorrosion coating featured by rapid self-healing functionality for the protection of magnesium alloy. ACS Applied Materials & Interfaces, 9(24): 21 034-21 047.
DOI:10.1021/acsami.7b06347 |
Dong Y H, Wang F, Zhou Q. 2014. Protective behaviors of 2-mercaptobenzothiazole intercalated Zn-Al-layered double hydroxide coating. Journal of Coatings Technology and Research, 11(5): 793-803.
DOI:10.1007/s11998-014-9568-9 |
Dou W W, Wu J J, Gu T Y, Wang P, Zhang D. 2018. Preparation of super-hydrophobic micro-needle CuO surface as a barrier against marine atmospheric corrosion. Corrosion Science, 131: 156-163.
DOI:10.1016/j.corsci.2017.11.012 |
Du Y J, Damron M, Tang G, Zheng H X, Chu C J, Osborne J H. 2001. Inorganic/organic hybrid coatings for aircraft aluminum alloy substrates. Progress in Organic Coatings, 41(4): 226-232.
DOI:10.1016/S0300-9440(01)00133-3 |
Eklund G S. 1974. Initiation of pitting at sulfide inclusions in stainless steel. Journal of the Electrochemical Society, 121(4): 467-473.
DOI:10.1149/1.2401840 |
Fan W J, Zhang Y, Li W H, Wang W, Zhao X D, Song L Y. 2019. Multi-level self-healing ability of shape memory polyurethane coating with microcapsules by induction heating. Chemical Engineering Journal, 368: 1 033-1 044.
DOI:10.1016/j.cej.2019.03.027 |
Fang L, Chen J M, Zou Y T, Chen S P, Fang T Y, Lu C H, Xu Z Z. 2017a. Self-healing epoxy coatings via focused sunlight based on photothermal effect. Macromolecular Materials and Engineering, 302(9): 1700059.
DOI:10.1002/mame.201700059 |
Fang Y L, Du X S, Du Z L, Wang H B, Cheng X. 2017b. Lightand heat-triggered polyurethane based on dihydroxyl anthracene derivatives for self-healing applications. Journal of Materials Chemistry A, 5(17): 8 010-8 017.
DOI:10.1039/c7ta00871f |
Feng C J, Yang X L, Sun Z L, Xue J, Sun L R, Wang J H, He Z L, Yu J Q. 2020. Dual interfacial synergism in Au-Pd/ZnIn2S4 for promoting photocatalytic selective oxidation of aromatic alcohol. Applied Surface Science, 501: 144018.
DOI:10.1016/j.apsusc.2019.144018 |
Feng Y C, Chen S G, Cheng Y F. 2017. Fabrication of SiO2 nanoparticle-polyelectrolyte nanocontainers with preloaded benzotriazole inhibitors and their self-releasing mechanism and kinetics. Journal of Materials Science, 52(14): 8 576-8 590.
DOI:10.1007/s10853-017-1074-x |
Fu J J, Chen T, Wang M D, Yang N W, Li S N, Wang Y, Liu X D. 2013a. Acid and alkaline dual stimuli-responsive mechanized hollow mesoporous silica nanoparticles as smart nanocontainers for intelligent anticorrosion coatings. ACS Nano, 7(12): 11 397-11 408.
DOI:10.1021/nn4053233 |
Fu J J, Chen T, Wang M D, Yang N W, Li S N, Wang Y, Liu X D. 2013b. Acid and alkaline dual stimuli-responsive mechanized hollow mesoporous silica nanoparticles as smart nanocontainers for intelligent anticorrosion coatings. ACS Nano, 7(12): 11 397-11 408.
DOI:10.1021/nn4053233 |
Gao L, He J L, Hu J, Wang C. 2015. Photoresponsive selfhealing polymer composite with photoabsorbing hybrid microcapsules. ACS Applied Materials & Interfaces, 7(45): 25 546-25 552.
DOI:10.1021/acsami.5b09121 |
González-García Y, Mol J M C, Muselle T, De Graeve I, Van Assche G, Scheltjens G, Van Mele B, Terryn H. 2011a. A combined mechanical, microscopic and local electrochemical evaluation of self-healing properties of shape-memory polyurethane coatings. Electrochimica Acta, 56(26): 9 619-9 626.
