Institute of Oceanology, Chinese Academy of Sciences
Article Information
- CHEN Chao, WANG Yi, ZHANG Dun
- Bifunctional nanozyme activities of layered double hydroxide derived Co-Al-Ce mixed metal oxides for antibacterial application
- Journal of Oceanology and Limnology, 38(4): 1233-1245
- http://dx.doi.org/10.1007/s00343-020-0041-6
Article History
- Received Jan. 21, 2020
- accepted in principle Mar. 12, 2020
- accepted for publication Mar. 21, 2020
2 University of Chinese Academy of Sciences, Beijing 100049, China;
3 Open Studio for Marine Corrosion and Protection, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266237, China;
4 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
Biofouling is a global issue that can cause increased hydrodynamic drag, fuel consumption, and maintenance costs for marine and industrial equipment (Banerjee et al., 2011; Zhu et al., 2019). However, most of the current antifouling materials would damage the environment through metal leaching and bacteria resistance that may be restricted for the application. Nanozyme has a huge application prospect for environmental and effective antifouling (Natalio et al., 2012; Herget et al., 2017).
Ceria (CeO2-x) nanoparticles have been demonstrated having strong haloperoxidase mimicry against the biofouling in natural environment (Herget et al., 2017). In addition, nanoceria has attracted widespread attention for their good catalytic properties in recent years (Valente et al., 2011; Seftel et al., 2015; Nayak and Parida, 2016; Zhou et al., 2020). Their different catalytic properties depend on difference of nanoceria themselves (e.g., sizes, morphologies, crystal structures and ratios of Ce3+/Ce4+) and the reaction condition (e.g., pH and so on) (Deshpande et al., 2005; Peng et al., 2011; Nicolini et al., 2015). Although most of studies focus on how to enhance the catalytic properties and optimize the synthetic method (Hong et al., 2017; Lebedeva et al., 2019; He et al., 2020), the uncontrolled tendency of nanoceria aggregation weakens the performance. Therefore, system to overcome the agglomeration of nanoceria nanozymes remains a huge challenge (Lin et al., 2014).
Mixed metal oxides (MMOs) are interesting choices resulting from their large surface area, phase purity, and structural stability (Genty et al., 2016; Hao et al., 2017; Smalenskaite et al., 2017). In our previous work, layered double hydroxide (LDH) was demonstrated as a precursor for preparing ultrathin 2D Ni-V MMO nanosheets with an intrinsic peroxidase (POx)-like catalysis activity (Chen et al., 2019). In this system, the agglomeration of active phase can be avoided effectively and thus the stability is improved. This pioneering work inspires us that Ce containing MMOs will be potential high activity nanozyme materials for marine antifouling. However, there is no report about this topic so far.
Considering that the ionic radius Ce3+ (102 pm) is larger than that of most used M3+ to form LDH layer with Co2+ (74 pm), a fully isomorphic substitution of M3+ by Ce3+ can induce a lattice distortion and lead to imperfections in the LDH layers (Wang and Zhang, 2016). Thus, in this paper, Co-Al-Ce LDH is chosen as the precursor to prepare Ce containing MMOs. Herein, we demonstrate the development of Co-Al-Ce MMOs composited of Co3O4 and CoAl2O4 and CeO2 as antibacterial nanozyme materials for the first time (Fig. 1). First, Co-Al-Ce LDH precursor has been synthesized via a hydrothermal route. After calcination, Co-Al-Ce MMOs show attracted performance of intrinsic enzyme-like activities under optimized conditions. Importantly, the enzyme-like activity of Co-Al-Ce MMOs can be tailored by adjusting the heating temperature and molar ratio of Ce. Owing to its high dispersion properties and high specific area, Co-Al-Ce MMOs with 2.5% Ce at 200℃ exhibited excellent catalytic antibacterial activity in near-neutral pH solution. This strategy will open a new route for the well-dispersed CeO2 nanoparticle decorated MMO matrix as nanozyme in marine antifouling.
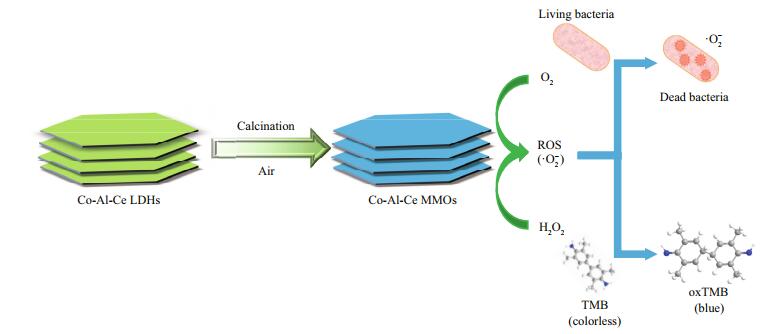
|
| Fig.1 The designed system based on Co-Al-Ce MMOs and H2O2 for the antibacterial and enzyme-like application |
3, 3', 5, 5'-tetramethylbenzidine (TMB), 2', 7'-dichlorodihydrofluorescein diacetate (DCFH-DA), propidium iodide (PI), and SYTO 9 were purchased from Sigma-Aldrich (USA). Hydroxyphenyl fluorescein (HPF) and dihydroethidium (DHE) were purchased from APExBIO (USA) and AAT Bioquest (USA). 30 wt% H2O2 aqueous solution, Co(NO3)2·6H2O, Al(NO3)3·9H2O, Ce(NO3)3·6H2O, urea, isopropanol (IPA), p-benzoquinone (BQ), and other chemicals were of analytical grade and bought from Sinopharm Chemical Reagent Co., Ltd. (China). Milli-Q water (Millipore, USA) was used for preparing all aqueous solutions.
2.2 Preparation of Co-Al-Ce MMOsThe Co-Al-Ce LDH precursor was synthesized using a urea hydrolysis method (Zhang et al., 2017). Co(NO3)2·6H2O (12 mmol), Al(NO3)3·9H2O (4–16×x mmol, x=0%, 1%, 2.5%, and 5%, respectively), Ce(NO3)3·6H2O (16×x mmol), and urea (40 mmol) were dissolved in 70 mL of water vigorous stirring for 20 min. Precursors with different Ce doping contents were denoted as 0CoAlCe, 1CoAlCe, 2.5CoAlCe, and 5CoAlCe. The solution was incubated in a 100-mL Teflon-lined autoclave at 110℃ for 12 h. After hydrothermal treatment, the Co-Al-Ce LDH precipitate was centrifuged and washed with water and anhydrous ethanol three times separately, and dried over 12 h under vacuum at 60℃. The precursors were heated at 200, 300, 400, 500, 600, and 800℃ in air for 2 h with a heating rate of 5℃/min, following cooled to room temperature naturally. Finally, Co-Al-Ce MMOs were formed and denoted as nCoAlCe-200, nCoAlCe-300, nCoAlCe-400, nCoAlCe-500, nCoAlCe-600, and nCoAlCe-800 (n=0, 1, 2.5, and 5), respectively.
