Institute of Oceanology, Chinese Academy of Sciences
Article Information
- WANG Pengpeng, ZHANG Fang, SUN Song, WANG Weicheng, WAN Aiyong, LI Chaolun
- Experimental clearance rates of Aurelia coerulea ephyrae and medusae, and the predation impact on zooplankton in Jiaozhou Bay
- Journal of Oceanology and Limnology, 38(4): 1256-1269
- http://dx.doi.org/10.1007/s00343-020-0024-7
Article History
- Received Jan. 14, 2020
- accepted in principle Apr. 19, 2020
- accepted for publication May. 25, 2020
2 Laboratory for Marine Ecology and Environmental Sciences, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China;
3 University of Chinese Academy of Sciences, Beijing 100049, China;
4 Jiaozhou Bay Marine Ecosystem Research Station, Chinese Academy of Sciences, Qingdao 266071, China;
5 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
Jellyfish are gelatinous zooplanktonic organisms, which play an important role in the material cycle and energy flow of marine ecosystems (Möller, 1984; Purcell, 1997). Dramatic changes in the distributions and abundances of some jellyfish, such as ctenophores and cnidarians (hydromedusae, siphonophores and scyphomedusae) (Båmstedt et al., 1994; Purcell and Cowan Jr., 1995; Purcell et al., 1999), have increased discussion about the impact of these organisms on pelagic food webs (Purcell, 1985; Purcell et al., 1999; Pitt et al., 2009).
Common bloom jellyfish, Aurelia are offshore scyphomedusae with worldwide distributions (Olesen et al., 1994; Olesen, 1995; Purcell, 2009). Global populations of Aurelia species have increased significantly in the past half-century (Kogovšek et al., 2010). Mass occurrences of Aurelia species have been reported from many parts of the world, including Japan (Yasuda, 1976; Omori et al., 1995), Sweden (Hernroth and Gröndahl, 1983), China (Dong et al., 2010), and Greece (Papathanassiou et al., 1987). Aurelia species are voracious predators, consuming a broad range of prey (Purcell, 1997; Hansson et al., 2005), including fish eggs and larvae, copepods, barnacles, gastropods, bivalves and polychaetes (Purcell, 1985, 1997; Ishii and Tanaka, 2001; Purcell and Arai, 2001). High densities of Aurelia species can seriously deplete populations of zooplankton and ichthyoplankton, both by direct predation and competition for the same resources (Purcell, 1997). For example, during highdensity blooms, Aurelia aurita medusae consumed about 2/3 of all daily secondary production in Kiel Bight Bay, Germany, leading to a noticeable decline of meso-zooplankton (Schneider and Behrends, 1994). Intraguild predation is also common in jellyfish, including Aurelia species (Purcell, 1991; Titelman and Hansson, 2006). Gut content analyses indicated that Aurelia, including its small medusae and ephyrae, consumed many hydromedusae (Sullivan et al., 1997).
Jiaozhou Bay is a semi-enclosed bay and locates on the southern side of the Shandong Peninsula that is strongly influenced by human activities (Wang and Sun, 2015). This bay is therefore an ideal system for studying the influence of ecological disasters on a marine ecosystem, with a particular focus on jellyfish blooms and the impact of these blooms on the compositions and quantities of zooplankton communities. Population explosions of Aurelia coerulea have been recorded in Jiaozhou Bay, with an especially large population outbreak in 2009 (Wan and Zhang, 2012; jellyfish bloom year, JBY). Jellyfish did not bloom in 2008 and 2010 (jellyfish non-bloom years, NJBYs). The explosions of A. coerulea population might cause imbalances to marine food webs and have negative effects on the marine ecology of Jiaozhou Bay (Wan and Zhang, 2012; Wang and Sun, 2015). Therefore, information on its trophic relationships with other zooplankton groups is required; we also wanted to know how this jellyfish functions within marine ecosystems.
Wan and Zhang (2012) observed A. coerulea ephyrae in Jiaozhou Bay during April, and reported that the population of A. coerulea medusae increased sharply in May, peaking in June. Wang and Sun (2015) found that A. coerulea strobilation occurs in early spring in Jiaozhou Bay as the water temperature begins to increase. In late May, young medusae (size: 9.74±1.7 mm) were observed. These medusae attained sexual maturity in mid-summer; in August, medusae numbers decreased, and reached zero by September (Wan and Zhang, 2012; Wang and Sun, 2015). It is likely that the A. coerulea population had its largest impact on zooplanktonic taxa from May to August in 2009. Previous studies found the highest abundances of A. coerulea in northern Jiaozhou Bay near the Hongdao (Fig. 1) (Wan and Zhang, 2012; Wang and Sun, 2015). In the present study, to investigate the influence of A. coerulea population on zooplankton, the abundances of each zooplankton group at a fixed station in northern Jiaozhou Bay from May to August were compared between NJBYs(2008, 2010) and JBY (2009). Clearance rates of A. coerulea medusae and ephyrae for different prey items were measured in laboratory experiments. The population clearance potential of A. coerulea was calculated in Jiaozhou Bay during mass occurrence of A. coerulea in May to August 2009. This was done by combining clearance experiments in the laboratory with field measurements of medusae size and abundance and the surface water temperature during the study period. The influences of A. coerulea blooms on the numbers and compositions of zooplankton communities in Jiaozhou Bay were determined.
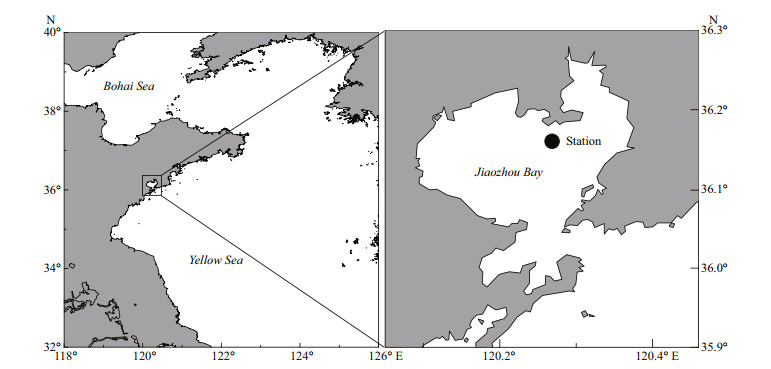
|
| Fig.1 Map of the study area Black dot in the right panel shows the fixed station that zooplankton samples being collected in Jiaozhou Bay. |
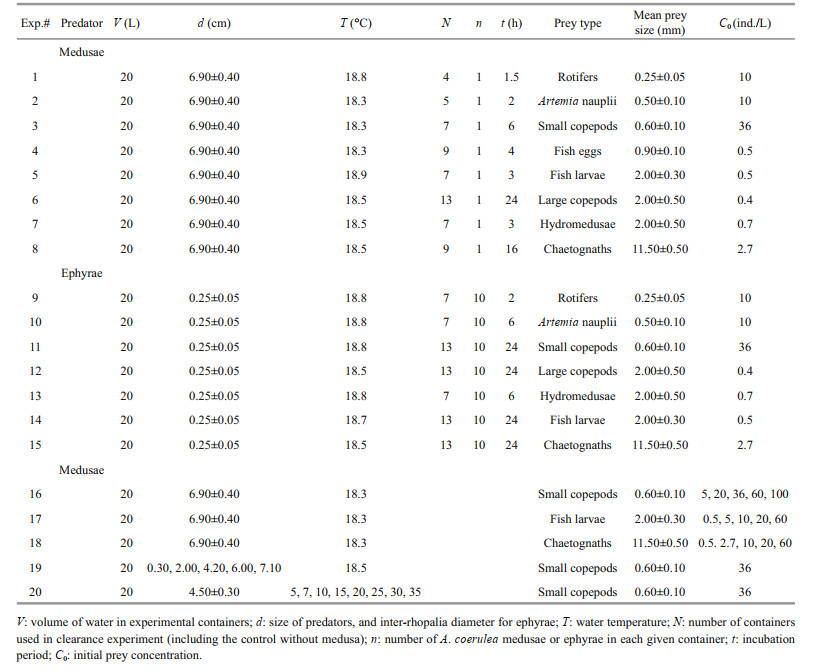
Aurelia coerulea ephyrae and medusae used in this experiment were cultivated in our jellyfish laboratory (Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China). Ephyrae (inter-rhopalia diameter 2.5±0.5 mm) were released by polyps, and then they were transferred to a cylindrical glass incubator (70-cm diameter, 50-cm high) with water volume of 120 L, and given Artemia nauplii twice a day. Experiments were conducted as the A. coerulea medusae grew.
