Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHONG Zhaoshan, WANG Minxiao, CHEN Hao, ZHENG Ping, LI Chaolun
- Gametogenesis and reproductive traits of the cold-seep mussel Gigantidas platifrons in the South China Sea
- Journal of Oceanology and Limnology, 38(4): 1304-1318
- http://dx.doi.org/10.1007/s00343-020-0027-4
Article History
- Received Jan. 14, 2020
- accepted in principle Mar. 31, 2020
- accepted for publication May. 18, 2020
2 Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China;
3 University of Chinese Academy of Sciences, Beijing 100049, China;
4 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China;
5 Center for Genomics and Biotechnology, Fujian Agriculture and Forestry University, Fuzhou 350002, China
Dense communities fueled by autotrophic bacteria are commonly found in deep-sea chemosynthetic ecosystems such as hydrothermal vents, cold seeps, and organic falls (Corliss et al., 1979; Van Dover et al., 2002; Jones et al., 2006; Lorion et al., 2009). The Bathymodiolinae mussel beds are one characteristic habitat in such ecosystems. Reproductive traits are key biotic factors for understanding how the mussels are recruited in these patchily distributed habitats (Laming et al., 2018).
Variations in light and temperature are considered the main abiotic factors shaping the seasonal rhythm of epibenthic species. However, deep-sea ecosystems are generally characterized by having a stable low temperature and no light penetration because of their depth (Lutz and Kennish, 1993). Mussels in shallow water have obvious reproductive cycles closely linked to water temperature cues and changes within a year (Bayne, 1976; Pieters et al., 1980; Newell et al., 1982; Lee, 1988; Borcherding, 1991; Jantz and Neumann, 1998), whereas for deep-sea mussels living more than 500 m below the surface, their reproductive cycles may well be different (Kennicutt II et al., 1985; Distel et al., 2000; Miyazaki et al., 2010). Interestingly, most Bathymodiolinae species studied thus far spawn seasonally like the coastal mussels. However, different conclusions of reproductive patterns were drawn, even within the same species Bathymodiolus thermophilus. Berg (1985) and Hessler et al. (1988) suggested the continuous gametogenesis in B. thermophilus based on considerable variations in oocyte size (and reviewed by Tyler and Young, 1999). The hypothesis was rejected by subsequent studies owing to the differences in the relative proportions of the acini and the inter-acinal connective tissue (Le Pennec and Beninger, 1997). The lack of continuous samples taken over a spawning period means these conclusions should be regarded with caution.
The genus Bathymodiolus was reclassified into three genera, Bathymodiolus, Gigantidas and 'Bathymodiolus like', according to updated molecular and morphological evidence (Lorion et al., 2013; Thubaut et al., 2013). Gigantidas platifrons (previously referred to Bathymodiolus platifrons) is one of the dominant species in chemosynthesis ecosystems formed by cold seeps and hydrothermal vents in the Northwest Pacific Ocean (NWP) (Hashimoto and Okutani, 1994; Xu et al., 2018, 2019). Gigantidas platifrons harbor methanotrophic bacteria in their gill epithelia cells (Takishita et al., 2017), and the bulk of energy (95%) needed for G. platifrons' nutrition is supplied by these endosymbionts (Wang, 2018). Until now, no direct evidence of the symbionts transfer mode is available for G. platifrons. In some Bathymodiolinae species, it was proved that their symbionts were horizontally transmitted (Fisher et al., 1993; Duperron et al., 2006; Picazo et al., 2019; Sayavedra et al., 2019). Recently, G. platifrons was utilized as a model organism for studying the organismal adaptations to extreme environments (Guo and Li, 2017; Zhang et al., 2017; Zheng et al., 2017; Du et al., 2018; Zhou et al., 2020), symbiosis (Sun et al., 2017b; Chen et al., 2019; Wang et al., 2019; Yu et al., 2019) and adaptive evolution (Sun et al., 2017a) in the deep-sea. However, surprisingly little is known about the reproductive biology of this important mussel species. In our study, we comprehensively analyzed histological sections of gonads and shell-length distribution of juvenile G. platifrons from the northwest Pacific to reveal the reproductive pattern of this species, with the aim of understanding the transfer mode of its endosymbionts, and to gain insights into biological features associated with the early life history of G. platifrons.
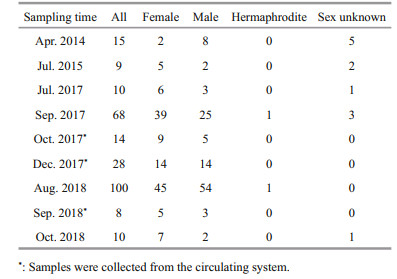
2 MATERIAL AND METHOD 2.1 Sample collectionAdult G. platifrons were sampled from the same cold seep location (119°17′08.043″E, 22°06′55.569″N; Fig. 1a, site F) in April 2014, July 2015, July and September 2017, and August 2018, by the remotely operated vehicle (ROV) Faxian (Discovery in Chinese) 4500 on R/V Kexue (Science in Chinese) and October 2018 by the ROV Haixing (Sea Star in Chinese) 6000 on the vessel Haiyang Shiyou (Ocean Oil in Chinese) 623 (Table 1). During September 2017 and August 2018, more than 100 mussels in a healthy condition were maintained in an onboard seawater circulating aquaculture system (Fig. 1c). They were kept at a constant low temperature (4.3 to 5.1℃) during the incubations and methane was bubbled into the tank twice a day as recommended in Sun et al. (2017b). The maximum methane concentration reached 338 μmol/L at the end of the bubbling period and dropped gradually to 253 μmol/L before the next bubbling period. Meanwhile, the dissolved oxygen (DO) concentration dropped to 2.38 mg/L during the methane bubbling and increased to ~7 mg/L again. The lowest DO fall was within the range (1.5–3.5 mg/L) of the DO inside the mussel bed of the Formosa cold seep (Du et al., 2018).
|
| Fig.1 Samples information a. map showing the Formosa cold seep (Site F) location in the South China Sea; b. larval traps settled in mussel beds in the Formosa cold seep (Site F) on July 4, 2016; c. inner view of circulating system; d. Gigantidas platifrons maintained in the circulating system; e. juvenile mussel attached on the nylon meshes in the larval trap; f juvenile mussel of G. platifrons detached from larval traps on 19th of July 2017, scale bar=2 μm. |
The gonad samples were collected in October and December 2017, and in September 2018, from the mussels in the same circulating aquiculture system with the same culture conditions onboard. The individual shell lengths of each G. platifrons were measured before dissection. After dissecting the individual specimen, the gonad samples of 1–2 mm3 were put into 4% paraformaldehyde (Solarbio Inc., Beijing, China) overnight and washed twice with 1× phosphate buffer solution (Solarbio Inc., Beijing, China). The fixed samples were then stored in 70% alcohol at -20℃, which ensured their preservation.
Juvenile G. platifrons were sampled by larval traps (Fig. 1b). The larval traps were filled with nylon meshes that were proved as the preferred materials for planktonic larvae settlement. The larval traps were settled in the bathymodioline beds of Formosa cold seep during the 2016 COLD SEEP CRUISE, with ROV 'Faxian 4500' equipped on R/V Kexue on 4th of July 2016, and were recovered from the same site 380 d later (19th of July 2017). After the larval traps was recovered on deck, the juvenile mussels were immediately removed from the attached substratum (Fig. 1e & f), and fixed in the fixative solution described above.
2.2 Histological and FISH analysisThe tissue samples of the mussels were dehydrated via a series of increasing alcohol concentrations (70%, 80%, 95%, and 100%), cleared in xylene and embedded in Paraplast Plus (Sigma, Inc., St. Louis, MO, USA). The 7 μm thick sections were stained using Hematoxylin and eosin (HE) stains and examined under a Nikon Eclipse Ni-U fluorescence microscope. The seven gonad stages were assigned followed the standards described by Dixon et al. (2006), including a sex-undifferentiated stage, initial gametogenesis stage, early gametogenesis stage, late gametogenesis stage, gamete maturation stage, spawning stage, and post-spawning stage. The gonadal index (GI) for each sample time was calculated according to Seed (1969):
GI=Σ(number of individual in stage x×x)/number of all staged individual,
where x is the numerical value of the stage.