DOI:10.1016/j.electacta.2011.03.081 |
González-García Y, Mol J, Muselle T, De Graeve I, Van Assche G, Scheltjens G, Van Mele B, Terryn H. 2011b. SECM study of defect repair in self-healing polymer coatings on metals. Electrochemistry Communications, 13(2): 169-173.
DOI:10.1016/j.elecom.2010.12.005 |
Gupta G, Birbilis N, Cook A B, Khanna A S. 2013. Polyanilinelignosulfonate/epoxy coating for corrosion protection of AA2024-T3. Corrosion Science, 67: 256-267.
DOI:10.1016/j.corsci.2012.10.022 |
Habault D, Zhang H J, Zhao Y. 2013. Light-triggered selfhealing and shape-memory polymers. Chemical Society Reviews, 42(17): 7 244-7 256.
DOI:10.1039/c3cs35489j |
Haddadi S A, Ramazani S A A, Mahdavian M, Taheri P, Mol J M C. 2018. Fabrication and characterization of graphenebased carbon hollow spheres for encapsulation of organic corrosion inhibitors. Chemical Engineering Journal, 352: 909-922.
DOI:10.1016/j.cej.2018.06.063 |
Hang T T X, Truc T A, Duong N T, Pébère N, Olivier M G. 2012. Layered double hydroxides as containers of inhibitors in organic coatings for corrosion protection of carbon steel. Progress in Organic Coatings, 74(2): 343-348.
DOI:10.1016/j.porgcoat.2011.10.020 |
He X D, Shi X M. 2009. Self-repairing coating for corrosion protection of aluminum alloys. Progress in Organic Coatings, 65(1): 37-43.
DOI:10.1016/j.porgcoat.2008.09.003 |
Hou B R, Li X G, Ma X M, Du C W, Zhang A W, Zheng M, Xu W C, Lu D Z, Ma F B. 2017. The cost of corrosion in China. NPJ Materials Degradation, 1(1): 4.
DOI:10.1038/s41529-017-0005-2 |
Huang M X, Yang J L. 2011. Facile microencapsulation of HDI for self-healing anticorrosion coatings. Journal of Materials Chemistry, 21(30): 11 123-11 130.
DOI:10.1039/c1jm10794a |
Jorcin J B, Scheltjens G, Van Ingelgem Y, Tourwé E, Van Assche G, De Graeve I, Van Mele B, Terryn H, Hubin A. 2010. Investigation of the self-healing properties of shape memory polyurethane coatings with the 'odd random phase multisine' electrochemical impedance spectroscopy. Electrochimica Acta, 55(21): 6 195-6 203.
DOI:10.1016/j.electacta.2010.01.027 |
Kaur R, Hasan A, Iqbal N, Alam S, Saini M K, Raza S K. 2014. Synthesis and surface engineering of magnetic nanoparticles for environmental cleanup and pesticide residue analysis: a review. Journal of Separation Science, 37(14): 1 805-1 825.
DOI:10.1002/jssc.201400256 |
Keller M W, White S R, Sottos N R. 2007. A self-healing poly(dimethyl siloxane) elastomer. Advanced Functional Materials, 17(14): 2 399-2 404.
DOI:10.1002/adfm.200700086 |
Kendig M, Hon M. 2005. A hydrotalcite-like pigment containing an organic anion corrosion inhibitor. Electrochemical and Solid State Letters, 8(3): B10-B11.
DOI:10.1149/1.1857743 |
Kermannezhad K, Chermahini A N, Momeni M M, Rezaei B. 2016. Application of amine-functionalized MCM-41 as pH-sensitive nano container for controlled release of 2-mercaptobenzoxazole corrosion inhibitor. Chemical Engineering Journal, 306: 849-857.
DOI:10.1016/j.cej.2016.08.004 |
Kowalski D, Ueda M, Ohtsuka T. 2010. Self-healing ionpermselective conducting polymer coating. Journal of Materials Chemistry, 20(36): 7 630-7 633.
DOI:10.1039/c0jm00866d |
Krishnamoorthy K, Jeyasubramanian K, Premanathan M, Subbiah G, Shin H S, Kim S J. 2014. Graphene oxide nanopaint. Carbon, 72: 328-337.
DOI:10.1016/j.carbon.2014.02.013 |
Kuroki H, Tokarev I, Nykypanchuk D, Zhulina E, Minko S. 2013. Stimuli-responsive materials with self-healing antifouling surface via 3D polymer grafting. Advanced Functional Materials, 23(36): 4 593-4 600.