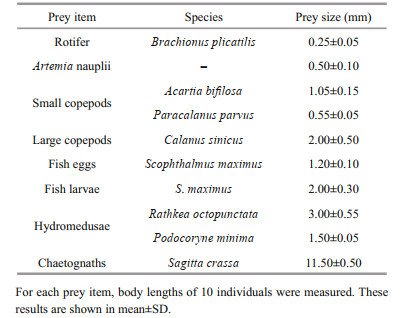
Prey items, including Artemia nauplii, rotifers, fish eggs and larvae, small copepods, large copepods, hydromedusae and chaetognaths, were used in feeding experiments. The main species and size of each prey item are shown in Table 1. Copepods, hydromedusae and chaetognaths were captured in Jiaozhou Bay using shallow water Ⅱ type plankton nets (net mesh: 160 μm; net mouth area: 0.08 m2) between early May and early June 2017. Captured zooplankton were immediately transported to the laboratory; small copepods (mean body length: 0.6±0.1 mm), large copepods (mean body length: 2±0.5 mm), hydromedusae, and chaetognaths were selected, respectively. Then these prey items were held in plastic buckets containing 20-L filtered seawater (20-μm mesh) with an air pump.
Artemia nauplii, rotifers, and fish eggs and larvae (turbot, Scophthalmus maximus) were also offered as prey in feeding experiments. Artemia nauplii and rotifers were maintained in the laboratory at 18℃ and salinity of 31. Fish used in the experiment were obtained from Jiaozhou Farm, Qingdao, China. To raise fish larvae, we incubated artificially fertilized fish eggs in artificial seawater at 18–19℃ and salinity of 31. After hatching, fish larvae were incubated in a tank with 1 000-L filtered seawater (20-μm mesh) and an air pump; the fish offered rotifers daily. Approximately one third of the tank water was exchanged daily.
2.2 Feeding experimentAurelia coerulea ephyrae and medusae that were visually undamaged and actively pulsing were used in the experiments. Selected specimens were starved for 24 h, and then transferred to experimental containers (30-cm diameter, 30-cm height) with 20-L filtered seawater (20-μm mesh). One A. coerulea medusa and ten ephyrae were placed in each experimental container. All jellyfish were allowed to acclimatize to experimental containers for at least 1 h under observation to ensure that their free-swimming behavior was normal. To know the exact number of each prey organisms, Artemia nauplii, copepods, and fish eggs were collected using a pipette; big drops of water with prey from the cultivation bucket were placed into a Petri dish and the number of prey in each drop was counted. Fish larvae, hydromedusae and chaetognaths were individually sorted out with a small handled sieve (0.1-mm mesh size), and transferred into 2-L beakers, respectively. Rotifer was counted under a dissecting microscope (Nikon, SMZ745, Japan).
Clearance rate was determined by measuring the volume of water cleared of prey organisms used in the present experiments per unit time. Thirteen containers (including the control) were employed in each experiment. The measurements of clearance rates were based on time series. Incubation time should be from the beginning of the experiment until the prey is completely cleared. However, rates of A. coerulea varied with different prey, the incubation time of different prey varied between 1 h and 24 h (Table 2), and the maximum incubation time was 24 h in this study. Sampling frequencies of different prey depended on the rate on which prey organisms were removed from the water by the predation of A. coerulea. The concentration of prey organisms in our laboratory experiment was determined according to the maximum densities of different zooplankton groups in the field (the maximum densities of zooplankton in the field: large copepods, 320.5 inds./m3; small copepods, 35 200.0 inds./m3; hydromedusae: 640.0 inds./m3; chaetognaths, 2 560.0 inds./m3). Fish larvae and eggs were not found in this field zooplankton survey, but according to previous study, densities of fish larvae and eggs were very low in Jiaozhou Bay (maximum density: fish egg, 5.02 inds./m3; larvae, 16.34 inds./m3, Huang et al., 2007). It is reasonable that the concentrations of fish larvae and eggs were determined to be 0.5 inds./L. Concentrations (100 small copepods/L, 60 fishes/L, 60 chaetognaths/L) used to study the relationship between prey concentration and clearance rate are higher than the average density of populations in natural to demonstrate that A. coerulea were not saturated at these high prey densities. Details of the specific conditions for each experiment are shown in Table 2.
The experiments were started by carefully adding the prey organisms to each container with jellyfish. As time went on, the number of prey organisms gradually declined, and the reduction in the number of prey organisms as a function of time was followed by removing the jellyfish from one container at periods of time intervals and filtering (20-μm mesh) all the water (i.e. data for each container represents 1 time). The retained prey organisms were counted by using stereomicroscope (Nikon, SMZ745). Feeding experiments were performed in triplicate. For each experiment, a container without jellyfish served as a control. All experiments were conducted at 18–19℃ and salinity of 31. The clearance rate (Cl, in L/h) was determined based on the exponential reduction in prey concentration (Ct, the prey concentration at time t):
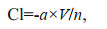 (1)
(1)where a is the slope of the fitted regression line of lnCt versus time t; n is the number of jellyfish in each experimental container; and V is the volume of seawater of the given water.
Four clearance experiments were conducted: (1) clearance rate of different prey organisms for medusae and ephyrae (Table 2, #1–15); (2) the relationship between prey concentration and clearance rate (Table 2, #16–18); copepods (small copepods), fish larvae and gelatinous organisms (chaetognaths) with different concentrations were offered as prey; (3) the effects of predator size on clearance rate (Table 2, #19); the cross-sectional area of A. coerulea (A, cm2) was used to represent the predator size, which was defined as π multiplied by the square of the radius of A. coerulea bell because of the round medusae body; and (4) the relationship between water temperature and clearance rate, and medusae were adapted to the specified experimental temperature over 3 h prior to incubation (Table 2, #20). Zooplankton was comprised of 71% copepods in Jiaozhou Bay. Therefore, copepods were selected as the prey for experiments (3) and (4).