2.3 Apparatus and characterizationThe as-prepared samples were characterized with the 2θ ranging from 5° to 80° using Cu K radiation (λ=0.154 18 nm) at 40 kV and 40 mA by X-ray diffractometer (XRD) (Ultima IV X-ray diffractometer, Rigaku, Japan). Transmission electron microscopy (TEM), high-resolution TEM (HRTEM) and selected area electron diffraction (SAED) were captured to analyze the morphological features of the sample by a JEM-2100 transmittance electron microscopy (JEOL, Japan). Energy-dispersive X-ray spectroscopy (EDS) was carried on image captured to investigate the chemical compositions and their content. Fourier transform infrared (FT-IR) spectra were carried out through the standard KBr disk method by a Nicolet iS10 Fourier transform spectrometer (Thermo Fisher Scientific, USA). The Brunauer-Emmett-Teller (BET) measurement was performed on a Micromeritics TriStar II 3020. The surface electronic state was investigated by X-ray photoelectron spectroscopy (XPS) spectrometer (ESCALAB 250, Thermo Fisher Scientific Inc., USA).
2.4 Enzyme-like activityStandard TMB liquid substrate system was used to evaluate enzyme-like activities of Co-Al-Ce MMOs at room temperature. An amount of 40 μg/mL Co-Al-Ce MMO suspension was added into 1 mL of NaAc-HAc buffer solution (pH=4.0) consisting of 1 mmol/L H2O2 and 0.4 mmol/L TMB as the substrate. The color changes can be observed with naked eyes immediately, as the substrate added. After 3 min reactions, the absorbance value at 652 nm of the mixed solution was monitored by an Infinite M1000Pro Tecan microplate spectrophotometer (Switzerland). All the samples were carried out in three parallels and the average values were given.
2.5 Steady-state kinetic analysisThe influence of pH and catalyst concentration was carried out to probe the dependence of the POx mimic catalytic activity of Co-Al-Ce MMOs. The steady-state kinetic analysis was performed under the optimized system of pH=4.0 and the catalyst suspension concentration of 50 μg/mL at room temperature. The kinetic analysis of 2.5CoAlCe-200 was carried out by varying concentrations of H2O2 at a fixed concentration of TMB or vice versa. The concentration of oxidation products derived by TMB were calculated following all obtained absorbance at 652 nm, through the Beer-Lambert' law (A=ɛbC). In the equation, b is the path length, 0.323 7 cm and ɛ is the molar absorption coefficient (39 000 M-1/cm). Then, kinetic parameters were calculated following the Michaelis-Menten formalism:
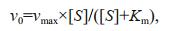
in which v0 is the initial conversion rate reached by the initial slope of absorbance changes with time, [S] is the substrate concentration, Km is the Michaelis constant, and vmax is the maximum conversion rate. Three parallel tests were conducted to ensure the accuracy of the results.
2.6 Bacteria culture and antibacterial activity testEscherichia coli (E. coli) (ATCC 6538) was from CAS Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences. After incubated in Luria-Bertani broth medium, the logarithmic phase bacteria were diluted to ~106 CFU/mL and then added to 1 mL of NaAc-HAc buffer solution (pH=4.0) with various concentrations of H2O2 and the 2.5CoAlCe-200 sample for 2 h. Then, the bacterial strains were diluted to 103 CFU/mL after treatment and cultured on agar plate for 36 h at 30℃. Three parallel plates corresponding to a particular sample were done to obtain the average for antibacterial efficiency.
2.7 Live/dead fluorescent stainingAfter treatment with H2O2 and 2.5CoAlCe-200, E. coli bacteria were cultured with SYTO 9 and PI for 15 min at room temperature in the dark. Then 10 μL of the stained bacterial suspension was added on the microscope slide covered with a coverslip. The live/ dead bacterial cells were then visualized by Olympus BX53M fluorescence microscopy (Japan).
2.8 Measurement of intracellular ROSThe intracellular reactive oxygen species (ROS) level was assessed by the oxidant-sensitive dye DCFH-DA. Then resuspended bacterial cells with 10 μmol/L DCFH-DA were indicated in the dark at room temperature for 30 min. Before detection, the stained bacterial solution was washed with PBS once. Fluorescent probes of DHE and HPF (10 μmol/L) were further used to investigate the specific type of free radicals, ∙O2ˉ and ∙OH. The intracellular ROS level was observed using Olympus BX53M fluorescence microscopy (Japan).
3 RESULT AND DISCUSSION 3.1 Synthesis and characterizations of Co-Al-Ce MMOsThe structural information of Co-Al-Ce LDH precursor with different Ce molar ratios is given by X-ray diffraction patterns. As shown in Fig. 2, all the diffraction peaks can be indexed at 2θ=11.6°, 23.5°, 34.6°, 39.2°, 46.7°, 60.2°, and 61.6°, which correspond to the (003), (006), (012), (015), (018), (110), and (113) reflections of the hexagonal hydrotalcite-like structure of Co-Al LDHs (JCPDS card No. 51-0045, R-3m(166)) (Genty et al., 2019). The d003 value is 0.758 nm, indicating that the major phase is attributed to the carbonate anion intercalated LDHs. Meanwhile, diffraction characteristic peaks of Co-Al LDHs created with Ce molar ratio of 1.0%, 2.5%, and 5.0% match well with Co-Al LDHs, demonstrating that the LDHs phase is well crystallized in these composites with doping Ce3+ less than 5%. This uniform composite structure has a great advantage to construct MMOs. The FT-IR spectra also further confirms the successful composition of Co-Al-Ce LDHs. As showing in Fig. 3, the peak assigned to the stretching vibration of the O-H bond was observed at 3 423/cm due to the metal-OH layer and/or water in the crystal. The peaks observed at 1 552, 1 359, 836, 800 are assigned to v(OCO2), C-O, δ(Co-OH), and δ(CO3), respectively (Zhu et al., 2013; Zhou et al., 2015). The peaks in the skeletal region (613, 548 and 426/cm) are typical M-O bonds of Co-Al LDHs. These results suggest that the precursor is Co-Al-Ce LDHs.