DOI:10.1002/adfm.201300363 |
Leal D A, Riegel-Vidotti I C, Ferreira M G S, Marino C E B. 2018. Smart coating based on double stimuli-responsive microcapsules containing linseed oil and benzotriazole for active corrosion protection. Corrosion Science, 130: 56-63.
DOI:10.1016/j.corsci.2017.10.009 |
Lee M W, An S, Jo H S, Yoon S S, Yarin A L. 2015. Selfhealing nanofiber-reinforced polymer composites. 1.tensile testing and recovery of mechanical properties.ACS Applied Materials & Interfaces, 7(35): 19 546-19 554.
DOI:10.1021/acsami.5b05998 |
Li G L, Zheng Z L, Mö hwald H, Shchukin D G. 2013. Silica/polymer double-walled hybrid nanotubes: synthesis and application as stimuli-responsive nanocontainers in selfhealing coatings. ACS Nano, 7(3): 2 470-2 478.
DOI:10.1021/nn305814q |
Li G Q, Nettles D. 2010. Thermomechanical characterization of a shape memory polymer based self-repairing syntactic foam. Polymer, 51(3): 755-762.
|
Liu J, Qiao S Z, Hu Q H, Lu G Q. 2011. Magnetic nanocomposites with mesoporous structures: synthesis and applications. Small, 7(4): 425-443.
DOI:10.1002/smll.201001402 |
Luo X F, Mather P T. 2013. Shape memory assisted selfhealing coating. ACS Macro Letters, 2(2): 152-156.
DOI:10.1021/mz400017x |
Luo X H, Yuan S, Pan X Y, Zhang C X, Du S, Liu Y L. 2017. Synthesis and enhanced corrosion protection performance of reduced graphene oxide nanosheet/ZnAl layered double hydroxide composite films by hydrothermal continuous flow method. ACS Applied Materials & Interfaces, 9(21): 18 263-18 275.
DOI:10.1021/acsami.7b02580 |
Lutz A, van den Berg O, van Damme J, Verheyen K, Bauters E, De Graeve I, Du Prez F E, Terryn H. 2014. A shaperecovery polymer coating for the corrosion protection of metallic surfaces. ACS Applied Materials & Interfaces, 7(1): 175-183.
DOI:10.1021/am505621x |
Mahajanam S P V, Buchheit R G. 2008. Characterization of inhibitor release from Zn-Al-. Corrosion, 64(3): 230-240.
DOI:10.5006/1.3278468 |
Mahmoudian M R, Alias Y, Basirun W J. 2012. Effect of narrow diameter polyaniline nanotubes and nanofibers in polyvinyl butyral coating on corrosion protective performance of mild steel. Progress in Organic Coatings, 75(4): 301-308.
DOI:10.1016/j.porgcoat.2012.08.004 |
Mahmoudian M, Nozad E, Kochameshki M G, et al. 2018. Preparation and investigation of hybrid self-healing coatings containing linseed oil loaded nanocapsules, potassium ethyl xanthate and benzotriazole on copper surface. Progress in Organic Coatings, 120: 167-178.
DOI:10.1016/j.porgcoat.2018.03.014 |
Makhlouf A S H. 2014. Handbook of Smart Coatings for Materials Protection. Elsevier, New York.
|
Meng Y, Yang J C, Lewis C L, Jiang J S, Anthamatten M. 2016. Photoinscription of chain anisotropy into polymer networks. Macromolecules, 49(23): 9 100-9 107.
DOI:10.1021/acs.macromol.6b01990 |
Michal B T, Jaye C A, Spencer E J, Rowan S J. 2013. Inherently photohealable and thermal shape-memory polydisulfide networks. ACS Macro Letters, 2(8): 694-699.
DOI:10.1021/mz400318m |
Nji J, Li G Q. 2012. Damage healing ability of a shapememory-polymer-based particulate composite with small thermoplastic contents. Smart Materials and Structures, 21(2): 025011.
DOI:10.1088/0964-1726/21/2/025011 |
Ostrowski N, Lee B, Enick N, Carlson B, Kunjukunju S, Roy A, Kumta P N. 2013. Corrosion protection and improved cytocompatibility of biodegradable polymeric layer-bylayer coatings on AZ31 magnesium alloys. Acta Biomaterialia, 9(10): 8 704-8 713.