2.3 Frequency of bell contractionThe relationship between temperature and frequency of bell contractions (beats/min) was measured with 10 inds. medusae (mean size: 4.5±0.3 cm) and temperatures (℃) of 5, 7, 10, 15, 20, 25, 30, and 35. A. coerulea medusae were placed in 20-L water volume (30-cm container diameter, 30-cm height), containing filtered seawater (20-μm mesh, salinity of 31). The number of pulses was counted for 10 min. Prior to the measurement, all medusae were allowed to acclimatize to experimental temperatures for 1–3 h under observation to ensure that their freeswimming behaviors were normal. Two series of these experiments were carried out. In the first series, the frequency of bell contractions was measured without any prey added. In the second series, small copepods were offered as prey, and the frequency of bell contractions of A. coerulea medusae was measured after the capture of 1 prey. We also observed the swimming directions of medusae. These experiments were conducted in a constant temperature incubator.
2.4 Population clearance potentialThe population clearance potential of A. coerulea (PCP, /d) in Jiaozhou Bay was estimated from May to August 2009. PCP is defined as the number of times per day that the water volume the total population of jellyfish could clear relative to the whole water column (Olesen, 1995). The effect of A. coerulea predation on zooplankton communities was further illustrated by calculating the theoretical mean residence time (t1/2) for copepods (referred to Olesen, 1995). The dominant group, copepods, can occupy more than 70% of total zooplankton in the Jiaozhou Bay (our field surveys). Thus, PCP and t1/2 were calculated for this dominant group, which could exactly reflect the effect of jellyfish predation on zooplankton. PCP and t1/2 were calculated from experimental clearance rates, and information obtained from Wan and Zhang (2012) and unpublished data on A. coerulea density and mean size and water temperature from May to August 2009. Clearance experiments on the relationship between medusae size and clearance rate were conducted at 18.5℃, whereas the in situ temperature ranged from 11 to 26℃. Corrections for all medusae sizes (0.3–7.1 cm) were made to be proportional with the trend with temperature measured for 4.5-cm A. coerulea. A temperature correction factor (tk) due to differences between temperatures in the laboratory and in situ was defined as: tk=CRk/CR18.5, therefore, Cl (L/h):
 (2)
(2)The population clearance potential of A. coerulea (PCP, /d) was defined as:
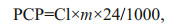 (3)
(3)where CRk and CR18.5 are the clearance rate (L/h) for 4.5 cm A. coerulea at in situ temperature (k, ℃) and 18.5℃, respectively; and A (cm2): the cross area of A. coerulea; m (inds./m3): abundance of A. coerulea in Jiaozhou Bay. Theoretical mean residence time of copepods t1/2 was calculated as:
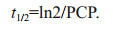 (4)
(4)It is assumed that the feeding rates were constant day and night, and the relations between individual sizes and clearance rates used in the present study were also valid for medusae larger than those used in the clearance experiments.
2.5 Zooplankton variation in Jiaozhou BayZooplankton samples from May to August at a fixed location in northern Jiaozhou Bay (36°15′45″N, 120°25′04″; Fig. 1) were compared between NJBYs(2008, 2010) and JBY (2009). Zooplankton samples were collected by a shallow water Ⅱ type plankton net (the net mesh: 160 μm; net mouth area: 0.08 m2) throughout the water column from bottom to surface; then zooplankton samples were immediately preserved in 5% neutral formalin. A Motoda sampler (Motoda, 1963) was used to subsample our zooplankton collection. Then each subsample was placed in a scan cell (11 cm×24 cm), and scanned the subsamples at 4 800 dpi with ZooScan (ZooScan, HYDROPTIC, France, L×W×H: 60 cm×54 cm×36 cm), following manufacturer instructions. Zooplankton samples in Jiaozhou Bay were classified into the organismal groups according to Sun et al. (2008). These were copepods, chaetognaths, medusae, appendicularians, Noctiluca scintllans and others. The "total zooplankton" included all organisms except N. scintllans, because there were large interannual variations in N. scintllans abundance. To study the effects of jellyfish on different zooplankton organisms in greater detail, total zooplankton (including copepods) and copepods alone were classified into three groups based on size: < 0.5 mm, 0.5–1 mm, and > 1 mm. The abundance (D, inds./m3) of each zooplankton group was determined on a per unit volume (m3) basis, which was calculated as: D=N/v, where N is the number of each zooplankton group; v (v=s×h) is the volume of water faltered by the plankton net; s is the area of the plankton net mouth; and h is the sampling depth.
2.6 Statistical methodSPSS v16.0 was conducted to statistical analysis of processing data. One-way analysis of variance (ANOVA) was used to determine the differences in clearance rates among different prey concentrations; normality and equal variances were checked before ANOVA analysis. The differences in clearance rates of different prey items and the variations in frequency of bell contraction at different feeding conditions were analyzed by the non-parametric Kruskal-Wallis test. Statistical significance was set at P < 0.05.
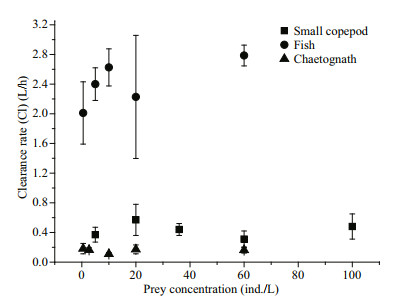
3 RESULT 3.1 Clearance rate and bell contraction of A.coerulea 3.1.1 Effects of prey type and predator size on the clearance rateClearance rates were not significantly different among the various prey concentrations, whether small copepods, fish larvae, or chaetognaths were offered as prey (ANOVA, small copepods: F=0.454, n=15, P=0.767; fish larvae: F=1.433, n=15, P=0.293; chaetognaths: F=1.039, n=15, P=0.434) (Fig. 2). Prey type has significant effect on clearance rate; for A. coerulea medusae of similar size (6.9±0.4 cm; Table 2), the estimated mean clearance rates were 4.78±1.34 L/h for rotifers, 1.12±0.29 L/h for Artemia, 2.55±0.29 L/h for fish larvae, 0.67±0.04 L/h for fish eggs, 1.58±0.03 L/h for hydromedusae, 0.37±0.03 L/h for small copepods, 0.04±0.01 L/h for large copepods, and 0.17±0.04 L/h for chaetognaths (Fig. 3a). Clearance rates for rotifers, fish larvae, hydromedusae, and Artemia were significantly higher than clearance rates for fish eggs and small copepods, followed by chaetognaths and large copepods (H=19.636, P=0.003). Ephyrae could not capture large copepods or chaetognaths. For ephyrae, the estimated mean clearance rates were 0.064±0.005 L/h for rotifers, 0.026±0.015 L/h for Artemia, 0.007±0.001 L/h for small copepods, 0.05±0.017 L/h for hydromedusae, and 0.001±0.0003 L/h for fish larvae. Clearance rates for rotifers and hydromedusae were significantly higher than the other prey (H=12.700, P=0.013) (Fig. 3b).