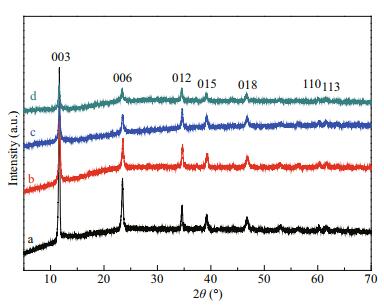
|
| Fig.2 XRD patterns of Co-Al-Ce LDHs with different Ce molar ratios a. 0.0%; b. 1.0%; c. 2.5%; d. 5.0% |
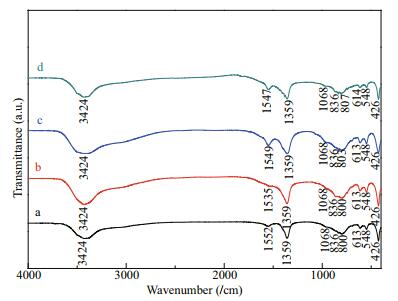
|
| Fig.3 FT-IR spectra of Co-Al-Ce LDHs with different Ce molar ratios a. 0.0%; b. 1.0%; c. 2.5%; d. 5.0% |
XRD patterns of the 2.5CoAlCe precursor heated at different temperatures are given by in Fig. 4. As the heating temperature increases, characteristic peaks of LDHs gradually disappear. Samples heated at 200 and 300℃ become essentially amorphous and the XRD pattern exhibits one set of strong diffraction peaks at 2θ=19.0°, 31.2°, 36.8°, 38.5°, 44.8°, 55.6°, 59.4°, and 65.2°, matching well with the (111), (220), (311), (222), (400), (422), (511), and (440) of cubic Co3O4 (JCPDS card No. 43-1003) and cubic CoAl2O4 (JCPDS card No. 44-0160). After calcined at a higher temperature, the XRD pattern exhibits two sets of diffraction peaks. The other new weak set is attributed to the (111), (220) and (311) reflection of cubic CeO2 (JCPDS card No. 34-0394), indicating that Co-Al-Ce MMOs is composited of CeO2, Co3O4, and CoAl2O4.
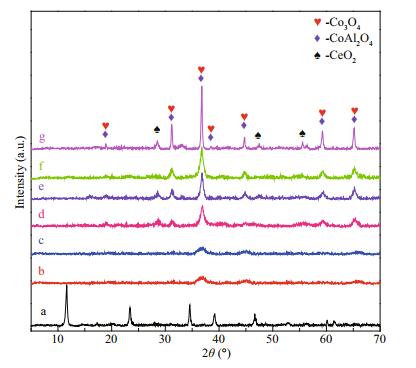
|
| Fig.4 XRD patterns a. 2.5CoAlCe; b. 2.5CoAlCe-200; c. 2.5CoAlCe-300; d. 2.5CoAlCe-400; e. 2.5CoAlCe-500; f. 2.5CoAlCe-600; g. 2.5CoAlCe-800. |
The morphologies and microstructures of 2.5CoAlCe-200 were obtained by TEM. From the TEM morphologies (Fig. 5a & b), it can be seen that 2.5CoAlCe-200 has rough surfaces so that it can offer the high specific surface area. As shown in Fig. 5c, HRTEM was used to investigate the detailed microstructure further. The HRTEM image of 2.5CoAlCe-200 displays clear lattice fringes of Co3O4/CoAl2O4 and CeO2. From the FFT pattern corresponding to the HRTEM image, the calculated distance between the adjacent lattice fringes is 0.244 nm. This value can be ascribed to the d spacing of (311) plane of Co3O4 and CoAl2O4 plane. The distance 0.310 nm agrees with the d spacing of (111) plane of CeO2. The SAED pattern of 2.5CoAlCe-200 (Fig. 5d) displays discontinuous diffused diffraction rings, confirming that CeO2 has been embedded in the Co3O4/CoAl2O4 successfully. The EDS mapping of 2.5CoAlCe-200 further describes the uniformity and high dispersion characteristics of CeO2 nanoparticles mixed with Co3O4/CoAl2O4 nanoparticles (Fig. 5e). According to the EDS analysis result (the insert of Fig. 5f), the actually Ce molar ratio in 2.5CoAlCe-200 is ~2.5, which is very similar to that in the precursor solution.
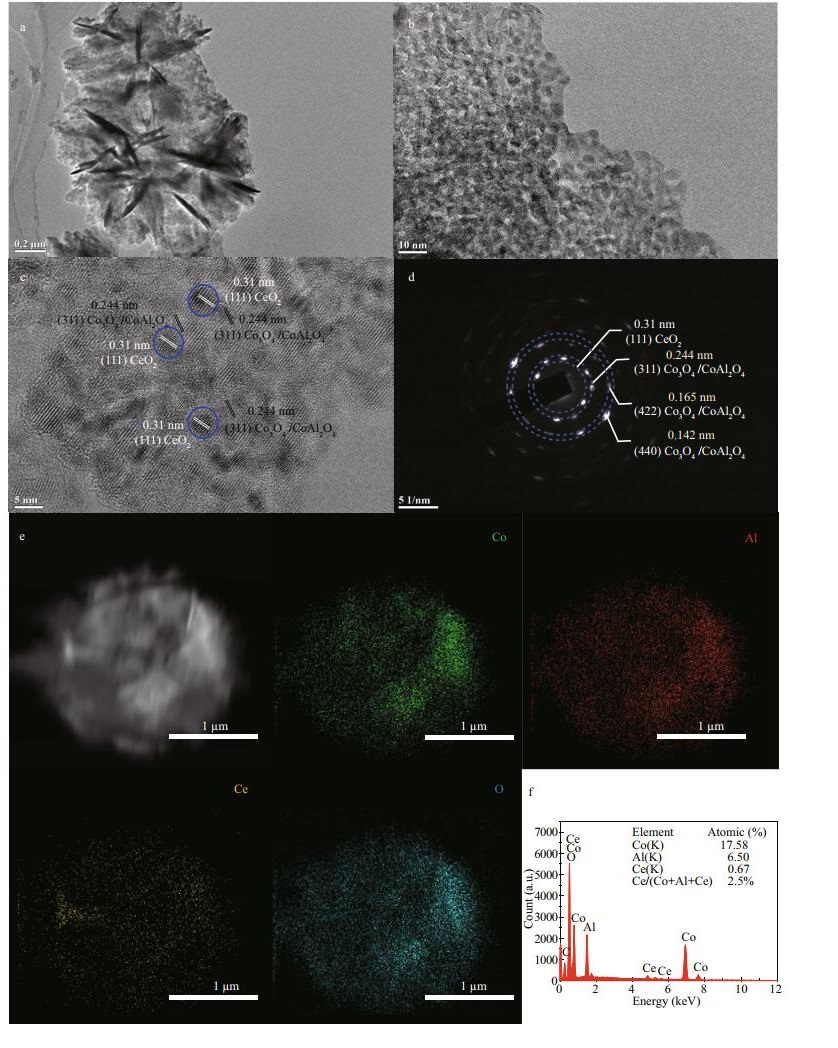
|
| Fig.5 The morphologies and microstructures a & b. TEM images; c. HRTEM image; d. SAED pattern; e. EDS elemental mappings of Co, Al, Ce, and O; f. EDS spectrum of 2.5CoAlCe-200. |
Nitrogen adsorption/desorption experiments were applied to investigate the effect of Ce molar ratios on the specific surface area further. From Fig. 6, when the Ce molar ratios is 2.5%, the specific surface area of 2.5CoAlCe-200 increases to the maximum (150.10±4.95 m2/g), which is higher than that of 0CoAlCe-200 (131.96±4.35 m2/g), 1CoAlCe-200 (98.18±3.09 m2/g), and 5CoAlCe-200 (78.73± 1.17 m2/g).