DOI:10.1016/j.actbio.2013.05.010 |
Park J H, Braun P V. 2010. Coaxial electrospinning of selfhealing coatings. Advanced Materials, 22(4): 496-499.
DOI:10.1002/adma.200902465 |
Poznyak S K, Tedim J, Rodrigues L M, Salak A N, Zheludkevich M L, Dick L F P, Ferreira M G S. 2009. Novel inorganic host layered double hydroxides intercalated with guest organic inhibitors for anticorrosion applications. ACS Applied Materials & Interfaces, 1(10): 2 353-2 362.
DOI:10.1021/am900495r |
Qian B, Michailidis M, Bilton M, Hobson T, Zheng Z L, Shchukin D. 2019a. Tannic complexes coated nanocontainers for controlled release of corrosion inhibitors in self-healing coatings. Electrochimica Acta, 297: 1 035-1 041.
DOI:10.1016/j.electacta.2018.12.062 |
Qian B, Zheng Z L, Michailids M, Fleck N, Bilton M, Song Y, Li G L, Shchukin D. 2019b. Mussel-inspired self-healing coatings based on polydopamine-coated nanocontainers for corrosion protection. ACS Applied Materials & Interfaces, 11(10): 10 283-10 291.
DOI:10.1021/acsami.8b21197 |
Qian H C, Xu D K, Du C W, Zhang D W, Li X G, Huang L Y, Deng L P, Tu Y C, Mol J M C, Terryn H A. 2017. Dualaction smart coatings with a self-healing superhydrophobic surface and anti-corrosion properties. Journal of Materials Chemistry A, 5(5): 2 355-2 364.
DOI:10.1039/C6TA10903A |
Serdechnova M, Kallip S, Ferreira M G S, Zheludkevich M L. 2014. Active self-healing coating for galvanically coupled multi-material assemblies. Electrochemistry Communications, 41: 51-54.
DOI:10.1016/j.elecom.2014.01.023 |
Singh H K, Yeole K V, Mhaske S T. 2016. Synthesis and characterization of layer-by-layer assembled magnesium zinc molybdate nanocontainer for anticorrosive application. Chemical Engineering Journal, 295: 414-426.
DOI:10.1016/j.cej.2016.03.055 |
Siva T, Sathiyanarayanan S. 2015. Self healing coatings containing dual active agent loaded urea formaldehyde(UF) microcapsules. Progress in Organic Coatings, 82: 57-67.
DOI:10.1016/j.porgcoat.2015.01.010 |
Skorb E V, Fix D, Andreeva D V, Möhwald H, Shchukin D G. 2009a. Surface-modified mesoporous sio2 containers for corrosion protection. Advanced Functional Materials, 19(15): 2 373-2 379.
DOI:10.1002/adfm.200801804 |
Skorb E V, Skirtach A G, Sviridov D V, Shchukin D G, Möhwald H. 2009b. Laser-controllable coatings for corrosion protection. ACS Nano, 3(7): 1 753-1 760.
DOI:10.1021/nn900347x |
Skorb E V, Sviridov D V, Möhwald H, Shchukin D G. 2009c. Light responsive protective coatings. Chemical Communications, (40): 6 041-6 043.
DOI:10.1039/b914257f |
Snihirova D, Lamaka S V, Taryba M, Salak A N, Kallip S, Zheludkevich M L, Ferreira M G S, Montemor M F. 2010. Hydroxyapatite microparticles as feedback-active reservoirs of corrosion inhibitors. ACS Applied Materials & Interfaces, 2(11): 3 011-3 022.
DOI:10.1021/am1005942 |
Song Y K, Chung C M. 2013. Repeatable self-healing of a microcapsule-type protective coating. Polymer Chemistry, 4(18): 4 940-4 947.
DOI:10.1039/c3py00102d |
Song Y K, Jo Y H, Lim Y J, Cho S Y, Yu H C, Ryu B C, Lee S I, Chung C M. 2013. Sunlight-induced self-healing of a microcapsule-type protective coating. ACS Applied Materials & Interfaces, 5(4): 1 378-1 384.