|
| Fig.2 Clearance rates as a function of prey concentrations during incubations Small copepods, fish larvae and chaetognaths were offered as prey. The mean size of A. coerulea medusae in these experiments was 6.9±0.4 cm. Data points are the mean values of three replicates. Error bars indicate SD. An ANOVA on raw data showed no significant change in clearance rate was associated with prey concentration (small copepods: F=0.454, n=15, P=0.767; fish larvae: F=1.433, n=15, P=0.293; chaetognaths: F=1.039, n=15, P=0.434). |

|
| Fig.3 Clearance rates of A. coerulea feeding on different prey organisms a. A. coerulea medusae (mean size: 6.9±0.4 cm); b. A. coerulea ephyrae (mean inter-rhopalia diameter: 0.25±0.05 cm). Error bars indicate SD. Experimental conditions are shown in Table 2. |
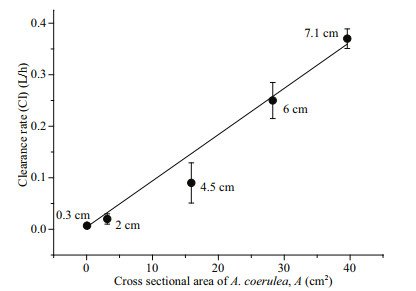
Predator size also had a significant effect on clearance rate. The relationship between the cross sectional area of A. coerulea and clearance rates in experiments with small copepods as prey is shown in Fig. 4. The clearance rate increased from 0.007 L/h for 0.3-cm A. coerulea to 0.37 L/h for 7.1-cm A. coerulea. By linear regression, clearance rate (Cl) linearly increased with the cross sectional area of A. coerulea (A): Cl=0.009A+0.0042 (F=271.107, P < 0.001, R2=0.985; Fig. 4).
|
| Fig.4 Relationship between the cross sectional area of A. coerulea and the clearance rates of the experiments with small copepods as prey organisms (F=271.107, P < 0.001, R2= 0.985) A (cm2): the cross sectional area of A. coerulea; Cl (L/h): clearance rate. The numbers with "cm" next to the data points indicate the size of experimental A. coerulea. |
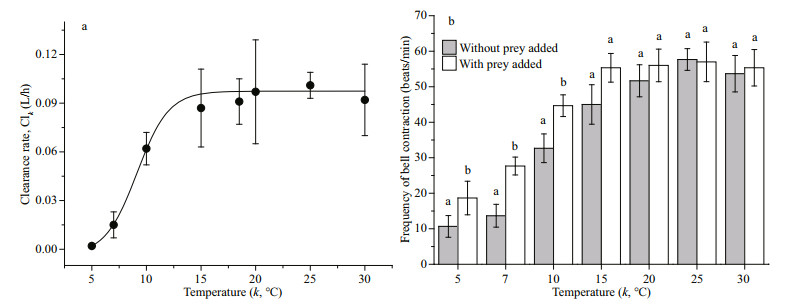
The relationship between temperature and clearance rate for A. coerulea medusae (mean size: 4.5±0.3 cm) with small copepods as prey is shown in Fig. 5a. The sigmoidal curve fit shows that clearance rate increased from 0.002 L/h at 5℃ to 0.093 L/h at 15–16℃, and clearance rate was constant at 17℃ to 30℃. When the temperature was raised above 33℃, the medusae died. The relationship is described by the following equation:
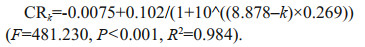
|
| Fig.5 Relationship between clearance rate, bell contraction, and temperature a. relationship between temperature and clearance rate for 4.5±0.3 cm medusae (F=481.23, P < 0.001, R2=0.984). Clk: clearance rate at temperature k (℃); b. relationship between temperature and mean frequency of bell contraction for 4.5±0.3 cm medusae with prey and without prey; different letters in (b) mean significant differences (P < 0.05) at the same temperature. Error bars indicate SD. |
The frequency of bell contraction as a function of water temperature is shown in Fig. 5b. The mean beat frequency of A. coerulea medusa without prey increased from 11±3 beats/min at 5℃ to 45±6 beats/ min at 15℃, and did not increase further as the temperature increased above 15–16℃. The mean beat frequency with prey was significantly higher than the frequency with no prey at 5℃, 7℃, and 10℃, and there was no significant difference above 15℃.
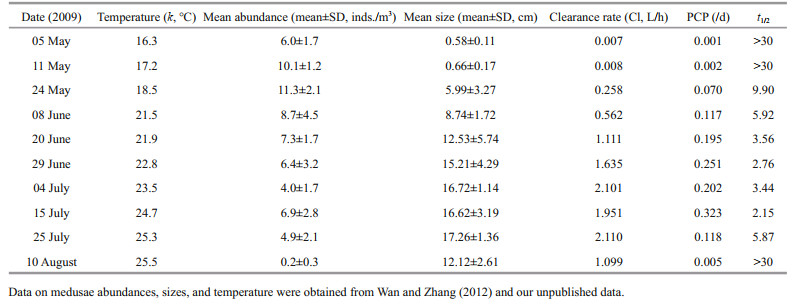
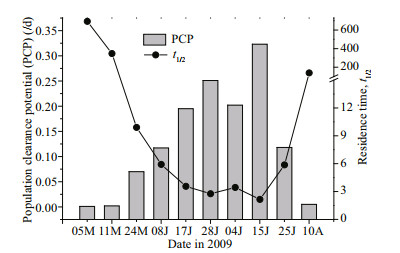
3.2 Population clearance potential in Jiaozhou BayThe surface water temperature varied from May to August in 2009. Temperature ranged from 16.3℃ to 25.5℃ during the survey period. The size of A. coerulea increased from 0.58±0.11 cm in early May to 17.26±1.36 cm in July, and decreased to 12.12±2.61 cm in August (Table 3). The water column PCP for A. coerulea in Jiaozhou Bay during May to August in 2009 is shown in Table 3 and Fig. 6. PCP was low in early May, but increased from late May to July, then decreased in August (Fig. 6). At the beginning of May, the population of A. coerulea could potentially clear the volume of water in the bay less than 0.001 times each day, and the theoretical mean residence time (t1/2) for copepods was more than 30 days. The PCP increased gradually at the end of May, and the t1/2 was relatively low (9.9 days). The maximum PCP was measured on 15 July, when the population of A. coerulea could clear a volume of water in the bay 0.323 times per day, and the t1/2 for copepods was only 2.15 days in this period. The theoretical mean residence time for copepods was estimated to be less than 6 days in June and July. In August, PCP was less than 0.01 times per day, and the t1/2 was greater than 30 days (Fig. 6; Table 3).
|
|
| Fig.6 Population clearance potential (PCP, /d) and residence time (t1/2) for copepod Data obtained from Table 3. M: May; 08J, 17J, and 28J: June; 04J, 15J, and 25J: July; A: August. |
On average, the zooplankton abundance at the sampling site was comprised of 71% copepods in abundance. Copepods are the dominant zooplankton community in Jiaozhou Bay. In NJBYs (2008 and 2010), the abundances of total zooplankton (< 1 mm) and copepods (< 1 mm) were relatively high during May to July, whereas they were significantly less in JBY (2009; Fig. 7a & b). In addition, the abundances of hydromedusae and chaetognaths also decreased in the JBY compared to the levels in the NJBYs (Fig. 7c & d).