The fluorescence in-situ hybridization (FISH) technique was performed by following Duperron et al. (2006). Briefly, after incubating samples in 1×phosphate buffered solution with tween-20 0.05% (v/v) (PBST, Solarbio Inc., Beijing, China) for 5 min, each slide was hybridized for 1 h at 46℃ in a hybridization buffer (0.9 mol/L NaCl, 0.02 mol/L of Tris-HCl, 0.01% SDS, and 20% formamide). The eubacteria probe Eub338 (5′-GCTGCCTCCCGTAGGAGT-3′) with 5′-labeled fluorescein isothiocyanate (FITC) was used to label the endosymbiont. The stained slides were separately examined under a Nikon Eclipse Ni-U fluorescence microscope.
2.3 Shell-length distribution of the juvenile musselThe shell lengths and shell heights of juvenile G. platifrons were measured through photographs by a stereoscopic microscope (ZEISS SteREO Discovery. V20). The shell length was plotted in 0.5 mm bins to show the shell length frequency histogram.
2.4 Data analysisThe oocyte diameters in each female mussel sample was measured with the Fiji.app (v.2.0.0 National Institutes of Health, Bethesda, Md, USA). The month was the fixed factor for one-way ANOVA analyses, and all tests were performed with the GraphPad Prism program (v.7.0.4, GraphPad Software, Inc., San Diego, CA, USA). Graphs were drawn in GraphPad Prism v.7.04 and Excel 2016. Data were represented by means±standard errors (SE).
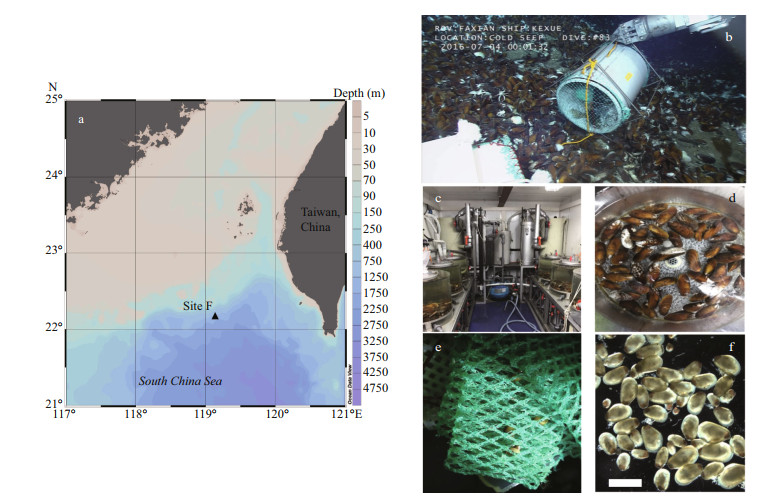
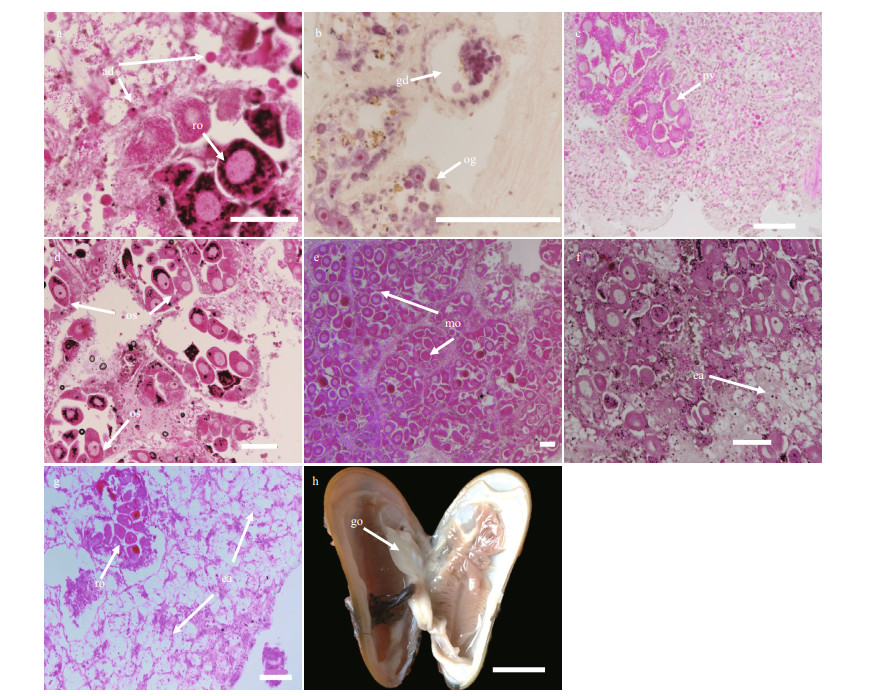
3 RESULT 3.1 Gonad gross morphologyGigantidas platifrons is a functional dioecious species, although two hermaphrodites were collected (Table 1). The sex of G. platifrons cannot be discriminated by the color of its mantle (Figs. 2h & 3h); and can only be determined by microscopy. For male acini, the germinal cells sat within the inner wall of the acini, with spermatocytes, spermatids, and spermatozoa developing toward the lumen. The number of acini increased within the mantle in reproductive seasons. For females, we could easily distinguish their oogonia (Fig. 2b), previtellogenic oocytes (Fig. 2c), and ripe primary oocytes (Fig. 2e). The progression of gametogenesis was uniform throughout the gonad and mantle. The gonadal ducts were more apparent in mussels in early gametogenesis. The gonadal duct consisted of fibrous ciliated cells, and these often contained residual oocytes or sperms (Figs. 2g & 3g).
|
| Fig.2 The process of G. platifrons female gonad oogenesis in stages 1 to 7 (a to g) a. stage 1, oogenesis with adipogranular cells and residual oocytes appearing in the ovary; b. stage 2, undifferentiated germ cells and oogonia on the acinus walls; c. stage 3, oogenesis with oogonia and previtellogenic oocytes on the periphery of the acinus; d. stage 4, oocytes begin to disengage from the acinus wall; e. stage 5, mature oocytes in the lumen, with adipogranular tissue greatly reduced; f. stage 6, the ovum in circular or ovoid in spawning time, and the acini are less densely in female's gonad; g. stage 7, after spawning, hemocytes enzymatically degrade most of the residual oocytes; h. a female G. platifrons mussel. Labels are: adipogranular cells (ad), empty acinus (ea), gonadal duct (gd), gonad (go), mature oocyte (mo), oogonia (og), oocyte with stalk (os), previtellogenic oocyte (pv), residual degrading oocyte (ro). Scale bars of a to g represent 100 μm, and that of h represents 1 cm. |
|
| Fig.3 The process of G. platifrons male gonad spermatogenesis in stages 1 to 7 (a to g) a. stage 1, adipogranular cells are dominant in the testis; b. stage 2, spermatogonia and spermatocytes appear around the periphery of the acinus; c. stage 3, some spermatocytes differentiate into spermatids and both spermatocytes and spermatids begin to occupy the lumen of the acinus; d. stage 4, spermatids fill most of the lumen and are connected by cytoplasmic material appearing as whorls within the acini; e. stage 5, spermatogonia and spermatocytes form a thin layer on the periphery of the acinus, with the lumen containing ripe spermatozoa and a greatly reduced amount of adipogranular tissue present; f. stage 6, male spawning occurs, in which the acini deflate, and spermatozoa density is reduced; g. stage 7, few spermatozoa are left in the acini; h. a male G. platifrons with its gill removed. Labels are: adipogranular cells (ad), deflated acinus (da), gonad (go), residual spermatozoa (rs), spermatocytes (sc), spermatogonia (sg), spermatids (st), spermatozoa (sz). Scale bars of a to g represent 100 μm and that of h represents 1 cm. |
Before gametogenesis began, only adipogranular cells were present in the ovary and mantle. If individuals had previously undergone a spawning event, the residual oocytes should be present in the deflated acini or gonadal ducts before oogenesis begins. In our study, most of the G. platifrons reproductive tissue was composed of adipogranular cells and most residual oocytes did show signs of degradation, including a grainy appearance, proximity to hemocytes, and absent nuclei (Stage 1; Fig. 2a). Oogenesis started in the gonadal part of the visceral mass and extended into the mantle lobes as gametogenesis proceeded. Undifferentiated germ cells on the acinus walls of the female mussels gave rise, through mitotic division, to definitive oogonia (10.64±2.42 μm) (Stage 2; Fig. 2b). The diameters of oogonia and previtellogenic oocytes were very small and all these cells attached to the acinus walls (Stage 3; Fig. 2c). As the oocyte grew and vitellogenesis began, its basal region was capable of extending itself from the acinus wall by means of a broad stalk (Stage 4; Fig. 2d). However, as the oocyte developed further and entered late vitellogenesis, the stalk became tenuous and eventually disappeared. These mature oocytes were then detached with the acinus wall and show a free state and their amount of adipogranular tissue was greatly reduced (Stage 5; Fig. 2e). Distinct nuclei and nucleoli were evident in ripe oocytes with diameters of about 54.93–86.84 μm (Stage 5; Fig. 2e). With more primary oocytes, however, the individual shape of oocytes became polygonal owing to many oocytes crowded in one acinus. During the spawning time, the acini became less dense in the female's gonad, so the ovum appeared circular or ovoid (Stage 6; Fig. 2f). After spawning, hemocytes enzymatically degraded most of the residual oocytes (Stage 7; Fig. 2g). Residual gametes may be present in all seven stages, within the acini or gonadal ducts.