DOI:10.1021/am302728m |
Sun D W, An J L, Wu G, Yang J L. 2015. Double-layered reactive microcapsules with excellent thermal and nonpolar solvent resistance for self-healing coatings. Journal of Materials Chemistry A, 3(8): 4 435-4 444.
DOI:10.1039/c4ta05339g |
Sun D W, Chong Y B, Chen K, Yang J L. 2018. Chemically and thermally stable isocyanate microcapsules having good self-healing and self-lubricating performances. Chemical Engineering Journal, 346: 289-297.
DOI:10.1016/j.cej.2018.04.046 |
Szabó T, Telegdi J, Nyikos L. 2015. Linseed oil-filled microcapsules containing drier and corrosion inhibitorTheir effects on self-healing capability of paints. Progress in Organic Coatings, 84: 136-142.
DOI:10.1016/j.porgcoat.2015.02.020 |
Tatiya P D, Hedaoo R K, Mahulikar P P, Gite V V. 2013. Novel polyurea microcapsules using dendritic functional monomer: synthesis, characterization, and its use in selfhealing and anticorrosive polyurethane coatings. Industrial & Engineering Chemistry Research, 52(4): 1 562-1 570.
DOI:10.1021/ie301813a |
Tavandashti N P, Ghorbani M, Shojaei A, Gonzalez-Garcia Y, Terryn H, Mol J M C. 2016a. pH responsive Ce(Ⅲ) loaded polyaniline nanofibers for self-healing corrosion protection of AA2024-T3. Progress in Organic Coatings, 99: 197-209.
DOI:10.1016/j.porgcoat.2016.04.046 |
Tavandashti N P, Ghorbani M, Shojaei A, Mol J M C, Terryn H, Baert K, Gonzalez-Garcia Y. 2016b. Inhibitor-loaded conducting polymer capsules for active corrosion protection of coating defects. Corrosion Science, 112: 138-149.
DOI:10.1016/j.corsci.2016.07.003 |
Tedim J, Poznyak S K, Kuznetsova A, Raps D, Hack T, Zheludkevich M L, Ferreira M G S. 2010. Enhancement of active corrosion protection via combination of inhibitor-loaded nanocontainers. ACS Applied Materials & Interfaces, 2(5): 1 528-1 535.
DOI:10.1021/am100174t |
Tedim J, Zheludkevich M L, Bastos A C, Salak A N, Lisenkov A D, Ferreira M G S. 2014. Influence of preparation conditions of Layered Double Hydroxide conversion films on corrosion protection. Electrochimica Acta, 117: 164-171.
DOI:10.1016/j.electacta.2013.11.111 |
Telitel S, Amamoto Y, Poly J, Morlet-Savary F, Soppera O, Lalevée J, Matyjaszewski K. 2014. Introduction of selfhealing properties into covalent polymer networks via the photodissociation of alkoxyamine junctions. Polymer Chemistry, 5(3): 921-930.
DOI:10.1039/c3py01162c |
Tran T H, Vimalanandan A, Genchev G, Fickert J, Landfester K, Crespy D, Rohwerder M. 2015. Regenerative Nanohybrid coating tailored for autonomous corrosion protection. Advanced Materials, 27(25): 3 825-3 830.
DOI:10.1002/adma.201501044 |
Twite R L, Bierwagen G P. 1998. Review of alternatives to chromate for corrosion protection of aluminum aerospace alloys. Progress in Organic Coatings, 33(2): 91-100.
DOI:10.1016/S0300-9440(98)00015-0 |
Vimalanandan A, Lv L P, Tran T H, Landfester K, Crespy D, Rohwerder M. 2013. Redox-responsive self-healing for corrosion protection. Advanced Materials, 25(48): 6 980-6 984.
DOI:10.1002/adma.201302989 |
Wang D H, Bierwagen G R. 2009. Sol-gel coatings on metals for corrosion protection. Progress in Organic Coatings, 64(4): 327-338.
DOI:10.1016/j.porgcoat.2008.08.010 |
Wang L T, Deng L P, Zhang D W, Qian H C, Du C W, Li X G, Mol J M C, Terryn H A. 2016. Shape memory composite(SMC) self-healing coatings for corrosion protection. Progress in Organic Coatings, 97: 261-268.
DOI:10.1016/j.porgcoat.2016.04.041 |
Wang M D, Liu M Y, Fu J J. 2015. An intelligent anticorrosion coating based on pH-responsive smart nanocontainers fabricated via a facile method for protection of carbon steel. Journal of Materials Chemistry A, 3(12): 6 423-6 431.