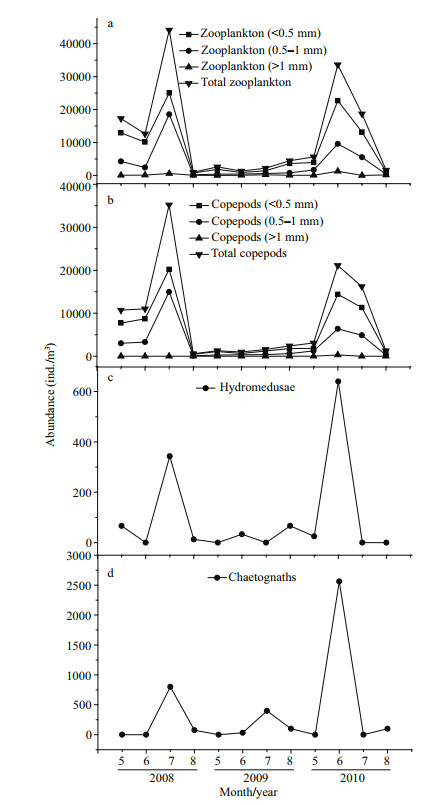
|
| Fig.7 Abundances of total zooplankton (a), copepods (b), hydromedusae (c), and chaetognaths (d) between May and August 2008, 2009, and 2010 in Jiaozhou Bay, at the fixed station shown in Fig. 1 Total zooplankton (including copepods) and copepods alone were divided into three groups in size: < 0.5 mm, 0.5-1 mm, and > 1 mm. |
The clearance rates of Aurelia species on different prey items have been well studied (Table 4). It is therefore of interest to compare our obtained clearance rates for different prey organisms with those of previous studies. Previous and present studies all indicate that the Aurelia clearance rates for fish larvae and hydromedusae were higher than the other prey items, and there was a lower clearance rate of copepods (Fig. 8). Predatory jellyfish have selective feeding behaviors that are based on several factors, including prey size, swimming speed and escape ability, predator tentacle length, width and spacing, predator swimming behavior and the resulting bellmargin water flow (Båmstedt, 1990; Purcell, 1992; Ford et al., 1997; Purcell et al., 1999; Suchman and Sullivan, 2000). Here, the clearance rate of hydromedusae was higher than the rate of copepods. This suggests that the low swimming speeds and poor escape abilities of hydromedusae increased their vulnerability to be attacked by both medusae and ephyrae. Although Anderson (1974) indicated that fast swimming copepods were more vulnerable to be ambushed by jellyfish, copepods have a characteristic escape jump (Hartline et al., 1996). Their rapid and vigorous escape behaviors are important adaptations for avoiding predation (Buskey et al., 2002; Waggett and Buskey, 2008). Fancett (1988) found that Cyanea capillata preferentially preyed upon fish eggs and larvae, whereas copepods were least preferred. We also found that the clearance rates of A. coerulea for fish larvae were generally higher than the rates for other prey. This result was consistent with previous studies, which suggested that fish larvae have relatively poor escape abilities compared to copepods (Purcell and Arai, 2001; Titelman and Hansson, 2006). Chaetognaths also have a characteristic escape jump, and the 'jumping' chaetognaths often escaped predation by A. coerulea in our study. Consequently, even if a successful encounter between these prey (copepods and chaetognaths) and A. coerulea (as a predator) happened, the stronger escape ability of copepods and chaetognaths, compared to fish larvae and hydromedusae, reduced the predation efficiency of A. coerulea.
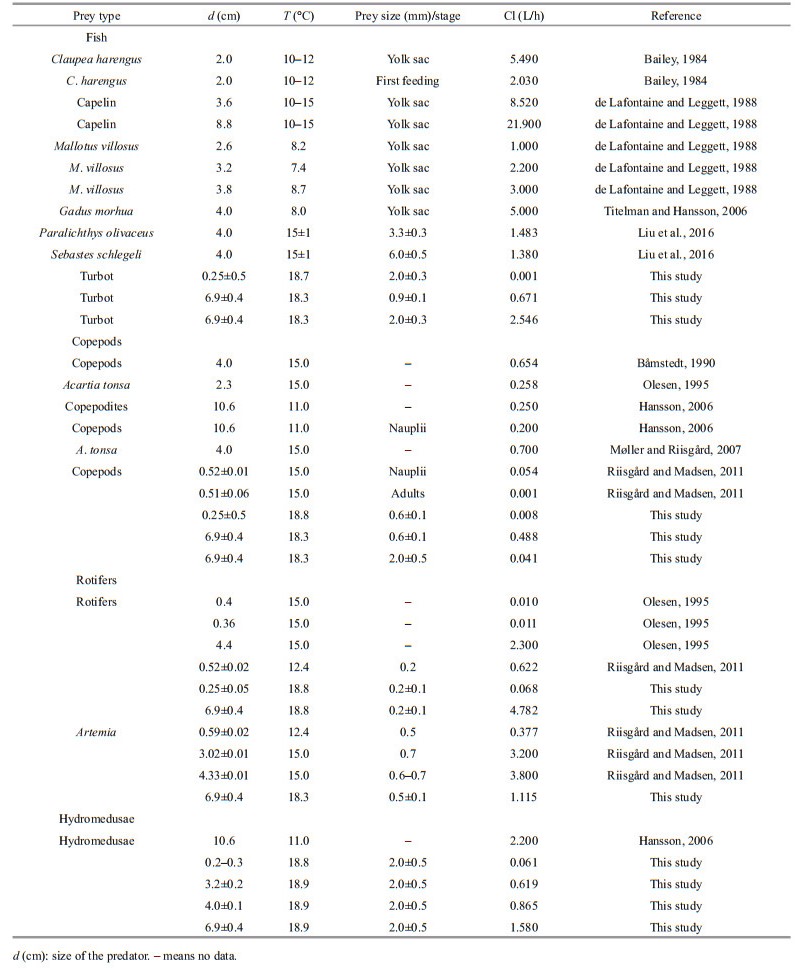
|

|
| Fig.8 Clearance rate of different prey types This figure combines the data from the present study and previous publications (Table 4). |
Water temperature is an important factor that influences the clearance rate for Aurelia (Olesen, 1995). Clearance rate for 4.5-cm A. coerulea medusae at 5℃ was only 2.2% of the clearance rate at 15℃ (Fig. 5a). The relationship between water temperature and clearance rate in our study can be explained by the increased beat frequency as water temperature increased (Fig. 5b). An increase in the frequency of bell contraction means an increase in the swimming speed of the same size medusa. Increased swimming speed allows the medusae to search more area, which causes the encounter rate of prey organisms to increase (Bailey and Batty, 1983); higher clearance rate was reasonable. In addition, compared to the frequency of bell contraction when no prey was added, the frequency of bell contraction increased when prey added (Fig. 5b). We speculated that this was related to A. coerulea feeding behaviors. Aurelia capture prey with external surface mucus or wrap prey through the constriction of bell (Costello and Colin, 1994). A. coerulea medusae capture small copepods after contact with their exumbrella surface, and small copepods would attempt to escape by making violent thrashing motions. These prey escape responses stimulate the constriction of bell more frequently, which was propitious to increase the success of predation. Bailey and Batty (1983) indicated that Aurelia medusae were relatively more active after capture of one fish larva and their "searching" path was altered. We found that an increase in beat frequency allowed medusa to swim vertically, rather that remaining at the bottom or top of the incubator when no prey were added. Hence, these responses allowed medusae to scan larger volumes of water and to increase encounter rates with prey.