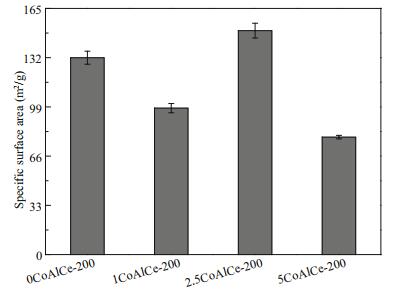
|
| Fig.6 Specific surface area of 0CoAlCe-200, 1CoAlCe-200, 2.5CoAlCe-200, and 5CoAlCe-200 |
The surface chemical compositions and chemical state of Co-Al-Ce MMOs were investigated by XPS. As shown in Fig. 7a, the signal of Co, Al, Ce, and O can be detected in the survey spectra, without any other impurities. As shown in Fig. 7b, the multiple peaks of Ce 3d spectrum are observed, which correspond to mixed valence states of Ce3+ and Ce4+ (Venugopal et al., 2013; Herget et al., 2017). The peaks appearing at 902.4 eV (u), 907.8 eV (u″), and 917.0 eV (u‴) are corresponding to Ce4+ 3d3/2 and the peaks occurring at 882.6 eV (ν), 888.7 eV (ν″), and 898.3 eV (ν‴) are due to Ce4+ 3d5/2. The four peaks namely u′, ν′, uo, and νo (904.7, 885.5, 900.7 and 881.5 eV) are corresponding to Ce3+. The peaks at 780.15 eV (2p3/2) and 795.21 eV (2p1/2) with two satellites located at 790.05 and 804.76.33 eV in Fig. 7c are characteristic of a Co2+ of Co3O4/CoAl2O4 (Xing et al., 2014). While at 779.16 and 794.89 eV for Co 2p3/2 and 2p1/2, transitions are ascribed to Co3+ of Co3O4 (Soundharrajan et al., 2016). The spin-energy separation between Co 2p3/2 and Co 2p1/2 orbits is ~15.2 eV, confirming the presence of Co3O4 (Schenck et al., 1983; Oku and Sato, 1992; Varghese et al., 2007). As the O 1s orbital spectrum showing in Fig. 7d, a strong peak at about 532.0 eV is assigned to the high-binding energy peak from chemisorbed and dissociated oxygen species, 531 eV is usually attributed to surface oxygen defect species, whereas 530.4 and 529.8 eV are associated with oxygen in the metal-oxygen bonds (Lü et al., 2014; Wang et al., 2020).
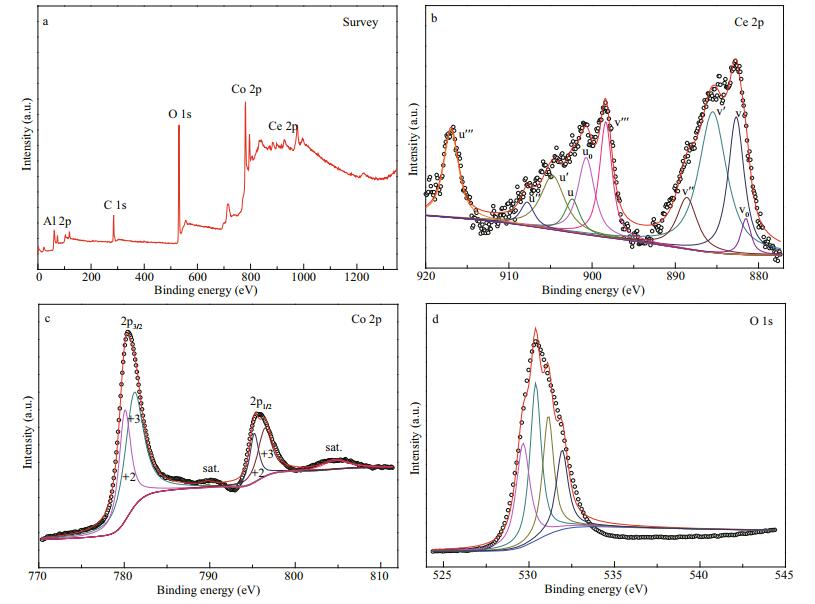
|
| Fig.7 XPS spectra of 2.5CoAlCe-200 a. survey; b. Ce 2p; c. Co 2p; d. O 1s. |
The natural POx can catalyze colorless TMB substrate into blue oxTMB rapidly with H2O2. The absorbance changes of oxTMB product at 652 nm can be monitored following the subsequent color reaction. Therefore, the standard TMB liquid substrate system was performed to evaluate the nanozyme-like activity of Co-Al-Ce MMOs further at room temperature. From Fig. 8, no obvious color changes are presented in the H2O2+TMB (a) and H2O2+2.5CoAlCe-200 (c) system. For other two TMB+2.5CoAlCe-200 (b) and H2O2+TMB+2.5CoAlCe-200 (d) systems, color changes can be observed. The color reaction indicates that 2.5CoAlCe-200 has both the POx-like activity and the oxidase (ODs)-like activity. The results of the POx mimic activity of Co-Al-Ce MMOs depending on the various molar ratio of Ce and the heating temperature was evaluated in Fig. 9. This indicates that Co-Al-Ce MMO samples obtained by calcination at 200℃ have the best POx mimic catalytic performance for the precursor samples with any Ce molar ratio, then the activity deteriorates as the calcination temperature increases. As poor crystallinity can result in more transportation channels, this can be favorable for improving the catalytic activity of material (Liu et al., 2014). Among the four samples of 0CoAlCe-200, 1CoAlCe-200, 2.5CoAlCe-200, and 5CoAlCe-200, the POx mimic catalytic performance of 2.5CoAlCe-200 is the best. Its good catalytic activity can be attributed to the good dispersion of catalytically active components and high specific surface area.