DOI:10.1039/c5ta00417a |
Wang P, Zhang D, Qiu R, Wu J J. 2014. Super-hydrophobic metal-complex film fabricated electrochemically on copper as a barrier to corrosive medium. Corrosion Science, 83: 317-326.
DOI:10.1016/j.corsci.2014.02.028 |
Wang T, Tan L H, Ding C D, Wang M D, Xu J H, Fu J J. 2017. Redox-triggered controlled release systems-based bilayered nanocomposite coating with synergistic selfhealing property. Journal of Materials Chemistry A, 5(4): 1 756-1 768.
DOI:10.1039/c6ta08547d |
Wang W, Li W H, Fan W J, Zhang X Y, Song L Y, Xiong C S, Gao X, Liu X J. 2018. Accelerated self-healing performance of magnetic gradient coating. Chemical Engineering Journal, 332: 658-670.
DOI:10.1016/j.cej.2017.09.112 |
Wang X, Jing C, Chen Y X, Wang X S, Zhao G, Zhang X, Wu L, Liu X Y, Dong B Q, Zhang Y X. 2020. Active corrosion protection of super-hydrophobic corrosion inhibitor intercalated Mg-Al layered double hydroxide coating on AZ31 magnesium alloy. Journal of Magnesium and Alloys, 8(1): 291-300.
DOI:10.1016/j.jma.2019.11.011 |
Wei H G, Ding D W, Wei S Y, Guo Z H. 2013. Anticorrosive conductive polyurethane multiwalled carbon nanotube nanocomposites. Journal of Materials Chemistry A, 1(36): 10 805-10 813.
DOI:10.1039/c3ta11966a |
Wei T, Tang Z C, Yu Q, Chen H. 2017. Smart antibacterial surfaces with switchable bacteria-killing and bacteriareleasing capabilities. ACS Applied Materials & Interfaces, 9(43): 37 511-37 523.
DOI:10.1021/acsami.7b13565 |
White S R, Sottos N R, Geubelle P H, Moore J S, Kessler M R, Sriram S R, Brown E N, Viswanathan S. 2001. Autonomic healing of polymer composites. Nature, 409(6822): 794-797.
DOI:10.1038/35057232 |
Williams G R, O'Hare D. 2006. Towards understanding, control and application of layered double hydroxide chemistry. Journal of Materials Chemistry, 16(30): 3 065-3 074.
DOI:10.1039/B604895A |
Williams G, McMurray H N. 2004. Inhibition of filiform corrosion on polymer coated AA2024-T3 by hydrotalcitelike pigments incorporating organic anions. Electrochemical and Solid-State Letters, 7(5): B13-B15.
DOI:10.1149/1.1691529 |
Xie T. 2011. Recent advances in polymer shape memory. Polymer, 52(22): 4 985-5 000.
DOI:10.1016/j.polymer.2011.08.003 |
Xiong W, Tang J N, Zhu G M, Han N X, Schlangen E, Dong B Q, Wang X F, Xing F. 2015. A novel capsule-based selfrecovery system with a chloride ion trigger. Scientific Reports, 5(1): 10 866.
DOI:10.1038/srep10866 |
Yang J L, Keller M W, Moore J S, White S R, Sottos N R. 2008. Microencapsulation of isocyanates for self-healing polymers. Macromolecules, 41(24): 9 650-9 655.
DOI:10.1021/ma801718v |
Yang T I, Peng C W, Lin Y L, Weng C J, Edgington G, Mylonakis A, Huang T C, Hsu C H, Yeh J M, Wei Y. 2012. Synergistic effect of electroactivity and hydrophobicity on the anticorrosion property of room-temperature-cured epoxy coatings with multi-scale structures mimicking the surface of Xanthosoma sagittifolium leaf. Journal of Materials Chemistry, 22(31): 15 845-15 852.
DOI:10.1039/c2jm32365f |
Yang X L, Yu J Q, Zhang Y, Peng Y H, Li Z, Feng C J, Sun Z L, Yu X F, Cheng J B, Wang Y. 2020. Visible-near-infraredresponsive g-C3N4Hx+ reduced decatungstate with excellent performance for photocatalytic removal of petroleum hydrocarbon. Journal of Hazardous Materials, 381: 120994.