4.2 Predation impact of A.coerulea in Jiaozhou BayAccurate assessment of the potential predation effects of A. coerulea bloom on zooplankton communities is important for understanding the plankton dynamics in marine ecosystems with mass occurrences of jellyfish. Predation by Aurelia during blooms can substantially affect the species composition and abundance of zooplankton in pelagic systems (Möller, 1980; Hansson et al., 2005; Møller and Riisgård, 2007). For example, Möller (1980) reported that increased A. aurita abundance was accompanied by a sharp decrease in copepod standing stock; Hansson et al. (2005) indicated that A. aurita was the main predator on zooplankton communities, and the mean half-life time (t1/2) of cirripede larvae in May was just hours to a few days; Møller and Riisgård (2007) indicated that A. aurita had a high potential for preying on Acartia tonsa from mid-May to August in Skive Fjord, and the t1/2 of Acartia tonsa only ranged from 1 to 6 d. The density of A. coerulea in Jiaozhou Bay was extremely high in 2009 and A. coerulea undoubtedly reduced zooplankton stocks. When the population explosion of jellyfish A. coerulea occurred in Jiaozhou Bay, the numbers and compositions of zooplankton communities were significantly changed.
The theoretically calculated PCP for A. coerulea in Jiaozhou Bay varied seasonally as a result of variation in medusae density, size and water temperature. PCP was high during June and July 2009 (0.12–0.32 times a day) indicating that A. coerulea exerted high predation pressure on zooplankton during this period (Table 3; Fig. 6). This came a time when a high number of relatively larger A. coerulea medusae (mean bell size from 8.74±1.72 cm to 17.26±1.36 cm) was present with a higher surface water temperature (21.5–25.5℃) (Table 3). In the present study, clearance rates increased linearly with the crosssectional area (Fig. 4), which was consistent with the results of Olesen (1995). This suggests that large medusae have stronger predation ability because large jellyfish have a high prey encounter rate and high prey capture success (Bailey and Batty, 1983; Titelman and Hansson, 2006). The clearance rate also increased with temperature (< 15℃; Fig. 5a). Consequently, a stronger capture ability of A. coerulea occurred during this period, and the A. coerulea population was able to clear the water volume of the bay 0.12–0.32 times per day during June and July 2009 (Table 3). A. coerulea predation exerted high predation pressure on copepods population in this period. The mean residence time (t1/2) for copepods typically ranged from 2–10 d, and the minimum t1/2 value of 2.15 d was found on 15 July. Estimated residence time for copepods was considerably shorter than the time that these copepodites spend as larvae in the water column, which is in several weeks. Although the abundance of A. coerulea was high in early May, the mean size was 0.66±0.17 cm. Ephyrae and metamedusae have a lower clearance for preying on copepods (Figs. 3b & 4). In August, a high proportion of A. coerulea was deteriorated and the mean size had decreased (Wang and Sun, 2015). Therefore, PCP was low in early May and August 2009, and the estimated mean t1/2 of copepods was more than 30 d (Table 3; Fig. 6). This indicated a lower level of jellyfish predation on copepods during these times. The A. coerulea population controlled the amounts of copepods in late May, June and July, but not during the other months in 2009.
The A. coerulea population control of zooplankton communities is supported by field zooplankton investigations. Zooplankton abundances varied in different seasons between JBY (2009) and NJBYs (2008 and 2010) (Fig. 7). Mean abundances of total zooplankton and copepods sharply decreased in the JBY compared to the NJBYs, especially small copepods (< 1 mm; Fig. 7a & b). The high predation pressure exerted by A. coerulea reduced the copepod community standing stock. This was also demonstrated by Wan and Zhang (2012). In addition, low abundances of hydromedusae and chaetognaths were observed in the field surveys whenever the abundance of A. coerulea medusae was high. Zooplankton organisms, in addition to copepods, might also be controlled by A. coerulea in Jiaozhou Bay, as Aurelia medusae are usually considered as voracious predators with high feeding efficiency (Purcell and Arai, 2001; Titelman and Hansson, 2006). Our feeding experiments indicated that hydromedusae were captured by A. coerulea ephyrae and medusae with considerably high efficiency (Fig. 3). Therefore, high predation pressure by A. coerulea on hydromedusae occurred during population outbreaks of A. coerulea in Jiaozhou Bay. Although the predation efficiency was lower on chaetognaths (Fig. 3), the high capture pressure by A. coerulea on the other zooplankton organisms, like copepods, would generate food competition with chaetognaths as a predator. Competition for prey between both planktivorous zooplankton and fish and jellyfish has been suggested in previous studies (Möller, 1980; Purcell and Arai, 2001; Hansson et al., 2005). This may also exist in Jiaozhou Bay when jellyfish population explodes. Variations in the zooplankton populations were probably not due to environmental changes, because of the stable environments in Jiaozhou Bay during 2008 and 2010 (temperature and salinity; Fig. 9). However, the concentration of chlorophyll a was obviously high with the mean value of 7.4 μg/L in July 2009 (Fig. 9). The peak concentration reached nearly 10 μg/L (Wan and Zhang, 2012). This was significantly higher than the normal concentration of chlorophyll a in Jiaozhou Bay. The function of A. coerulea as a key organism may manifest itself not only as affecting the great variations in zooplankton communities, but also in the abundance of phytoplankton in Jiaozhou Bay. Release of phytoplankton from the control of zooplankton grazing due to the predation pressure by A. coerulea may result in a pronounced phytoplankton increase. Møller and Riisgård (2007) also suggested this effect of A. aurita in Skive Fjord. Thus, A. coerulea bloom can change the structure of the marine ecosystems in Jiaozhou Bay.
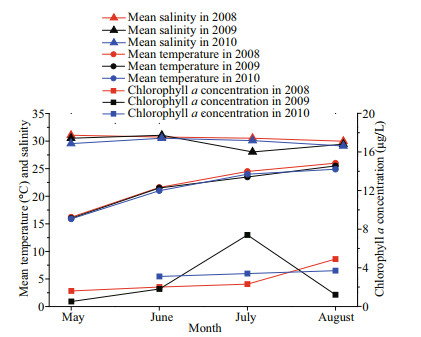
|
| Fig.9 Variation in monthly mean temperature, salinity, and chlorophyll a concentration in Jiaozhou Bay during May to August 2008–2010 Data of environments in 2008 were obtained from Sun et al. (2011). Data of environments in 2009 were obtained from Wan and Zhang (2012). Data of environments in 2010 were obtained from Wang et al. (2012). |
The population clearance potential by A. coerulea on fish eggs and larvae has not been established in this study, but the feeding experiments indicated the effectiveness of A. coerulea in capturing fish eggs and larvae compared to other prey items (Fig. 3). Clearance rates of fish larvae and eggs were up to 7 times and 2 times greater, respectively, compared to small copepods. This implies that A. coerulea bloom could cause high mortality to fish eggs and larvae in Jiaozhou Bay, as was also indicated by Hansson et al. (2005) in Limfjorden, Denmark.