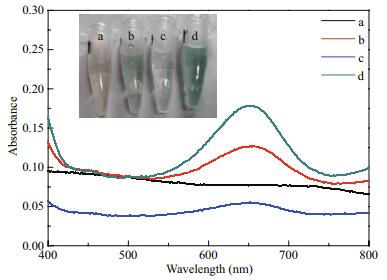
|
| Fig.8 Color changes in different reaction systems a. H2O2+TMB; b. TMB+2.5CoAlCe-200; c. H2O2+2.5CoAlCe-200; d. H2O2+TMB+2.5CoAlCe-200. |
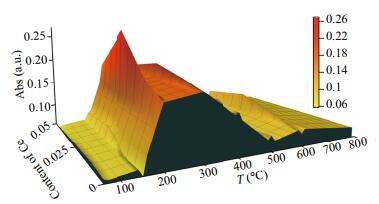
|
| Fig.9 The POx mimic catalytic activity of samples with different Ce molar ratios at different calcination temperatures |
According to the enzyme kinetic theory and method, the POx mimic catalytic mechanism and kinetic parameters of 2.5CoAlCe-200 were further investigated on the optimized condition. The results in Fig. 10 indicate that the oxidation reaction of TMB catalyzed by 2.5CoAlCe-200 is dependent on pH and the concentrations of the catalyst. Figure 11 shows the double reciprocal plots of POx mimic catalytic activity of 2.5CoAlCe-200. As shown, the parallel lines indicate that the slopes are similar and demonstrate that the catalytic mechanism of 2.5CoAlCe-200 follows the ping-pang mechanism. This is similar with natural horseradish peroxidase (HRP). The Km value is calculated using the MichaelisMenten equation. The Km value of 2.5CoAlCe-200 with TMB as the substrate is 0.273 mmol/L, which suggests that 2.5CoAlCe-200 has a higher affinity for TMB compared with HRP (0.434 mmol/L). On the other hand, the Km value for 2.5CoAlCe-200 with H2O2 as the substrate is 32.9 mmol/L, higher than that of 3.7 mmol/L for HRP (Gao et al., 2007).
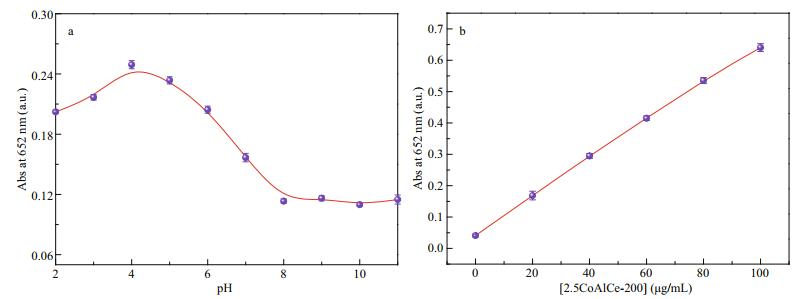
|
| Fig.10 The optimizing experiment of pH (a) and the concentration of 2.5CoAlCe-200 (b) while the concentration of H2O2 and TMB were set as 1 mmol/L and 0.4 mmol/L, respectively |
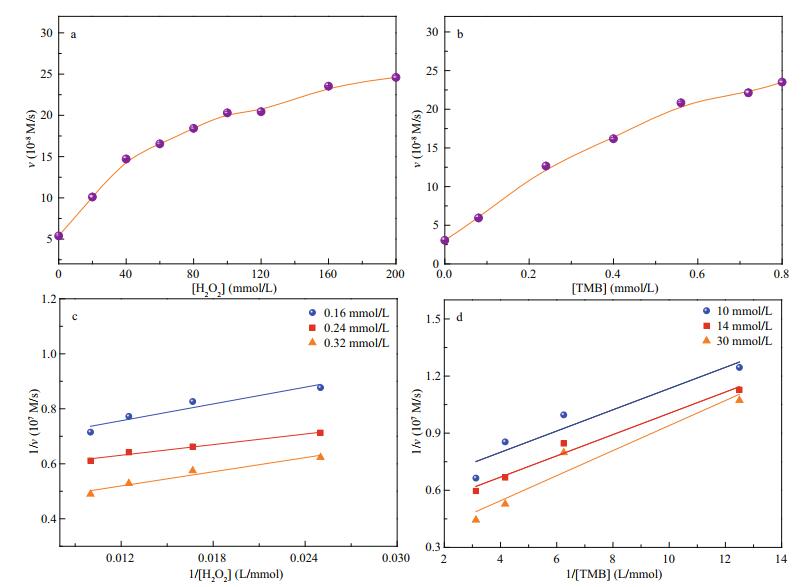
|
| Fig.11 Steady-state kinetic assays and catalytic mechanism of 2.5CoAlCe-200 a. the concentration of TMB was fixed at 0.4 mmol/L and the concentration of H2O2 varied; b. the concentration of H2O2 was fixed at 1 mmol/L and the concentration of TMB varied; c and d: double reciprocal plots of activity of 2.5CoAlCe-200 with the concentration of one substrate (H2O2 or TMB) fixed and the other varied. |
To investigate the POx-like catalytic mechanism of 2.5CoAlCe-200, IPA, and BQ was used to eliminate ∙OH and ∙O2ˉ and put into the optimal system with other conditions remained still to determine corresponding radicals produced. As shown in Fig. 12, the addition of BQ can reduce the absorbance at 652 nm significantly. As shown in Fig. 12, the system added IPA shows almost no signal of the absorbance at 652 nm as Control-IPA, and the characteristic signal shows poor reduction by adding 2.5CoAlCe-200 as IPA, suggesting that 2.5CoAlCe-200 possesses the POx mimic ability to decompose H2O2 to generate reactive ∙O2ˉ radicals. As a result, it can be deduced that the strong oxidizing ∙O2ˉ radicals play major roles in the ODs-like and POx-like catalytic reaction.