3.3 SpermatogenesisIn G. platifrons males, the general morphology of the testis before gametogenesis resembled that of the ovary. Only adipogranular cells could be found in the male testis and mantle (Stage 1; Fig. 3a). If individuals previously underwent a spawning event, residual spermatozoa should be present in their deflated acini or gonadal ducts before spermatogenesis begins. Spermatogenesis started with a proliferation of spermatogonia and spermatocytes near the periphery of the acinus (Stage 2; Fig. 3b). Then, some spermatocytes differentiated into spermatids and both spermatocytes and spermatids begin to occupy the lumen of the acinus (Stage 3; Fig. 3c). As spermatogenesis proceeded, the spermatids filled most of the acinus' lumen, and the acinus resembled a whorl (Stage 4; Fig. 3d). The spermatids differentiated into ripe spermatozoa having a cap-like acrosome atop them, and the gamete density in the acinus greatly increased. Yet the spermatogonia and spermatocytes only form a very thin layer on the periphery of the acinus, for which the amount of adipogranular tissue was greatly reduced (Stage 5; Fig. 3e). During spawning, the acini deflate and the spermatozoa density was reduced further (Stage 6; Fig. 3f). After spawning was completed, only residual spermatozoa could be found in the acini (Stage 7, Fig. 3g).
3.4 Rare samples display hermaphroditismInterestingly, two hermaphrodites were observed in the 262 gonadal samples of this study. The cooccurrence/span of both male spermatozoa in the lumen and oocytes in the acini provided solid evidences of occasional hermaphroditism in the deepsea mussel G. platifrons (Fig. 4c & d). In both samples, the ovarian follicles were filled with ripe spermatozoa and immature oocytes were positioned alongside the stratum basales. In addition, emitted sperm were also observed in these tissue sections.

|
| Fig.4 Male, female, and hermaphrodite gonadal histology of G. platifrons a. observation of a normal mature seminiferous tubule. Spermatogonia (sg), spermatocyte (sc), spermatid (st), and spermatozoa (sz); b. a normal ovarian follicle with a high number of mature oocytes (mo) and immature oocytes (im); c. gonads of a hermaphrodite with mature lumen spermatozoa and ovarian follicles (of); d. immature oocytes (im) and spermatozoa (sz) in ovarian follicles. Scale bars of a and d are 50 μm, and b and c are 100 μm. |
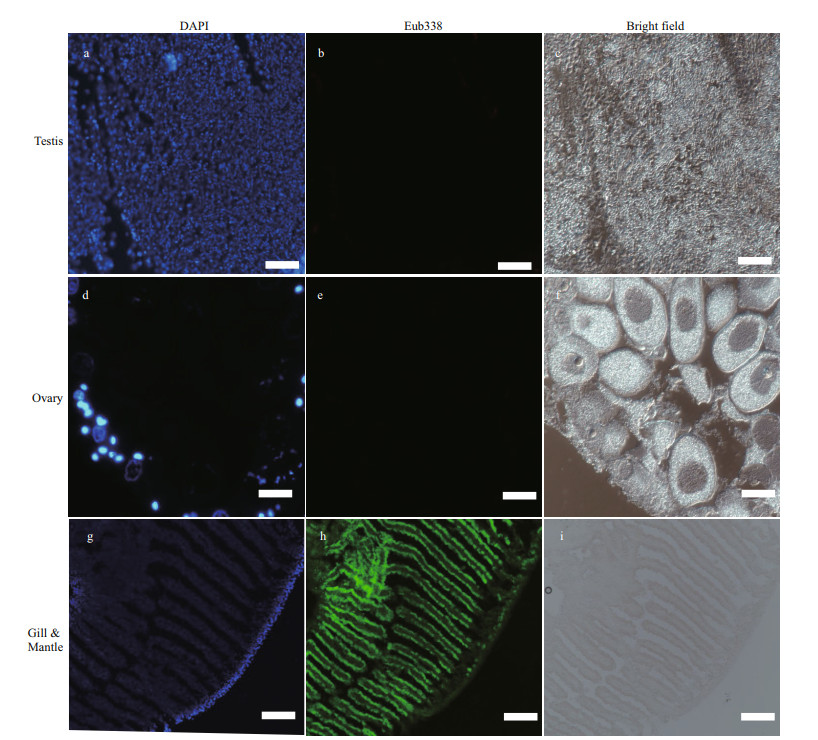
Although an obvious bacteria signal was detected on gill and mantle (Fig. 5h), no Eub338 signals were observed in the gonadal sections (Fig. 5b & e), supporting that symbiotic bacteria absent in the testis and ovary of the mussel. This result supported the hypothesis that bacteria were not transferred from spawning parents to progeny via the gonads, but rather horizontally through the local aquatic environment.
|
| Fig.5 FISH analysis of bacteria in G. platifrons testis and ovary a. DAPI staining of testis; b. no Eub338 signals in the testis; c. bright field of testis; d. DAPI staining of ovary; e. no Eub338 signals in the ovary; f. bright field of ovary; g. DAPI staining of the gill and mantle; h. Eub338 in the bacteriocyte on the gill filaments and mantle; i. bright field of gill and mantle. Scale bars of a to f represent 30 μm and g to i represents 300 μm. |
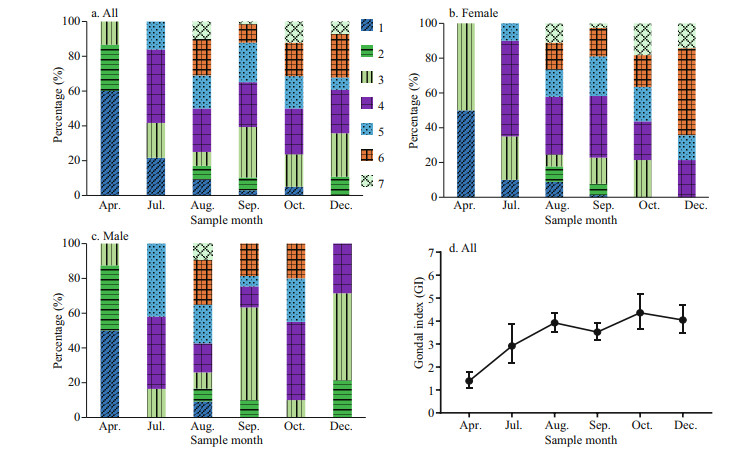
According to the ANOVAs testing, the gonad index was not significantly different among samples from the same months in different years. Thus, we pooled data for the same month, namely July of 2015 and 2017, September of 2017 and 2018, and October of 2017 and 2018. Figure 6a shows the gametogenesis periodicity of G. platifrons inferred from the percentage at each gonad stage. The majority of gonads remained in an early stage until April (Stages 1 to 3) and ripened in late July, when the proportion of Stage 4 gonads increased progressively. Most G. platifrons attained maturity (Stage 5) and spawned (Stage 6 and 7) from August to December. After spawning, most G. platifrons mussels were in Stage 7 or 1, and they were preparing for the next gametogenesis.
|
| Fig.6 The percentage at each gonad stage of all samples, female, and male samples, respectively (a–c); GI (mean±SE) of all samples pooled together in different months (d) |
The percentage at each gonad stage of female and male G. platifrons are shown in Fig. 6b & c, respectively. Their gonadal development was synchronous from August to October; and we found the spawning of the males finished earlier than the females as the stage 6 gonads were only available in females in December. Ripe gonads could be observed in female samples from September to December, but they were absent in male samples after October. The GI of all samples pooled together increased from April to December (Fig. 6d); the April GI value was significantly lower than the GI of other months (χ2=42.51, P < 0.000 1).
