Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LI Xinxin, FENG Xiuni, LUO Kai, MA Shuoli, DENG Junming, ZHANG Wenbing, MAI Kangsen
- Effects of vitamin C deficiency or excess on growth performance, anti-oxidative response and fatty acid composition of juvenile abalone Haliotis discus hannai Ino
- Journal of Oceanology and Limnology, 38(6): 1936-1944
- http://dx.doi.org/10.1007/s00343-019-9183-9
Article History
- Received Aug. 3, 2019
- accepted in principle Sep. 5, 2019
- accepted for publication Nov. 14, 2019
2 Jiaozhou Fisheries Technology Promotion Station, Qingdao 266003, China;
3 College of Animal Science and Technology, Yunnan Agricultural University, Kunming 650201, China
Vitamin C, also known as ascorbic acid, is a water-soluble vitamin that involves many physiological processes including growth, collagen formation, iron metabolism and haematology, reproduction, and wound healing (NRC, 2011). It can also scavenge harmful free radicals produced by cells (Bendich et al., 1986; Ames et al., 1993; Shao et al., 2018), and inhibit the destructive effect of reactive oxygen species (ROS) on biological macromolecules (Berger et al., 1997) to protect cells from oxidative damage (Halver, 1995; Chew, 1996). In addition, numerous studies have shown that vitamin C could improve the innate immunity in many ways, such as protecting surrounding phagocyte and promoting serum bactericidal activity (Ren et al., 2008; Chen et al., 2015). In aquatic animals, vitamin C can improve the anti-oxidative ability and innate immunity of fish and shrimp, such as Wuchang bream Megalobrama amblycephala Yih (Ming et al., 2012), cobia Rachycentron canadum (Zhou et al., 2012), loach Misgurnus anguillicaudatus Cantor (Zhao et al., 2017) and white shrimp Litopenaeus vannamei (López et al., 2003; Wu et al., 2016).
Vitamin C activates the lipid soluble system and protects lipid membranes from free radical peroxidation (Kosutarak et al., 1995; Hwang and Lin, 2002; Wang and Huang, 2015). In aquatic animals, researches have shown that dietary vitamin C increased the lipid contents in yellow drum Nibea albiflora (Wang et al., 2017), and effectively prevented the lipid peroxidation in common carp (Hwang and Lin, 2002). Moreover, Gao et al. (2013) observed that incremental dietary vitamin C increased percentages of EPA, DHA, 22:5n-3, and total n-3 fatty acids in both liver and muscle of red sea bream Pagrus major. On the contrary, the percentages of 14:0, 18:0, and 18:1n-9 decreased with increased dietary vitamin C levels.
Abalone of the archaeogastropod genus of mollusca is one of the most important mariculture mollusk species in China, with more than 148 539 tons produced in 2017 (Fishery Bureau, Ministry of Agriculture, People's Republic of China, 2018). Among the abalone farmed in China, Haliotis discus hannai Ino is the most important species. A previous study showed that supplementation of vitamin C from 0 to 800 mg/g in diet had no significant effects on the growth of juvenile abalone, but significantly affected the accumulation of ascorbic acid in the soft body (Mai, 1998). The purpose of the present study was to explore the effect of dietary vitamin C deficiency or excess on growth performance, anti-oxidative response and fatty acid composition of juvenile abalone. It provides basic data to better understand the protective effects of vitamin C on abalone.