Like other studies that extrapolate empirical laboratory results to processes at the ecosystem level, this study assumes that the measurements of laboratory clearance rate with monospecific diets yield clearance rate values comparable to a field situation where the medusae experience a variety of prey species. The mixed prey assemblage might generate an effect that one prey species reacts to the presence of another species with changed swimming behaviors, leading to a modified encounter rate with jellyfish predator (Hansson et al., 2005). However, total zooplankton at our sampling site was mainly comprised of 71% copepods and this likely generated only a minor effect on the clearance rate of copepods at low densities of the rest of prey items. Hence, the copepod population was used as a model prey group to estimate the collective predation impact by A. coerulea, and the predation effects on other zooplankton populations were not calculated in the present study. Moreover, when comparisons of results among containers sizes have been made, feeding has always been lower in the smaller containers (de Lafontaine and Leggett, 1987; Purcell, 1997). Thus, clearance rates of A. coerulea in the present study should be considered as conservative estimates, and the population clearance potentials may be underestimated.
5 CONCLUSIONPrey type and predator size both have significant effects on clearance rate. A. coerulea captured rotifers, fish larvae, and hydromedusae more efficiently than fish eggs, copepods, and chaetognaths. Clearance rate linearly increased with the cross sectional area of A. coerulea. Water temperature also had marked effect on clearance rate and this may due to the increased beat frequency of A. coerulea as the water temperature increased. The A. coerulea population potentially cleared the volume of water in the Bay more than 0.3 times a day in July, and the residence time (t1/2) for copepods was less than 6 d; the minimum t1/2-value was only 2.15 d in June and July in 2009. Abundances of copepods, hydromedusae, and chaetognaths were extremely low in 2009 compared to 2008 and 2010. Large predation pressure by A. coerulea population occurs to control zooplankton in Jiaozhou Bay, at least during June and July.
6 DATA AVAILABILITY STATEMENTThe authors declare that the data supporting the findings of this study are available within the article.
7 ACKNOWLEDGMENTWe thank the captain and crew of the R/V Haiou. We also thank ZHAO Zengxia, WANG Shiwei, LIU Mengtan, and XU Zhiqiang for help with sampling in Jiaozhou Bay. We thank WANG Junjian and CHENG Fangping for help in identification of zooplankton communities. We are grateful to the anonymous reviewers and the editor for their valuable comments.
Anderson E. 1974. Trophic Interactions among Ctenophores and Copepods in St. Margaret's Bay, Nova Scotia.Dalhousie University, Halifax. 85p.
|
Bailey K M, Batty R S. 1983. A laboratory study of predation by Aurelia aurita on larval herring (Clupea harengus):Experimental observations compared with model predictions. Marine Biology, 72(3): 295-301.
DOI:10.1007/BF00396835 |
Bailey K M. 1984. Comparison of laboratory rates of predation of five species of marine fish larvae by three planktonic invertebrates: effects of larval size on vulnerability. Marine Biology, 79(3): 303-309.
DOI:10.1007/BF00393262 |
Båmstedt U, Martinussen M B, Matsakis S. 1994. Trophodynamics of the two scyphozoan jellyfishes, Aurelia aurita and Cyanea capillata, in Western Norway. ICES Journal of Marine Science, 51(4): 369-382.
DOI:10.1006/jmsc.1994.1039 |
Båmstedt U. 1990. Trophodynamics of the scyphomedusae Aurelia aurita. Predation rate in relation to abundance, size and type of prey organism. Journal of Plankton Research, 12(1): 215-229.
DOI:10.1093/plankt/12.1.215 |
Buskey E J, Lenz P H, Hartline D K. 2002. Escape behavior of planktonic copepods in response to hydrodynamic disturbances: high speed video analysis. Marine Ecology Progress Series, 235: 135-146.
DOI:10.3354/meps235135 |
Costello J H, Colin S P. 1994. Morphology, fluid motion and predation by the scyphomedusa Aurelia aurita. Marine Biology, 121(2): 327-334.
DOI:10.1007/BF00346741 |
de Lafontaine Y, Leggett W C. 1987. Effect of container size on estimates of mortality and predation rates in experiments with macrozooplankton and larval fish. Canadian Journal of Fisheries and Aquatic Sciences, 44(9): 1 534-1 543.
DOI:10.1139/f87-185 |
de Lafontaine Y, Leggett W C. 1988. Predation by jellyfish on larval fish: an experimental evaluation employing in situ enclosures. Canadian Journal of Fisheries and Aquatic Sciences, 45(7): 1 173-1 190.
DOI:10.1139/f88-140 |
Dong Z J, Liu D, Keesing J K. 2010. Jellyfish blooms in China:dominant species, causes and consequences. Marine Pollution Bulletin, 60(7): 954-963.
DOI:10.1016/j.marpolbul.2010.04.022 |
Fancett M S. 1988. Diet and prey selectivity of scyphomedusae from Port Phillip Bay, Australia. Marine Biology, 98(4): 503-509.
DOI:10.1007/BF00391541 |
Ford M D, Costello J H, Heidelberg K B, Purcell J E. 1997. Swimming and feeding by the scyphomedusa Chrysaora quinquecirrha. Marine Biology, 129(2): 355-362.
DOI:10.1007/s002270050175 |
Hansson L J, Moeslund O, Kiørboe T, Riisgård H U. 2005. Clearance rates of jellyfish and their potential predation impact on zooplankton and fish larvae in a neritic ecosystem (Limfjorden, Denmark). Marine Ecology Progress Series, 304: 117-131.
DOI:10.3354/meps304117 |
Hansson L J. 2006. A method for in situ estimation of prey selectivity and predation rate in large plankton, exemplified with the jellyfish Aurelia aurita (L.). Journal of Experimental Marine Biology and Ecology, 328(1): 113-126.
DOI:10.1016/j.jembe.2005.07.002 |
Hartline D K, Lenz P H, Herren C M. 1996. Physiological and behavioral studies of escape responses in calanoid copepods. Marine and Freshwater Behaviour and Physiology, 27(2-3): 199-212.
DOI:10.1080/10236249609378965 |
Hernroth L, Gröndahl F. 1983. On the biology of Aurelia aurita (L.) 1. Release and growth of Aurelia aurita (L.)ephyrae in the Gullmar Fjord, Western Sweden, 1982-83. Ophelia, 22(2): 189-199.
DOI:10.1080/00785326.1983.10426595 |
Huang F P, Huang J Z, Yang Y L, Wang Z L, Chen S Q. 2007. Distributions of fish eggs and larval fish in the Jiaozhou Bay. Advances in Marine Science, 25(4): 468-473.
(in Chinese with English abstract) |
Ishii H, Tanaka F. 2001. Food and feeding of Aurelia aurita in Tokyo Bay with an analysis of stomach contents and a measurement of digestion times. Hydrobiologia, 451(1-3): 311-320.
|
Kogovšek T, Bogunović B, Malej A. 2010. Recurrence of bloom-forming scyphomedusae: wavelet analysis of a 200-year time series. In: Purcell J E, Angel D L eds.Jellyfish Blooms: New Problems and Solutions. Springer, Dordrecht. p.81-96.
|
Liu C S, Zhuang Z M, Chen S Q, Yan J P, Liu C L, Chen Z T. 2016. Predation of three juvenile scyphomedusa species to Paralichthys olivaceus and Sebastes schlegeli larvae. Journal of Fishery Sciences of China, 23(2): 436-446.