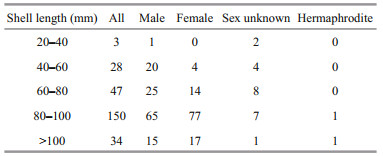
3.7 Sex-ratio patterns and oocyte diameter distributionThe sex distribution among the five shell size categories was compared to test whether the sex ratio was influenced by mussel ages (Table 2). For sex of small individuals less than 40 mm could not be determined except for one male mussel (30.1 mm). More males were observed in samples with a shell length between 40 mm and 60 mm, and the smallest female shell length was 55.7 mm. For those individuals with a shell length above 60 mm, their sex ratio approached 1 (mostly female). The shell lengths of the two hermaphrodite samples exceeded 80 mm.
|
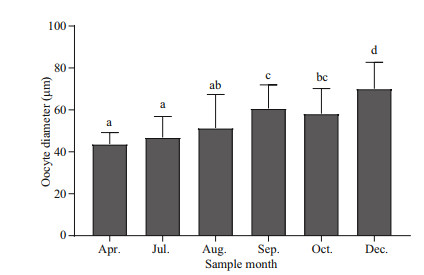
The oocyte diameter (~60 μm) of G. platifrons did not change with shell length. However, this diameter did significantly differ among months. The oocyte diameter in September to December was larger than that in April and July (Fig. 7). Furthermore, oocyte sizes of G. platifrons maintained in the laboratory were similar to the counterparts immediately dissected onboard the research vessel at sea.
|
| Fig.7 Oocyte diameters of G. platifrons (mean±SE) in different months Different lowercase letters denote a significance difference between months (P < 0.05). |
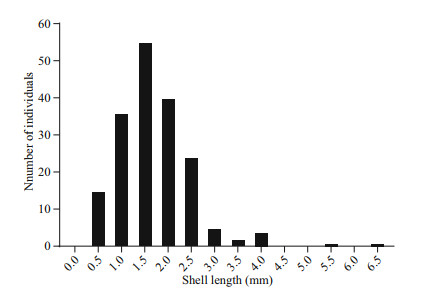
The shell length of the trapped juveniles ranged from 0.48 to 6.45 mm (n=183). The frequency histograms followed a clear unimodal pattern where the most recruited individuals (155 individuals) fell within 1–2.5 mm (Fig. 8). To give a rough estimation of the growth rate, we supposed the longest individual was trapped immediately after the setting of the larval traps. The minimum growth rate of the juvenile was estimated as 0.51 mm/month, which is comparable to other Bathymodiolines, such as G. childressi in Arellano and Young (2009) and I. modiolaeformis in Gaudron et al. (2012). Thus, the settlement of the larvae about 1.96 to 4.90 months before the recovery of the larval traps (from February to May), and the planktonic larval duration was about two to nine months.
|
| Fig.8 Shell length-distribution of G. platifrons juveniles trapped through 4th July 2016 to 19th July 2017 at the Formosa cold seep |
The biological sex can be distinguished in littoral mussels by the color of their gonads, with white for males and orange for females. The high concentration of the carotenoid pigment, which is accumulated from the phytoplankton food, makes the ovary orange (Campbell, 1970; Mikhailov et al., 1995). Neritic bivalves can accumulate carotenoids through filterfeeding on microalgae. Many of the carotenoids present in bivalves are metabolites of fucoxanthin, diatoxanthin, diadinoxanthin, and alloxanthin, which originate from microalgae (Maoka, 2011). However, methanotrophic endosymbiotic bacteria serve as the food resources in G. platifrons (Wang, 2018). To the best of our knowledge, the carotenoid biosynthesis pathway is incomplete in the methanotrophic bacteria (Schweiggert and Carle, 2016). The limited accumulation of carotenoids may result in the similar color of male and female gonads (Figs. 2h & 3h). Gigantidas platifrons is functionally dioecious, given the extremely low (0.76%) percentage of hermaphrodites found in the sampled population. A low-level occurrence of the hermaphrodites is a common phenomenon of Bathymodiolinae mussels (Berg, 1985; Le Pennec and Beninger, 1997; Dixon et al., 2006). The earlier occurrence of males may be the result of their earlier maturity, as recently found for B. septemdierum (Rossi and Tunnicliffe, 2017). However, all Idas species, in which gametogenesis has been studied, are reported as protandric hermaphrodites. Idas modiolaeformis are all males between 2.35 to 3.6 mm, and the sexual transition occurs in mussels between 3.6 to 11.6 mm in length (Laming et al., 2014). Protandrous hermaphroditism is often linked to adaptation to finite nutritional resources (Tyler et al., 2009; Ockelmann and Dinesen, 2010; Gaudron et al., 2012; Laming et al., 2014, 2015, 2018). Compared with the organic-falls ecosystems, cold seeps can last much longer (Smith and Amy, 2003; Levin et al., 2016). The reliable and adequate energy supplies in the Formosa cold seep (Feng and Chen, 2015; Niu et al., 2017; Du et al., 2018) could provide enough fixed carbons for G. platifrons, possibly giving rise to a smaller proportion of hermaphrodites in our study, as suggested by above mentioned finite-nutritionhermaphrodite hypothesis.
4.2 Reproductive patterns of deep-sea musselDetailed observations of gametogenesis can yield clues for a better understanding of the reproductive patterns of Bathymodiolinae mussels. Considering that breeding was controlled by energy availability, rapid growth, and continuous reproduction would be possible in cold seeps where the continuous food supplies were available (Tyler et al., 2007). However, we found that the GI of G. platifrons significantly differed between April and the second half of the year. As defined by Tyler et al. (1982), discontinuous reproduction was observed in G. platifrons as was found in other deep-sea mussels like the B. thermophilus at the 13°N site in the East Pacific, B. puteoserpentis and B. azoricus at the Snake Pit site of the mid-Atlantic ridge, B. elongatus from the North Fiji Basin, and G. childressi at the Gulf of Mexico (Le Pennec and Beninger, 1997; Dixon et al., 2006; Tyler et al., 2007). We observed asynchronized development of the male and female gonads in December samples with stage 6 gonads found in the females but absent in the males. Rather than collected from the cold seeps, the gonads in December were collected from the maintained mussels in the circulating aquarium. We observed spermiation during the first few days of aquaculture in September 2017. Mucus-like secreta with swimming sperms alone were observed (Supplementary Fig.S1). Thus, we supposed that the absence of ripe testis (stage 6) in December samples was the result of induced spawning of the males at the beginning of incubation. The periodic variation of GI and oocyte diameters suggested the occurrence of seasonal spawning of G. platifrons as shown in Figs. 6d & 7. In April, both a reduced GI and small oocyte diameters indicated an early stage of gametogenesis, suggesting the recent termination of the spawning or a period recovery before gonad development occurred again. The gonads of this deepsea species then developed to maturity, with males and females ready to spawn in the second half of the year as the percentage of ripe germ cells increased or were maintained at high levels from August to December. Thus, a spawning cycle was proposed for the G. platifrons, which was also confirmed by the unimodal distribution of shell lengths of the trapped juveniles in one year (Fig. 8). A similar unimodal shell length distribution of juvenile mussel has been described in deep-sea mussel from cold seeps in the Gulf of Mexico (Arellano and Young, 2009).