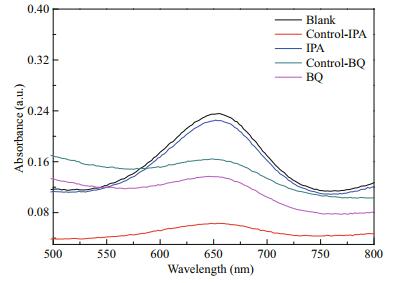
|
| Fig.12 UV-vis spectra of different reaction systems in the absence (blank) or presence (control-IPA/ BQ: without 2.5CoAlCe-200; IPA/BQ: with 2.5CoAlCe-200) of scavengers |
We have revealed that 2.5CoAlCe-200 exhibits ODs and POx like properties by significantly induced H2O2 species subsequently to convert into ∙O2ˉ radicals. As 2.5CoAlCe-200 is highly expected to kill bacteria by inducing the formation of ROS, we evaluated its antibacterial activities. Especially, concentrations of H2O2 will cause high toxicity and harm other organisms. The depending on concentrations of H2O2 of materials against Gram-negative E. coli bacteria were tested further. As shown in Fig. 13, E. coli in the blank group remain alive well at pH=4 and the inhibition rate of 2.5CoAlCe-200 against E. coli is 87.45% in the absence of H2O2. After the addition of H2O2, the inhibition rate against E. coli increases gradually. As the concentration of H2O2 increasing to 0.3 mmol/L, the inhibition rate of E. coli can arrive at 99.98%. It means that 2.5CoAlCe-200 can kill E. coli with the biologically relevant concentrations of H2O2.
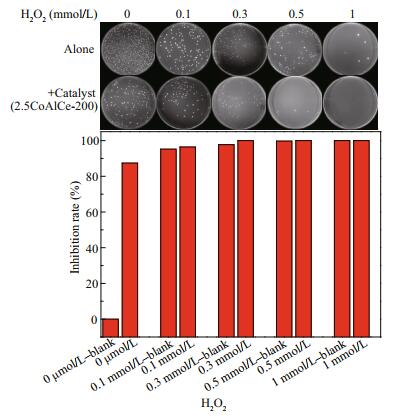
|
| Fig.13 Antibacterial activity of 2.5CoAlCe-200 at pH=4 depending on concentration of H2O2 |
Especially, pH is still a key parameter which have an effect on the efficiency of POx activity. To further examine the antibacterial activities of 2.5CoAlCe-200 in pH=6, fluorescence-based Live/Dead assays were performed. As shown in Fig. 14a, the green fluorescence and the lack of red fluorescence indicated that most bacteria in the control group remained alive. Addition of 5 mmol/L of H2O2 without 2.5CoAlCe-200 (Fig. 14b) or addition of 2.5CoAlCe-200 without H2O2 (Fig. 14c), most bacteria remained alive. While, 2.5CoAlCe-200 as well as 5 mmol/L of H2O2 both added in the system (Fig. 14d), the result shows the intensification of the red fluorescence signal, which indicates a perceptible increase in the number of dead cells.
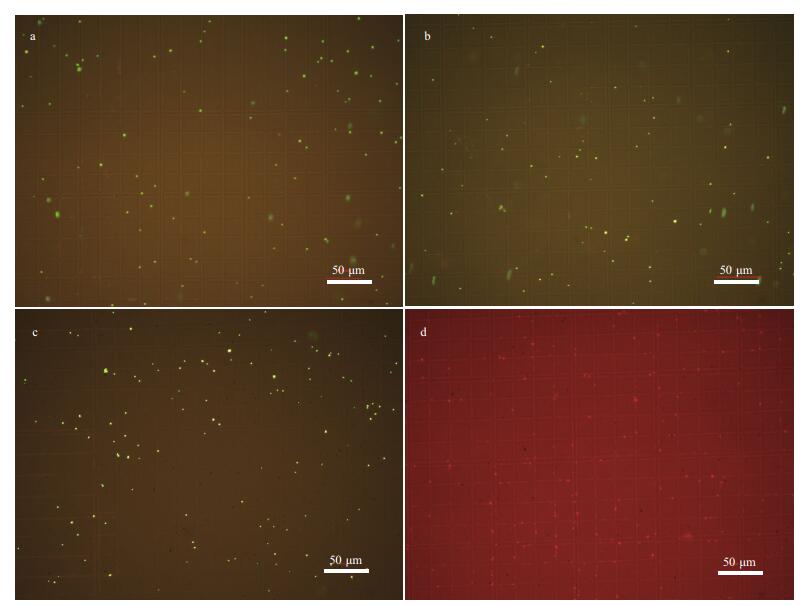
|
| Fig.14 Representative fluorescence images of live (green) and dead E. coli cells (red) after different treatments in the pH=6 system a. blank; b. 5 mmol/L H2O2; c. 2.5CoAlCe-200; d. 5 mmol/L H2O2+2.5CoAlCe-200. |
Next, to examine whether the bacterial death is caused by oxidative damage (oxidative stress), the intracellular ROS level was determined. From Fig. 15 the representative fluorescence images in E. coli cells confirm that the antibacterial property is caused by the generation of ROS catalyzed by 2.5CoAlCe-200, which is indicated by the clear green fluorescence signal (Fig. 15d). From Fig. 16a, the clear intracellular red fluorescence signals were detected obviously, which further proves that the generated ∙O2ˉ promotes the antibacterial property.