DOI:10.1016/j.jhazmat.2019.120994 |
Yasakau K A, Kallip S, Zheludkevich M L, Ferreira M G S. 2013. Active corrosion protection of AA2024 by sol-gel coatings with cerium molybdate nanowires. Electrochimica Acta, 112: 236-246.
DOI:10.1016/j.electacta.2013.08.126 |
Ying H Z, Zhang Y F, Cheng J J. 2014. Dynamic urea bond for the design of reversible and self-healing polymers. Nature Communications, 5(1): 3 218.
DOI:10.1038/Ncomms4218 |
Yuan L, Liang G Z, Xie J Q, Li L, Guo J. 2006. Preparation and characterization of poly(urea-formaldehyde) microcapsules filled with epoxy resins. Polymer, 47(15): 5 338-5 349.
DOI:10.1016/j.polymer.2006.05.051 |
Zahidah K A, Kakooei S, Ismail M C, Raja P B. 2017. Halloysite nanotubes as nanocontainer for smart coating application: a review. Progress in Organic Coatings, 111: 175-185.
DOI:10.1016/j.porgcoat.2017.05.018 |
Zeng R C, Cui L Y, Jiang K, Liu R, Zhao B D, Zheng Y F. 2016. In vitro corrosion and cytocompatibility of a microarc oxidation coating and poly(L-lactic acid)composite coating on Mg-1Li-1Ca alloy for orthopedic implants. ACS Applied Materials & Interfaces, 8(15): 10 014-10 028.
DOI:10.1021/acsami.6b00527 |
Zeng X, Tao W, Liu G, Mei L. 2017. Polydopamine-based surface modification of copolymeric nanoparticles as a targeted drug delivery system for cancer therapy. Journal of Controlled Release, 259: e150-e151.
DOI:10.1016/j.jconrel.2017.03.303 |
Zhang B B, Zhu Q J, Li Y T, Hou B R. 2018a. Facile fluorinefree one step fabrication of superhydrophobic aluminum surface towards self-cleaning and marine anticorrosion. Chemical Engineering Journal, 352: 625-633.
DOI:10.1016/j.cej.2018.07.074 |
Zhang C, Wang H R, Zhou Q X. 2018b. Preparation and characterization of microcapsules based self-healing coatings containing epoxy ester as healing agent. Progress in Organic Coatings, 125: 403-410.
DOI:10.1016/j.porgcoat.2018.09.028 |
Zhang F, Ju P F, Pan M Q, Zhang D W, Huang Y, Li G L, Li X G. 2018c. Self-healing mechanisms in smart protective coatings: a review. Corrosion Science, 144: 74-88.
DOI:10.1016/j.corsci.2018.08.005 |
Zhao Y H, Vuluga D, Lecamp L, Burel F. 2016. Photoinitiated thiol-epoxy addition for the preparation of photoinduced self-healing fatty coatings. RSC Advances, 6(38): 32 098-32 105.
DOI:10.1039/C6RA03693G |
Zhao Y, Berger R, Landfester K, Crespy D. 2015. Double redox-responsive release of encoded and encapsulated molecules from patchy nanocapsules. Small, 11(25): 2 995-2 999.
DOI:10.1002/smll.201402521 |
Zheludkevich M L, Poznyak S K, Rodrigues L M, Raps D, Hack T, Dick L F, Nunes T, Ferreira M G S. 2010. Active protection coatings with layered double hydroxide nanocontainers of corrosion inhibitor. Corrosion Science, 52(2): 602-611.
DOI:10.1016/j.corsci.2009.10.020 |
Zheludkevich M L, Shchukin D G, Yasakau K A, Möhwald H, Ferreira M G S. 2007. Anticorrosion coatings with selfhealing effect based on nanocontainers impregnated with corrosion inhibitor. Chemistry of Materials, 19(3): 402-411.
DOI:10.1021/cm062066k |
Zheng Z L, Huang X, Schenderlein M, Borisova D, Cao R, Möhwald H, Shchukin D. 2013. Self-healing and antifouling multifunctional coatings based on pH and sulfide ion sensitive nanocontainers. Advanced Functional Materials, 23(26): 3 307-3 314.
DOI:10.1002/adfm.201203180 |
 2020, Vol. 38
2020, Vol. 38