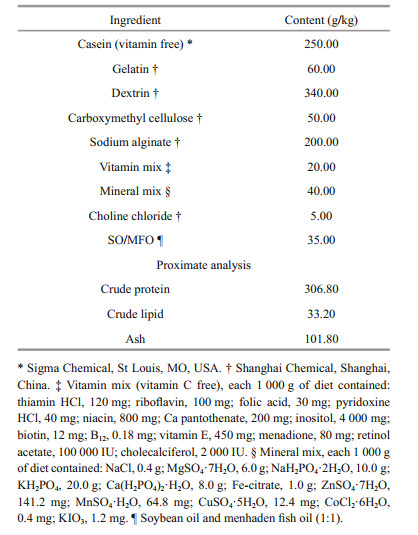
2 MATERIAL AND METHOD 2.1 Experimental dietsThe basal diet formulation was based on Wu et al.(2010, 2011), and the ingredients are shown in Table 1. The basal diet contained 306.8 g/kg of crude protein from casein (vitamin free) and gelatin, and 33.2 g/kg of crude lipid from soybean oil and menhaden fish oil (1:1). Vitamin C was added to the diets at levels of 0, 100, and 10 000 mg/kg in the form of L-ascorbyl-2-monophosphate, and the corresponding vitamin C contents in diets were 0.00, 94.52, and 9 649.58 mg/kg, respectively.
Abalone juveniles (initial shell length: 1.74±0.03 cm, initial weight: 0.93±0.00 g) were collected from Damai Island in Qingdao. Before the start of the feeding trial, all abalones were acclimated to the experimental conditions for 14 days with normal diet with no vitamin C supplementation. After body weight was measured, abalones were randomly distributed into nine tanks and 55 individuals per tank. Each treatment had three replicates (tanks). The feeding trial was carried out in an indoor recirculating water system. The pre-weighted experimental diets were fed to abalone once a day (17:00) at a satiation level for 240 days. Feces and excess feeds were removed at 8:00 every morning to maintain water quality. During the feeding trial, water temperature ranged from 17.5–19.0℃, salinity 31–34, pH 7.4–7.9, and dissolved oxygen was above 7 mg/L.
2.3 LeachingThe leaching test for dietary vitamin C was carried out according to the method used by Zhang et al. (2003). Pre-weighed diet was placed onto 100-μm mesh screens and allowed it to the bottom of experimental glass aquaria. Temperature was adjusted to match that of the experiment (18±1℃). At allotted time (0, 2, 4, 6, and 12 h, respectively), the remaining diet was removed from the glass aquaria and lyophilized. Dried diet was submitted to analyses of vitamin C contents. The leaching of dietary vitamin C was expressed as retention efficiency (RE):
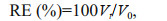
where Vt and V0 are final (0, 2, 4, 6, or 12 h) and initial dietary vitamin C content, respectively.
2.4 Sample collection and analysisAll animal care and handling procedures were approved by the Animal Care Committee of Ocean University of China. Before sampling, all the abalones were fasted for 3 days. Abalones in the same tank were counted one by one and weighed together. The viscera and muscle were collected and stored at -80℃ for later analysis. The crude protein and crude lipid content of diets and soft body of abalone were quantified by the method of AOAC (1995).
2.4.1 Ascorbic acid analysisThe concentrations of ascorbic acid in abalones and diets were determined by high performance liquid chromatography (HPLC, Agilent 1100). The mobile phase was 0.05 mol/L KH2PO4 at pH 2.8 and the flow rate was 0.6 mL/min. Weighed samples were homogenized in 5% cold metaphosphoric acid, homogenates were centrifuged at 2 739×g for 6 min, and supernatants were analyzed on HPLC after being filtered through a 0.22-μm pore size syringe filter. The detection wavelength was 254 nm, column temperature was 30℃, and dissolving appropriate amount of ascorbic acid (Sigma Chemicals Co.) in ultrapure water was used as standard sample.
2.4.2 Activities of enzymesActivities of acid phosphatase (ACP), alkaline phosphatase (AKP), lysozyme(LZ), phenoloxidase (PO), catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPX), glutathione S-transferase (GST) and glutathione reductase (GR) were determined by the commercial reagent kits (Nanjing Jiancheng Bioengineering Institute) according to the protocol instructions.