It was initially hypothesized that the reproduction rhythm of the deep-sea bivalves is linked to the seepage activity or seasonal temperature variation of surrounding environment (Momma et al., 1995; Philip et al., 2016). However, our long-term in-situ observation indicated that the environments (at least regarding temperature) of the cold seep was stable during our sampling periods (Fig. 9 and Supplementary Table S1). In addition, the seepage activity of methane changed over a smaller temporal scale (days or even hours) (Bayrakci et al., 2014; Philip et al., 2016). Thus, other factors should function as signals regulating the spawning rhythm.
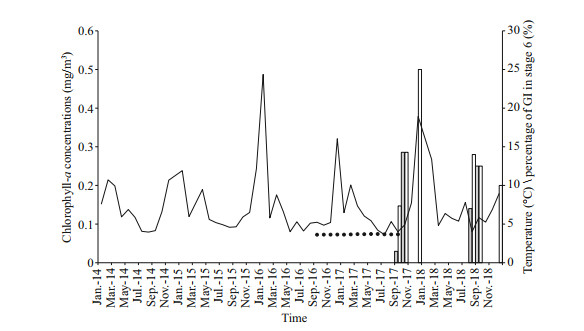
|
| Fig.9 Chlorophyll-a concentrations in the surface water upon the Formosa cold in the South China Sea (SCS) Data provided by Asia-Pacific Data Research Center in MODIS Aqua SST level 3 (http://apdrc.soest.hawaii.edu/). Solid line represents the changes in chlorophyll-a concentration, spots represent the changes in water temperature of G. platifrons' mussel bed around one year, and the gray and white bars represent the percentage of male and female in stage 6 respectively. |
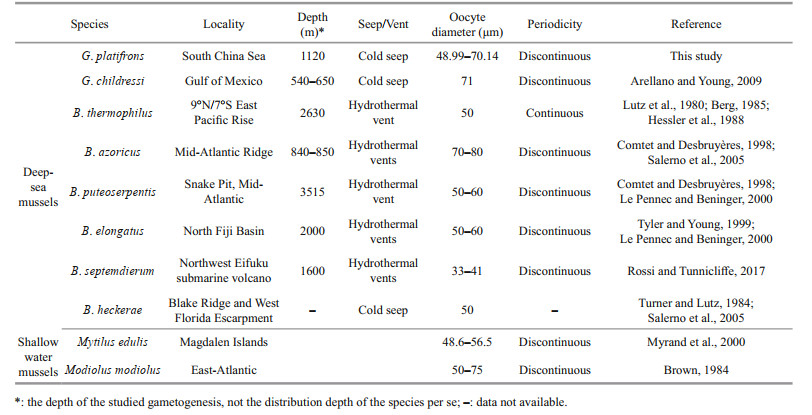
Another hypothesis is that the spawning of deepsea mussels at sites less than 1 000 m is regulated by seasonal fluctuations in the production of epipelagic organic matter, in which the phytoplankton sink to the bottom (Colaço et al., 2006; Dixon et al., 2006; Tyler et al., 2007). However, in our studied area, the depth is more than 1 000 m and the chlorophyll-a, which is a predictor of photosynthetic primary production, peaked around January (Fig. 9, the solid line), two months later than the proposed spawning period of G. platifrons. Thus, our results do not support the hypothesis that the spawning of the G. platifrons is triggered by falling organic matters. As shown in Table 3, similar conclusions have been drawn for other bathymodiolines dwelling at sites deeper than 2 000 m, where the availability of sunken organic material drops to very low levels (Khripounoff and Alberic, 1991; Rossi and Tunnicliffe, 2017).
|
We observed a time lag of two to nine months between spawning and settlement, supporting the idea that G. platifrons may have a long planktonic period. As observed in other bathymodiolines (Dixon et al., 2006; Arellano et al., 2014), the planktonic larvae of G. platifrons may migrate upwards to find food in the photic zone, which would support the development of the planktonic larvae. Primary production in this region is comprised of ultraplankton (0.2–5 μm) (Jiang et al., 2014) and diatoms (Wang et al., 2018; Ndah et al., 2019), which may supply food for the larvae of mussels (Jørgensen, 1981). G. platifrons spawned before the peak abundance of phytoplankton in the surface water. Similarly, the planktotrophic larvae of B. azoricus settled at a time when the concentration of chlorophyll-a reached its minimum (Colaço et al., 2006), providing enough food for the planktonic larvae. Thus, our results support the hypothesis that the seasonal reproduction of the bathymodiolines may be an ancestral characteristic of mussels, which was mentioned in Eckelbarger and Watling (1995) and in Tyler and Young (1999).
The oocyte diameters measured in G. platifrons fell within the ranges reported for other deep-sea Bathymodiolinae mussel species (i.e., 33 to 80 μm) (Table 3). Due to little oocyte diameter we can infer that G. platifrons larvae should employ the planktotrophic developmental mode (Le Pennec and Beninger, 2000). The larvae of G. childressi have been found in surface waters, and have a planktonic duration that may last up to 13 months and 3 000 km (Arellano and Young, 2009; Arellano et al., 2014). High dispersal and a lack of population differentiation have been found in G. platifrons from all habitats across the NWP (Miyazaki et al., 2013; Xu et al., 2018), supporting the notion of a long planktonic duration of G. platifrons. The long larval duration of deep-sea mussels has been found in many species (Arellano and Young, 2009; Young et al., 2012; Arellano et al., 2014). Furthermore the long planktonic duration is especially important for population recruitment in ephemeral and patchily distributed chemosynthetic ecosystems, and would have led to the wide distribution of G. platifrons.
4.3 Horizontal transmission of endosymbiontsThe successful establishment of endosymbionts is the key stepping stone for Bathymodiolinae species to colonize and survive the deep-sea chemosynthetic environments (Barry et al., 2002; Kádár et al., 2005; Laming et al., 2015). We provided evidences for horizontal transmission of endosymbionts in G. platifrons by FISH observation of gonads. According to our results, the gonads of both female and male lacked bacteria (Fig. 5). Thus, the vertical transfer hypothesis, where symbionts are acquired through gametes, was not supported for G. platifrons in our study.
A longer planktonic larvae stage up to 13 months has been observed in some deep-sea mussels (Arellano and Young, 2009; Arellano et al., 2014). Limited reductive materials are available in the upper waters where larvae live. Based on the on-board incubation studies, the abundance of the methanotrophic symbiont dropped to a very low level after 34 days (Sun et al., 2017b). Thus, we assume that it was inefficient for maintaining large amounts of symbionts through the planktonic phase of the larvae. However, we cannot rule out the possibility that some seed endosymbiont in a dormant state survived in the planktonic larvae. Nevertheless, based on the microscopy and isotopic results, the symbiotic relationships between the chemosynthetic bacteria and the mussels were found to be established only after attachment (Dubilier et al., 1998; Won et al., 2003; Salerno et al., 2005; Picazo et al., 2019).
During horizontal transmission, the symbionts are re-acquired from the local environment in each new generation. This should enhance the opportunity for increased genetic variation to arise, thereby facilitating adaptation of the mussels to different or changing environments.
5 CONCLUSIONThis is the first integrated study to report gametogenesis and reproductive traits of the deep-sea mussel Gigantidas platifrons in the NWP. Gigantidas platifrons is functionally dioecious and develops through a planktonic stage. Our study confirms that G. platifrons has discontinuous gametogenesis and a seasonal reproductive cycle. Our study also provide evidence to support the hypothesis of horizontal symbiont transmission in G. platifrons. This study of gametogenesis and the reproductive cycle will be helpful to elucidate the population structure and population recruitment strategies of G. platifrons in the NWP. Further studies should address the problems of planktonic larval capture, the long-term in-situ observation of reproduction and recruitment activities, and the artificial breeding of this species to make it model species for deep-sea adaptation studies.
6 DATA AVAILABILITY STATEMENTThe data that support the findings of this study are available from the corresponding author upon reasonable request.
7 ACKNOWLEDGMENTWe thank all the crews on R/V Kexue, R/V Haiyang Shiyou 623, ROV Faxian 4500, and ROV Haixing 6000 for their assistance in sample collection and all the laboratory members for continuous technical advice and helpful discussions.