(in Chinese with English abstract) |
Möller H. 1980. Scyphomedusae as predators and food competitors of larval fish. Meeresforschung, 28: 90-100.
|
Möller H. 1984. Reduction of a larval herring population by jellyfish predator. Science, 224(4649): 621-622.
DOI:10.1126/science.224.4649.621 |
Møller L F, Riisgård H U. 2007. Population dynamics, growth and predation impact of the common jellyfish Aurelia aurita and two hydromedusae, Sarsia tubulosa and Aequorea vitrina, in Limfjorden (Denmark). Marine Ecology Progress Series, 346: 153-165.
DOI:10.3354/meps06960 |
Motoda S. 1963. Devices of simple plankton apparatus Ⅱ. Bulletin of the Faculty of Fisheries, Hokkaido University, 14(3): 152-162.
|
Olesen N J, Frandsen K, Riisgård H U. 1994. Population dynamics, growth and energetics of jellyfish Aurelia aurita in a shallow fjord. Marine Ecology Progress Series, 105: 9-18.
DOI:10.3354/meps105009 |
Olesen N J. 1995. Clearance potential of jellyfish Aurelia aurita, and predation impact on zooplankton in a shallow cove. Marine Ecology Progress Series, 124: 63-72.
DOI:10.3354/meps124063 |
Omori M, Ishii H, Fujinaga A. 1995. Life history strategy of Aurelia aurita (Cnidaria, Scyphomedusae) and its impact on the zooplankton community of Tokyo Bay. ICES Journal of Marine Science, 52(3-4): 597-603.
DOI:10.1016/1054-3139(95)80074-3 |
Papathanassiou E, Panayotidis P, Anagnostaki K. 1987. Notes on the biology and ecology of the jellyfish Aurelia aurita Lam. in Elefsis Bay (Saronikos Gulf, Greece). Marine Ecology, 8(1): 49-58.
|
Pitt K A, Connolly R M, Meziane T. 2009. Stable isotope and fatty acid tracers in energy and nutrient studies of jellyfish:a review. Hydrobiologia, 616: 119-132.
DOI:10.1007/s10750-008-9581-z |
Purcell J E, Arai M N. 2001. Interactions of pelagic cnidarians and ctenophores with fish: a review. Hydrobiologia, 451(1-3): 27-44.
|
Purcell J E, Båmstedt U, Båmstedt A. 1999. Prey, feeding rates, and asexual reproduction rates of the introduced oligohaline hydrozoan Moerisia lyonsi. Marine Biology, 134(2): 317-325.
DOI:10.1007/s002270050549 |
Purcell J E, Cowan Jr J H. 1995. Predation by the scyphomedusan Chrysaora quinquecirrha on Mnemiopsis leidyi ctenophores. Marine Ecology Progress Series, 129: 63-70.
DOI:10.3354/meps129063 |
Purcell J E. 1985. Predation on fish eggs and larvae by pelagic cnidarians and ctenophores. Bulletin of Marine Science, 37(2): 739-755.
|
Purcell J E. 1991. A review of cnidarians and ctenophores feeding on competitors in the plankton. Hydrobiologia, 216-217: 335-342.
DOI:10.1007/BF00026483 |
Purcell J E. 1992. Effects of predation by the scyphomedusan Chrysaora quinquecirrha on zooplankton populations in Chesapeake Bay, USA. Marine Ecology Progress Series, 87: 65-76.
DOI:10.3354/meps087065 |
Purcell J E. 1997. Pelagic cnidarians and ctenophores as predators: selective predation, feeding rates, and effects on prey populations. Annales de l'Institute Oceanographique, 73(2): 125-137.
|
Purcell J E. 2009. Extension of methods for jellyfish and ctenophore trophic ecology to large-scale research. Hydrobiologia, 616(1): 23-50.
DOI:10.1007/s10750-008-9585-8 |
Riisgård H U, Madsen C V. 2011. Clearance rates of ephyrae and small medusae of the common jellyfish Aurelia aurita offered different types of prey. Journal of Sea Research, 65(1): 51-57.
|
Schneider G, Behrends G. 1994. Population dynamics and the trophic role of Aurelia aurita medusae in the Kiel Bight and Western Baltic. ICES Journal of Marine Science, 51(4): 359-367.
DOI:10.1006/jmsc.1994.1038 |
Suchman C L, Sullivan B K. 2000. Effect of prey size on vulnerability of copepods to predation by the scyphomedusae Aurelia aurita and Cyanea sp. Journal of Plankton Research, 22(12): 2 289-2 306.
DOI:10.1093/plankt/22.12.2289 |
Sullivan B K, Suchman C L, Costello J H. 1997. Mechanics of prey selection by ephyrae of the scyphomedusa Aurelia aurita. Marine Biology, 130(2): 213-222.
DOI:10.1007/s002270050241 |
Sun S, Li C L, Zhang G T, Sun X X, Yang B. 2011. Long-term changes in the zooplankton community in the Jiaozhou Bay. Oceanologia et Limnologia Sinica, 42(5): 625-631.
(in Chinese with English abstract) |
Sun S, Zhou K, Yang B, Zhang Y S, Ji P. 2008. Ecology of zooplankton in the Jiaozhou Bay Ⅰ. species composition. Oceanologia et Limnologia Sinica, 39(1): 1-7.
(in Chinese with English abstract) |
Titelman J, Hansson L J. 2006. Feeding rates of the jellyfish Aurelia aurita on fish larvae. Marine Biology, 149(2): 297-306.
DOI:10.1007/s00227-005-0200-5 |
Waggett R J, Buskey E J. 2008. Escape reaction performance of myelinated and non-myelinated calanoid copepods. Journal of Experimental Marine Biology and Ecology, 361(2): 111-118.
DOI:10.1016/j.jembe.2008.05.006 |
Wan A Y, Zhang G T. 2012. Annual occurrence of moon jellyfish Aurelia sp. 1 in the Jiaozhou Bay and its impacts on zooplankton community. Oceanologia et Limnologia Sinica, 43(3): 494-501.
(in Chinese with English abstract) |
Wang S W, Zhang G T, Sun S, Wang Y T, Zhao Z X. 2012. Population dynamics of three scyphozoan jellyfish species during summer of 2011 in Jiaozhou Bay. Oceanologia et Limnologia Sinica, 43(3): 471-479.
(in Chinese with English abstract) |
Wang Y T, Sun S. 2015. Population dynamics of Aurelia sp. 1 ephyrae and medusae in Jiaozhou Bay, China. Hydrobiologia, 754: 147-155.
DOI:10.1007/s10750-014-2021-3 |
Yasuda T. 1976. Ecological studies on the jelly-fish, Aurelia aurita in Urazoko Bay, Fukui Prefecture-XⅢ: vertical distribution of ephyrae and metephyrae. Nippon Suisan Gakkaishi, 42(7): 743-752.
DOI:10.2331/suisan.42.743 |
 2020, Vol. 38
2020, Vol. 38