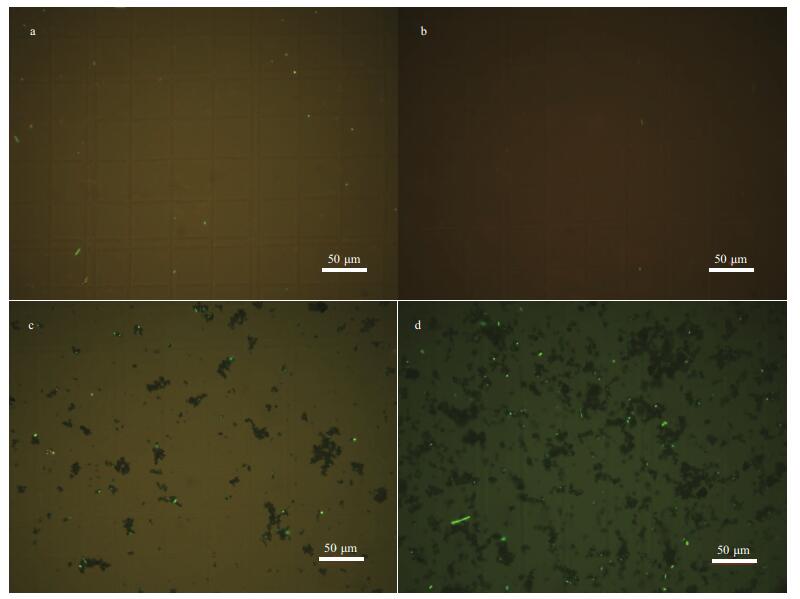
|
| Fig.15 Representative fluorescence images of ROS levels in E. coli cells after different treatments in the pH=6 system a. blank; b. H2O2; c. 5 mmol/L 2.5CoAlCe-200; d. 5 mmol/L 2.5CoAlCe-200+H2O2. |
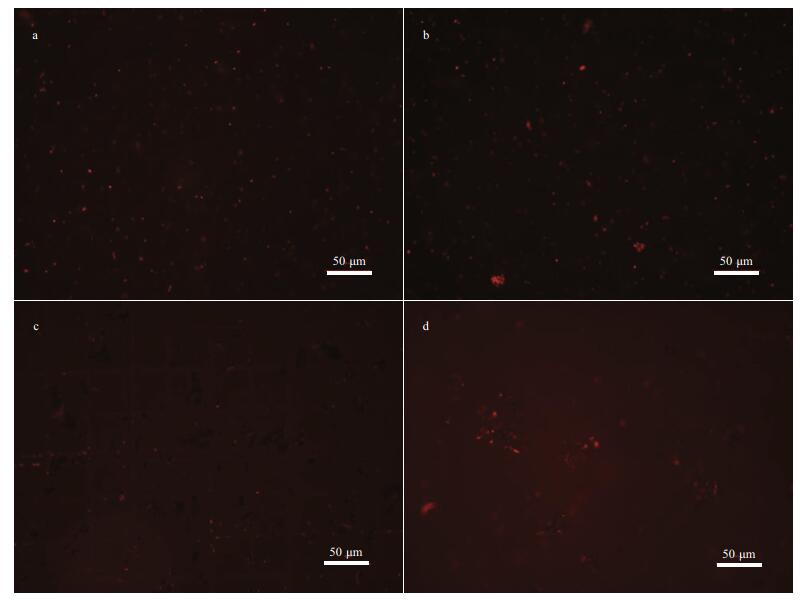
|
| Fig.16 Representative fluorescence images of ∙O2ˉ levels in E. coli cells after different treatment in the pH=6 system a. blank; b. H2O2; c. 5 mmol/L 2.5CoAlCe-200; d. 5 mmol/L 2.5CoAlCe-200+H2O2. |
In summary, LDH derived Co-Al-Ce MMOs composed of CeO2 compounding with Co3O4 and CoAl2O4 have been successfully synthesized by a rational approach. When the molar ratio of Ce is 2.5% and calcination temperature is 200℃, the obtained 2.5CoAlCe-200 sample exhibits the best POx-like activity and possess dual enzyme-like activities similar to those of ODs and POx. Co-Al-Ce MMOs with dual enzyme-like activities can catalyze H2O2 and O2 to generate ROS, mainly ∙O2ˉ. Owing to the excellent antibacterial capacity of ∙O2ˉ, Co-Al-Ce MMOs exhibit antibacterial activity even in near-neutral pH solution. Our results provide strong evidence that Ce containing-MMO can be utilized as the potential green marine antifouling nanozyme material.
5 DATA AVAILABILITY STATEMENTAll data of this project are public. The entire data have been annotated building process in the paper. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Banerjee I, Pangule R C, Kane R S. 2011. Antifouling coatings:recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Advanced Materterials, 23(6): 690-718.
DOI:10.1002/adma.201001215 |
Chen C, Wang Y, Yang Z Q, Zhang D. 2019. Layered double hydroxide derived ultrathin 2D Ni-V mixed metal oxide as a robust peroxidase mimic. Chemical Engineering Journal, 369: 161-169.
DOI:10.1016/j.cej.2019.03.070 |
Deshpande S, Patil S, Kuchibhatla S V, Seal S. 2005. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Applied Physics Letters, 87(13): 133113.
DOI:10.1063/1.2061873 |
Gao L Z, Zhuang J, Nie L, Zhang J B, Zhang Y, Gu N, Wang T H, Feng J, Yang D L, Perrett S, Yan X Y. 2007. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nature Nanotechnology, 2(9): 577-583.
DOI:10.1038/nnano.2007.260 |
Genty E, Brunet J, Pequeux R, Capelle S, Siffert S, Cousin R. 2016. Effect of Ce substituted hydrotalcite-derived mixed oxides on total catalytic oxidation of air pollutant. Materials Today-Proceedings, 3(2): 277-281.
DOI:10.1016/j.matpr.2016.01.069 |
Genty E, Siffert S, Cousin R. 2019. Investigation of reaction mechanism and kinetic modelling for the toluene total oxidation in presence of CoAlCe catalyst. Catalysis Today, 333: 28-35.
DOI:10.1016/j.cattod.2018.03.018 |
Hao J Q, Qu T, Wang Q F, Zhao Z B. 2017. Preparation and visible light responsive photocatalytic activity of Fe3O4/Ni-Al-Ce LDH/Bi2WO6 composites. Quimica Nova, 40(8): 849-853.
DOI:10.21577/0100-4042.20170062 |
He J J, Xu Y H, Wang W, Hu B, Wang Z J, Yang X, Wang Y, Yang L W. 2020. Ce(ó) nanocomposites by partial thermal decomposition of Ce-MOF for effective phosphate adsorption in a wide pH range. Chemical Engineering Journal, 379: 122431.
DOI:10.1016/j.cej.2019.122431 |
Herget K, Hubach P, Pusch S, Deglmann P, Götz H, Gorelik T E, Gural'skiy I A, Pfitzner F, Link T, Schenk S, Panthöfer M, Ksenofontov V, Kolb U, Opatz T, André R, Tremel W. 2017. Haloperoxidase mimicry by CeO2-x nanorods combats biofouling. Advanced Materterials, 29(4): 1 603 823.
DOI:10.1002/adma.201603823 |
Hong Q S, Lu H M, Wang J R. 2017. CuO nanoplatelets with highly dispersed Ce-doping derived from intercalated layered double hydroxides for synergistically enhanced oxygen reduction reaction in Al-Air batteries. ACS Sustainable Chemistry & Engineering, 5(10): 9169-9175.
DOI:10.1021/acssuschemeng.7b02076 |
Lebedeva O E, Rylotsova I G, Yapryntsev M N, Golovin S N, Veligzhanin A A. 2019. Stabilization of Cerium(ó) in the structure of hydrotalcite-like layered double hydroxides. Petroleum Chemistry, 59(7): 751-755.
DOI:10.1134/s0965544119070089 |
Lin Y H, Ren J S, Qu X G. 2014. Catalytically active nanomaterials: a promising candidate for artificial enzymes. Accounts of Chemical Research, 47(4): 1097-1105.
DOI:10.1021/ar400250z |
Liu M C, Kong L B, Kang L, Li X H, Walsh F C, Xing M, Lu C, Ma X J, Luo Y C. 2014. Synthesis and characterization of M3V2O8 (M = Ni or Co) based nanostructures: a new family of high performance pseudocapacitive materials. Journal of Materials Chemistry A, 2(14): 4919-4926.