Arellano S M, Van Gaest A L, Johnson S B, Vrijenhoek R C, Young C M. 2014. Larvae from deep-sea methane seeps disperse in surface waters. Proceedings of the Royal Society B: Biological Sciences, 281(1786): 20133276.
DOI:10.1098/rspb.2013.3276 |
Arellano S M, Young C M. 2009. Spawning, development, and the duration of larval life in a deep-sea cold-seep mussel. The Biological Bulletin, 216(2): 149-162.
DOI:10.1086/BBLv216n2p149 |
Barry J P, Buck K R, Kochevar R K, Nelson D C, Fujiwara Y, Goffredi S K, Hashimoto J. 2002. Methane-based symbiosis in a mussel, Bathymodiolus platifrons, from cold seeps in Sagami Bay, Japan. Invertebrate Biology, 121(1): 47-54.
|
Bayne B L. 1976. The biology of mussel larvae. In: Bayne B L ed. Marine Mussels: Their Ecology and Physiology.Cambridge University Press, Cambridge. p.81-120.
|
Bayrakci G, Scalabrin C, Dupré S, Leblond I, Tary J B, Lanteri N, Augustin J M, Berger L, Cros E, Ogor A, Tsabaris C, Lescanne M, Géli L. 2014. Acoustic monitoring of gas emissions from the seafloor. Part Ⅱ: a case study from the Sea of Marmara. Marine Geophysical Research, 35(3): 211-229.
DOI:10.1007/s11001-014-9227-7 |
Berg C J. 1985. Reproductive strategies of mollusks from abyssal hydrothermal vent communities. Bulletin of the Biological Society of Washington, 6: 185-197.
|
Borcherding J. 1991. The annual reproductive cycle of the freshwater mussel Dreissena polymorpha Pallas in lakes. Oecologia, 87(2): 208-218.
DOI:10.1007/BF00325258 |
Brown R A. 1984. Geographical variations in the reproduction of the horse mussel, Modiolus modiolus (Mollusca: Bivalvia). Journal of the Marine Biological Association of the United Kingdom, 64(4): 751-770.
DOI:10.1017/S0025315400047214 |
Campbell S A. 1970. The carotenoid pigments of Mytilus edulis and Mytilus californianus. Comparative Biochemistry and Physiology, 32(1): 97-115.
DOI:10.1016/0010-406X(70)90159-3 |
Chen H, Wang M X, Zhang H, Wang H, Lv Z, Zhou L, Zhong Z S, Lian C, Cao L, Li C L. 2019. An LRR-domain containing protein identified in Bathymodiolus platifrons serves as intracellular recognition receptor for the endosymbiotic methane-oxidation bacteria. Fish & Shellfish Immunology, 93: 354-360.
|
Colaço A, Martins I, Laranjo M, Pires L, Leal C, Prieto C, Costa V, Lopes H, Rosa D, Dando P R, Serrão-Santos R. 2006. Annual spawning of the hydrothermal vent mussel, Bathymodiolus azoricus, under controlled aquarium, conditions at atmospheric pressure. Journal of Experimental Marine Biology and Ecology, 333(2): 166-171.
DOI:10.1016/j.jembe.2005.12.005 |
Comtet T, Desbruyères D. 1998. Population structure and recruitment in mytilid bivalves from the Lucky Strike and Menez Gwen hydrothermal vent fields (37°17'N and 37°50'N on the Mid-Atlantic Ridge). Marine Ecology Progress Series, 163: 165-177.
DOI:10.3354/meps163165 |
Corliss J B, Dymond J, Gordon L I, Edmond J M, von Herzen R P, Ballard R D, Green K, Williams D, Bainbridge A, Crane K, van Andel T H. 1979. Submarine thermal springs on the Galápagos Rift. Science, 203(4385): 1 073-1 083.
DOI:10.1126/science.203.4385.1073 |
Distel D L, Baco A R, Chuang E, Morrill W, Cavanaugh C, Smith C R. 2000. Do mussels take wooden steps to deepsea vents?. Nature, 403(6711): 725-726.
|
Dixon D R, Lowe D M, Miller P I, Villemin G R, Colaço A, Serrão-Santos R, Dixon L R J. 2006. Evidence of seasonal reproduction in the Atlantic vent mussel Bathymodiolus azoricus, and an apparent link with the timing of photosynthetic primary production. Journal of the Marine Biological Association of the United Kingdom, 86(6): 1 363-1 371.
DOI:10.1017/S0025315406014391 |
Du Z F, Zhang X, Luan Z D, Wang M X, Xi S C, Li L F, Wang B, Cao L, Lian C, Li C L, Yan J. 2018. In situ raman quantitative detection of the cold seep vents and fluids in the chemosynthetic communities in the South China Sea. Geochemistry, Geophysics, Geosystems, 19(7): 2 049-2 061.
DOI:10.1029/2018GC007496 |
Dubilier N, Windoffer R, Giere O. 1998. Ultrastructure and stable carbon isotope composition of the hydrothermal vent mussels Bathymodiolus brevior and B. sp. affinis brevior from the North Fiji Basin, western Pacific. Marine Ecology Progress Series, 165: 187-193.
DOI:10.3354/meps165187 |
Duperron S, Bergin C, Zielinski F, Blazejak A, Pernthaler A, McKiness Z P, DeChaine E, Cavanaugh C M, Dubilier N. 2006. A dual symbiosis shared by two mussel species, Bathymodiolus azoricus and Bathymodiolus puteoserpentis (Bivalvia: Mytilidae), from hydrothermal vents along the northern Mid-Atlantic Ridge. Environmental Microbiology, 8(8): 1 441-1 447.
DOI:10.1111/j.1462-2920.2006.01038.x |
Eckelbarger K J, Watling L. 1995. Role of phylogenetic constraints in determining reproductive patterns in deepsea invertebrates. Invertebrate Biology, 114(3): 256-269.
DOI:10.2307/3226880 |
Feng D, Chen D F. 2015. Authigenic carbonates from an active cold seep of the northern South China Sea: new insights into fluid sources and past seepage activity. Deep Sea Research Part Ⅱ: Topical Studies in Oceanography, 122: 74-83.
DOI:10.1016/j.dsr2.2015.02.003 |
Fisher C R, Brooks J M, Vodenichar J S, Zande J M, Childress J J, Burke Jr R A. 1993. The co-occurrence of methanotrophic and chemoautotrophic sulfur-oxidizing bacterial symbionts in a deep-sea mussel. Marine Ecology, 14(4): 277-289.
DOI:10.1111/j.1439-0485.1993.tb00001.x |
Gaudron S M, Demoyencourt E, Duperron S. 2012. Reproductive traits of the cold-seep symbiotic mussel Idas modiolaeformis: gametogenesis and larval biology. The Biological Bulletin, 222(1): 6-16.
DOI:10.1086/BBLv222n1p6 |
Guo X Y, Li C L. 2017. Biochemical components of cold seep mussel Bathymodiolus platifrons from South China Sea and comparison with hydrothermal vent and offshore mussels. Marine Sciences, 41(6): 65-71.
(in Chinese with English abstract) |
Hashimoto J, Okutani T. 1994. Four new mytilid mussels associated with deep sea chemosynthetic communities around Japan. Venus, 53(2): 61-83.
|
Hessler R R, Smithey W M, Boudrias M A, Keller C H, Lutz R A, Childress J J. 1988. Temporal change in megafauna at the Rose Garden hydrothermal vent (Galapagos Rift; eastern tropical Pacific). Deep Sea Research Part A.Oceanographic Research Papers, 35(10-11): 1 681-1 709.
DOI:10.1016/0198-0149(88)90044-1 |
Jantz B, Neumann D. 1998. Growth and reproductive cycle of the zebra mussel in the River Rhine as studied in a river bypass. Oecologia, 114(2): 213-225.
DOI:10.1007/s004420050439 |
Jiang Z Y, Wang Y S, Sun F L. 2014. Spatial structure of eukaryotic ultraplankton community in the northern South China Sea. Biologia, 69(5): 557-565.
|
Jones W J, Won Y J, Maas PAY, Smith P J, Lutz R A, Vrijenhoek R C. 2006. Evolution of habitat use by deep-sea mussels. Marine Biology, 148(4): 841-851.
DOI:10.1007/s00227-005-0115-1 |
Jørgensen C B. 1981. Mortality, growth, and grazing impact of a cohort of bivalve larvae, Mytilus edulis L. Ophelia, 20(2): 185-192.
DOI:10.1080/00785236.1981.10426570 |
Kádár E, Bettencourt R, Costa V, Santos R S, Lobo-da-Cunha A, Dando P. 2005. Experimentally induced endosymbiont loss and re-acquirement in the hydrothermal vent bivalve Bathymodiolus azoricus. Journal of Experimental Marine Biology and Ecology, 318(1): 99-110.
DOI:10.1016/j.jembe.2004.12.025 |
Kennicutt Ⅱ M C, Brooks J M, Bidigare R R, Fay R R, Wade T L, McDonald T J. 1985. Vent-type taxa in a hydrocarbon seep region on the Louisiana slope. Nature, 317(6035): 351-353.
DOI:10.1038/317351a0 |
Khripounoff A, Alberic P. 1991. Settling of particles in a hydrothermal vent field (East Pacific Rise 13°N) measured with sediment traps. Deep Sea Research Part A.Oceanographic Research Papers, 38(6): 729-744.
DOI:10.1016/0198-0149(91)90009-5 |
Laming S R, Duperron S, Cunha M R, Gaudron S M. 2014. Settled, symbiotic, then sexually mature: adaptive developmental anatomy in the deep-sea, chemosymbiotic mussel Idas modiolaeformis. Marine Biology, 161(6): 1 319-1 333.
DOI:10.1007/s00227-014-2421-y |
Laming S R, Duperron S, Gaudron S M, Hilário A, Cunha M R. 2015. Adapted to change: the rapid development of symbiosis in newly settled, fast-maturing chemosymbiotic mussels in the deep sea. Marine Environmental Research, 112: 100-112.
DOI:10.1016/j.marenvres.2015.07.014 |
Laming S R, Gaudron S M, Duperron S. 2018. Lifecycle ecology of deep-sea chemosymbiotic mussels: a review. Frontiers in Marine Science, 5: 282.
DOI:10.3389/fmars.2018.00282 |
Le Pennec M, Beninger P G. 1997. Ultrastructural characteristics of spermatogenesis in three species of deep-sea hydrothermal vent mytilids. Canadian Journal of Zoology, 75(2): 308-316.
DOI:10.1139/z97-039 |
Le Pennec M, Beninger P G. 2000. Reproductive characteristics and strategies of reducing-system bivalves. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 126(1): 1-16.
|
Lee S Y. 1988. The reproductive cycle and sexuality of the green mussel Perna viridis (L.) (Bivalvia: Mytilacea) in Victoria Harbour, Hong Kong. Journal of Molluscan Studies, 54(3): 317-323.
DOI:10.1093/mollus/54.3.317 |
Levin L A, Baco A R, Bowden D A, Colaco A, Cordes E E, Cunha M R, Demopoulos A W J, Gobin J, Grupe B M, Le J, Metaxas A, Netburn A N, Rouse G W, Thurber A R, Tunnicliffe V, Van Dover C L, Vanreusel A, Watling L. 2016. Hydrothermal vents and methane seeps: rethinking the sphere of influence. Frontiers in Marine Science, 3: 72.
|
Lorion J, Duperron S, Gros O, Cruaud C, Samadi S. 2009. Several deep-sea mussels and their associated symbionts are able to live both on wood and on whale falls. Proceedings of the Royal Society B: Biological Sciences, 276(1654): 177-185.
DOI:10.1098/rspb.2008.1101 |
Lorion J, Kiel S, Faure B, Kawato M, Ho S Y W, Marshall B, Tsuchida S, Miyazaki J I, Fujiwara Y. 2013. Adaptive radiation of chemosymbiotic deep-sea mussels. Proceedings of the Royal Society B: Biological Sciences, 280(1770): 20131243.
DOI:10.1098/rspb.2013.1243 |
Lutz R A, Jablonski D, Rhoads D C, Turner R D. 1980. Larval dispersal of a deep-sea hydrothermal vent bivalve from the Galápagos Rift. Marine Biology, 57(2): 127-133.
DOI:10.1007/BF00387378 |
Lutz R A, Kennish M J. 1993. Ecology of deep-sea hydrothermal vent communities: a review. Reviews of Geophysics, 31(3): 211-242.
DOI:10.1029/93RG01280 |
Maoka T. 2011. Carotenoids in marine animals. Marine Drugs, 9(2): 278-293.
DOI:10.3390/md9020278 |
Mikhailov A T, Torrado M, Méndez J. 1995. Sexual differentiation of reproductive tissue in bivalve molluscs:identification of male associated polypeptide in the mantle of Mytilus galloprovincialis Lmk. International Journal of Developmental Biology, 39(3): 545-548.
|
Miyazaki J I, Beppu S, Kajio S, Dobashi A, Kawato M, Fujiwara Y, Hirayama H. 2013. Dispersal ability and environmental adaptability of deep-sea mussels Bathymodiolus (Mytilidae: Bathymodiolinae). Open Journal of Marine Science, 3(1): 31-39.
DOI:10.4236/ojms.2013.31003 |
Miyazaki J I, de Oliveira Martins L, Fujita Y, Matsumoto H, Fujiwara Y. 2010. Evolutionary process of deep-sea Bathymodiolus mussels. PLoS One, 5(4): e10363.
DOI:10.1371/journal.pone.0010363 |
Momma H, Mitsuzawa K, Kaiho Y, Iwase R, Fujiwara Y. 1995. Long-term deep sea floor observation off Hatsushima Island in Sagami Bay: one year in the Calyptogena soyoae clam colony. JAMSTEC Journal of Deep Sea Research, 11: 249-268.
|
Myrand B, Guderley H, Himmelman J H. 2000. Reproduction and summer mortality of blue mussels Mytilus edulis in the Magdalen Islands, southern Gulf of St. Lawrence.Marine Ecology Progress Series, 197: 193-207.
DOI:10.3354/meps197193 |
Ndah A B, Dagar L, Becek K, Odihi J O. 2019. Spatio-temporal dynamics of phytoplankton functional groups in the South China Sea and their relative contributions to marine primary production. Regional Studies in Marine Science, 29: e100598.
DOI:10.1016/j.rsma.2019.100598 |
Newell R I, Hilbish T J, Koehn R K, Newell C J. 1982. Temporal variation in the reproductive cycle of Mytilus edulis L. (Bivalvia, Mytilidae) from localities on the east coast of the United States. The Biological Bulletin, 162(3): 299-310.
DOI:10.2307/1540985 |
Niu M Y, Liang Q Y, Feng D, Wang F P. 2017. Ecosystems of cold seeps in the South China Sea. In: Kallmeyer J ed.Life at Vents and Seeps. De Gruyter, Berlin, Boston.p.139-160.
|
Ockelmann K W, Dinesen G E. 2010. Life on wood-the carnivorous deep-sea mussel Idas argenteus(Bathymodiolinae, Mytilidae, Bivalvia). Marine Biology Research, 7(1): 71-84.
|
Philip B T, Denny A R, Solomon E A, Kelley D S. 2016. Timeseries measurements of bubble plume variability and water column methane distribution above Southern Hydrate Ridge, Oregon. Geochemistry, Geophysics, Geosystems, 17(3): 1 182-1 196.
DOI:10.1002/2016GC006250 |
Picazo D R, Dagan T, Ansorge R, Petersen J M, Dubilier N, Kupczok A. 2019. Horizontally transmitted symbiont populations in deep-sea mussels are genetically isolated. The ISME Journal, 13(12): 2 954-2 968.
DOI:10.1038/s41396-019-0475-z |
Pieters H, Kluytmans J H, Zandee D I, Cadée G C. 1980. Tissue composition and reproduction of Mytilus edulis in relation to food availability. Netherlands Journal of Sea Research, 14(3-4): 349-361.
DOI:10.1016/0077-7579(80)90008-3 |
Rossi G S, Tunnicliffe V. 2017. Trade-offs in a high CO2 habitat on a subsea volcano: condition and reproductive features of a bathymodioline mussel. Marine Ecology Progress Series, 574: 49-64.