DOI:10.1039/c4ta00582a |
Lü Y Y, Zhan W W, He Y, Wang Y T, Kong X J, Kuang Q, Xie Z X, Zheng L S. 2014. MOF-templated synthesis of porous Co3O4 concave nanocubes with high specific surface area and their gas sensing properties. ACS Applied Materials & Interfaces, 6(6): 4186-4195.
DOI:10.1021/am405858v |
Natalio F, André R, Hartog A F, Stoll B, Jochum K P, Wever R, Tremel W. 2012. Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation. Nature Nanotechnology, 7(8): 530-535.
DOI:10.1038/nnano.2012.91 |
Nayak S, Parida K M. 2016. Nanostructured CeO2/MgAl-LDH composite for visible light induced water reduction reaction. International Journal of Hydrogen Energy, 41(46): 21 166-21 180.
DOI:10.1016/j.ijhydene.2016.08.062 |
Nicolini V, Gambuzzi E, Malavasi G, Menabue L, Menziani M C, Lusvardi G, Pedone A, Benedetti F, Luches P, D'Addato S, Valeri S. 2015. Evidence of catalase mimetic activity in Ce3+/Ce4+ doped bioactive glasses. The Journal of Physical Chemistry B, 119(10): 4 009-4 019.
DOI:10.1021/jp511737b |
Oku M, Sato Y. 1992. In-situ X-ray photoelectron spectroscopic study of the reversible phase transition between CoO and Co3O4 in oxygen of 10-3 Pa. Applied Surface Science, 55(1): 37-41.
DOI:10.1016/0169-4332(92)90378-B |
Peng Y F, Chen X J, Yi G S, Gao Z Q. 2011. Mechanism of the oxidation of organic dyes in the presence of nanoceria. Chemical Communications, 47(10): 2 916-2 918.
DOI:10.1039/c0cc04679e |
Schenck C V, Dillard J G, Murray J W. 1983. Surface analysis and the adsorption of Co(ò) on goethite. Journal of Colloid and Interface Science, 95(2): 398-409.
DOI:10.1016/0021-9797(83)90199-6 |
Seftel E M, Puscasu M C, Mertens M, Cool P, Carja G. 2015. Fabrication of CeO2/LDHs self-assemblies with enhanced photocatalytic performance: a case study on ZnSn-LDH matrix. Applied Catalysis B: Environmental, 164: 251-260.
DOI:10.1016/j.apcatb.2014.09.035 |
Smalenskaite A, Vieira D E L, Salak A N, Ferreira M G S, Katelnikovas A, Kareiva A. 2017. A comparative study of co-precipitation and sol-gel synthetic approaches to fabricate cerium-substituted Mg-Al layered double hydroxides with luminescence properties. Applied Clay Science, 143: 175-183.
DOI:10.1016/j.clay.2017.03.036 |
Soundharrajan V, Sambandam B, Song J J, Kim S, Jo J, Kim S, Lee S, Mathew V, Kim J. 2016. Co3V2O8 sponge network morphology derived from metal-organic framework as an excellent lithium storage anode material. ACS Applied Materials & Interfaces, 8(13): 8 546-8 553.
DOI:10.1021/acsami.6b01047 |
Valente J S, Tzompantzi F, Prince J. 2011. Highly efficient photocatalytic elimination of phenol and chlorinated phenols by CeO2/MgAl layered double hydroxides. Applied Catalysis B: Environmental, 102(1-2): 276-285.
DOI:10.1016/j.apcatb.2010.12.009 |
Varghese B, Teo C H, Zhu Y, Reddy M V, Chowdari B V R, Wee A T S, Tan V B C, Lim C T, Sow C H. 2007. Co3O4 nanostructures with different morphologies and their field-emission properties. Advanced Functional Materials, 17(12): 1 932-1 939.
DOI:10.1002/adfm.200700038 |
Venugopal A K, Venugopalan A T, Kaliyappan P, Raja T. 2013. Oxidative dehydrogenation of ethyl benzene to styrene over hydrotalcite derived cerium containing mixed metal oxides. Green Chemistry, 15(11): 3 259-3 267.
DOI:10.1039/c3gc41321g |
Wang Y, Chen C, Zhang D, Wang J. 2020. Bifunctionalized novel Co-V MMO nanowires: intrinsic oxidase and peroxidase like catalytic activities for antibacterial application. Applied Catalysis B: Environmental, 261: 118 256.
DOI:10.1016/j.apcatb.2019.118256 |
Wang Y, Zhang D. 2016. Application of layered inorganic functional materials in marine anticorrosion and antifouling field. Science Press, Beijing. (in Chinese)
|
Xing M, Kong L B, Liu M C, Liu L Y, Kang L, Luo Y C. 2014. Cobalt vanadate as highly active, stable, noble metal-free oxygen evolution electrocatalyst. Journal of Materials Chemistry A, 2(43): 18 435-18 443.
DOI:10.1039/c4ta03776f |
Zhang X, Wang S L, Xu L, He T B, Lu F X, Li H C, Ye J H. 2017. Controllable synthesis of cross-linked CoAl-LDH/NiCo2S4 sheets for high performance asymmetric supercapacitors. Ceramics International, 43(16): 14 168-14 175.
DOI:10.1016/j.ceramint.2017.07.159 |
Zhou X L, Zhong Y R, Yang M, Zhang Q, Wei J P, Zhou Z. 2015. Co2(OH)2CO3 nanosheets and CoO nanonets with tailored pore sizes as anodes for lithium ion batteries. ACS Applied Materials & Interfaces, 7(22): 12 022-12 029.
DOI:10.1021/acsami.5b02152 |
Zhou Y, He J L, Zhang C L, Li J, Fu X X, Mao W R, Li W M, Yu C. 2020. Novel Ce(ó)-metal organic framework with a luminescent property to fabricate an electrochemiluminescence immunosensor. ACS Applied Materials & Interfaces, 12(1): 338-346.
DOI:10.1021/acsami.9b19246 |
Zhu C, Li H, Wang H B, Yao B W, Huang H, Liu Y, Kang Z H. 2019. Negatively charged carbon nanodots with bacteria resistance ability for high-performance antibiofilm formation and anticorrosion coating design. Small, 15(23): 1 900 007.
DOI:10.1002/smll.201900007 |
Zhu L P, Wen Z, Mei W M, Li Y G, Ye Z Z. 2013. Porous CoO nanostructure arrays converted from rhombic Co(OH)F and needle-like Co(CO3)0.5(OH)·0.11H2O and their electrochemical properties. The Journal of Physical Chemistry C, 117(40): 20 465-20 473.
DOI:10.1021/jp406146b |
 2020, Vol. 38
2020, Vol. 38