DOI:10.3354/meps12196 |
Salerno J L, Macko S A, Hallam S J, Bright M, Won Y J, McKiness Z, van Dover C L. 2005. Characterization of symbiont populations in life-history stages of mussels from chemosynthetic environments. The Biological Bulletin, 208(2): 145-155.
DOI:10.2307/3593123 |
Sayavedra L, Ansorge R, Rubin-Blum M, Leisch N, Dubilier N, Petersen J M. 2019. Horizontal acquisition followed by expansion and diversification of toxin-related genes in deep-sea bivalve symbionts. BioRxiv, 605386, https://doi.org/10.1101/605386
|
Schweiggert R M, Carle R. 2016. Carotenoid production by bacteria, microalgae, and fungi. In: Kaczor A, Baranska M eds. Carotenoids: Nutrition, Analysis and Technology.Wiley, Washington. p.217-240.
|
Seed R. 1969. The ecology of Mytilus edulis L.(Lamellibranchiata) on exposed rocky shores: I. Breeding and settlement. Oecologia, 3(3): 277-316.
|
Smith C R, Amy R B. 2003. Ecology of whale falls at the deepsea floor. Oceanography and Marine Biology, 41: 311-354.
|
Sun J, Zhang Y, Xu T, Zhang Y, Mu H W, Zhang Y J, Lan Y, Fields C J, Hui J H L, Zhang W P, Li R S, Nong W Y, Cheung F K M, Qiu J W, Qian P Y. 2017a. Adaptation to deep-sea chemosynthetic environments as revealed by mussel genomes. Nature Ecology & Evolution, 1(5): 0121.
|
Sun Y, Wang M X, Li L L, Zhou L, Wang X C, Zheng P, Yu H Y, Li C L, Sun S. 2017b. Molecular identification of methane monooxygenase and quantitative analysis of methanotrophic endosymbionts under laboratory maintenance in Bathymodiolus platifrons from the South China Sea. PeerJ, 5(2): e3565.
|
Takishita K, Takaki Y, Chikaraishi Y, Ikuta T, Ozawa G, Yoshida T, Ohkouchi N, Fujikura K. 2017. Genomic evidence that methanotrophic endosymbionts likely provide deep-sea Bathymodiolus mussels with a sterol intermediate in cholesterol biosynthesis. Genome Biology and Evolution, 9(5): 1 148-1 160.
DOI:10.1093/gbe/evx082 |
Thubaut J, Puillandre N, Faure B, Cruaud C, Samadi S. 2013. The contrasted evolutionary fates of deep-sea chemosynthetic mussels (Bivalvia, Bathymodiolinae). Ecology and Evolution, 3(14): 4 748-4 766.
DOI:10.1002/ece3.749 |
Turner R D, Lutz R. 1984. Growth and distribution of mollusks at deep-sea vents and seeps. Oceanus, 27(3): 54-62.
|
Tyler P A, Grant A, Pain S L, Gage J D. 1982. Is annual reproduction in deep-sea echinoderms a response to variability in their environment?. Nature, 300(5894): 747-750.
DOI:10.1038/300747a0 |
Tyler P A, Marsh L, Baco-Taylor A, Smith C R. 2009. Protandric hermaphroditism in the whale-fall bivalve mollusc Idas washingtonia. Deep Sea Research Part Ⅱ: Topical Studies in Oceanography, 56(19-20): 1 689-1 699.
DOI:10.1016/j.dsr2.2009.05.014 |
Tyler P A, Young C M, Dolan E, Arellano S M, Brooke S D, Baker M. 2007. Gametogenic periodicity in the chemosynthetic cold-seep mussel "Bathymodiolus" childressi. Marine Biology, 150(5): 829-840.
DOI:10.1007/s00227-006-0362-9 |
Tyler P A, Young C M. 1999. Reproduction and dispersal at vents and cold seeps. Journal of the Marine Biological Association of the United Kingdom, 79(2): 193-208.
DOI:10.1017/S0025315499000235 |
Van Dover C L, German C R, Speer K G, Parson L M, Vrijenhoek R C. 2002. Evolution and biogeography of deep-sea vent and seep invertebrates. Science, 295(5558): 1 253-1 257.
DOI:10.1126/science.1067361 |
Wang H, Zhang H, Wang M X, Chen H, Lian C, Li C L. 2019. Comparative transcriptomic analysis illuminates the hostsymbiont interactions in the deep-sea mussel Bathymodiolus platifrons. Deep Sea Research Part Ⅰ:Oceanographic Research Papers, 151: 103082.
DOI:10.1016/j.dsr.2019.103082 |
Wang X C. 2018. Nutritional Sources Analysis and the HeavyMetal Enrichment of the Macrofauna from the Deep-Sea Chemotrophic Ecosystem. Institute of Oceanology, Chinese Academy of Sciences, Qingdao. p.28-34. (in Chinese with English abstract)
|
Wang Y, Kang J H, Liang Q Y, He X B, Wang J J, Lin M. 2018. Characteristics of phytoplankton communities and their biomass variation in a gas hydrate drilling area in the northern South China Sea. Marine Pollution Bulletin, 133: 606-615.
DOI:10.1016/j.marpolbul.2018.06.018 |
Won Y J, Hallam S J, O'Mullan G D, Pan I L, Buck K R, Vrijenhoek R C. 2003. Environmental acquisition of thiotrophic endosymbionts by deep-sea mussels of the genus Bathymodiolus. Applied and Environmental Microbiology, 69(11): 6 785-6 792.
DOI:10.1128/AEM.69.11.6785-6792.2003 |
Xu T, Feng D, Tao J, Qiu J W. 2019. A new species of deep-sea mussel (Bivalvia: Mytilidae: Gigantidas) from the South China Sea: morphology, phylogenetic position, and gillassociated microbes. Deep Sea Research Part Ⅰ:Oceanographic Research Papers, 146: 79-90.
DOI:10.1016/j.dsr.2019.03.001 |
Xu T, Sun J, Watanabe H K, Chen C, Nakamura M, Ji R B, Feng D, Lv J, Wang S, Bao Z M, Qian P Y, Qiu J W. 2018. Population genetic structure of the deep-sea mussel Bathymodiolus platifrons (Bivalvia: Mytilidae) in the Northwest Pacific. Evolutionary Applications, 11(10): 1 915-1 930.
DOI:10.1111/eva.12696 |
Young C M, He R Y, Emlet R B, Li Y Z, Qian H, Arellano S M, Van Gaest A, Bennett K C, Wolf M, Smart T I, Rice M E. 2012. Dispersal of deep-sea larvae from the intraAmerican seas: simulations of trajectories using ocean models. Integrative and Comparative Biology, 52(4): 483-496.
DOI:10.1093/icb/ics090 |
Yu J J, Wang M X, Liu B Z, Yue X, Li C L. 2019. Gill symbionts of the cold-seep mussel Bathymodiolus platifrons:composition, environmental dependency and immune control. Fish & Shellfish Immunology, 86: 246-252.
|
Zhang X, Du Z F, Luan Z D, Wang X J, Xi S C, Wang B, Li L F, Lian C, Yan J. 2017. In situ Raman detection of gas hydrates exposed on the seafloor of the South China Sea. Geochemistry, Geophysics, Geosystems, 18(10): 3 700-3 713.
DOI:10.1002/2017GC006987 |
Zheng P, Wang M X, Li C L, Sun X Q, Wang X C, Sun Y, Sun S. 2017. Insights into deep-sea adaptations and hostsymbiont interactions: a comparative transcriptome study on Bathymodiolus mussels and their coastal relatives. Molecular Ecology, 26(19): 5 133-5 148.
DOI:10.1111/mec.14160 |
Zhou L, Cao L, Wang X C, Wang M X, Wang H N, Zhong Z S, Xu Z, Chen H, Li L L, Li M N, Wang H, Zhang H, Lian C, Sun Y, Li C L. 2020. Metal adaptation strategies of deepsea Bathymodiolus mussels from a cold seep and three hydrothermal vents in the West Pacific. Science of the Total Environment, 707: 136046.
DOI:10.1016/j.scitotenv.2019.136046 |
 2020, Vol. 38
2020, Vol. 38