2.4.3 Fatty acid analysisFatty acid methyl esters (FAMEs) were prepared by esterification using 2% sulfuric acid methanol as described in Metcalfe et al. (1966). The FAMEs were separated and quantified using HP 5890II gas chromatograph (Agilent) equipped with a fused silica capillary column (007-CW). The temperature program was: initial temperature 150℃, 15℃/min rise to 200℃, then 2℃/min rise to 250℃. Nitrogen was used as carrier gas. The fatty acid quantification was identified by comparison of retention time and peak area with standard FAMEs (Sigma Chemicals Co.).
2.5 Calculations and statistical analysisThe growth performances of abalone were expressed as weight gain ratio (WGR), the daily increment in shell length (DISL) and survival rate (SR). They were calculated as follows:
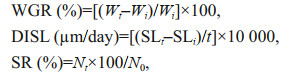
where Wt and Wi are final and initial body weight of abalone respectively; SLt and SLi are final and initial shell length (cm) of abalone, respectively; t is feeding trial duration in day; Nt and N0 are final and initial numbers of abalone in each replicate, respectively.
Statistical analysis was performed using SPSS 25.0 and Microsoft Excel 2010. All the data were presented as means±SE. Data were analyzed by one-way ANOVA. Differences between the means were tested by Tukey's test at level of 0.05.
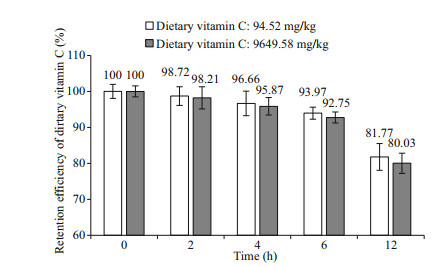
3 RESULT 3.1 LeachingThe results of the leaching test with experimental diets are illustrated in Fig. 1. The retention efficiency (RE) of dietary 94.52 mg/kg vitamin C at different interval immersed in seawater for 0, 2, 4, 6, and 12 h were 100%, 98.72%, 96.66%, 93.97%, and 81.77%, respectively. In the diet of 9 649.58 mg/kg vitamin C, the RE at different interval immersed in seawater for 0, 2, 4, 6, and 12 h were 100%, 98.21%, 95.87%, 92.75%, and 80.03%, respectively.
|
| Fig.1 The retention efficiency of vitamin C in diets at different interval immersed in seawater (0, 2, 4, 6, and 12 h) |
The results of the growth performance of abalone are shown in Table 2. There were no significant differences in WGR, DISL, and SR among all the groups (P > 0.05). The WGR were ranged 201.56%– 234.38%, the DISL 42.24–46.90 μm/d, and the SR 69.09%–72.73%.
|
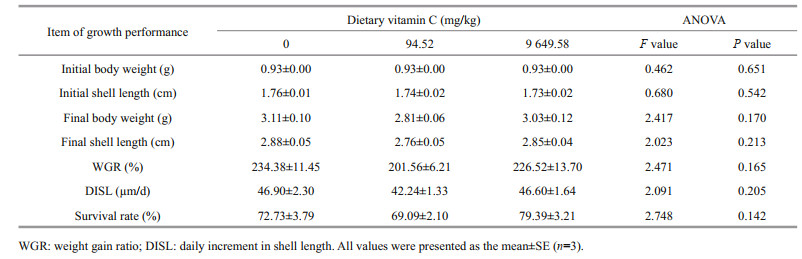
The contents of crude lipid and crude protein in the soft body of abalone are shown in Fig. 2. The highest value of crude lipid content was in the group with 9 649.58 mg/kg of dietary vitamin C (P < 0.05), but there was no significant difference between the other two groups (P > 0.05). The significantly lowest value of crude protein was in the group with 0 mg/kg of dietary vitamin C (P < 0.05).
|
| Fig.2 Effects of dietary vitamin C on crude lipid (a) and crude protein (b) contents in the soft body of abalone Haliotis discus hannai Ino after a 240-day feeding trial Data were presented in mean±SE (n=3). Bars bearing different letters are significantly different (P < 0.05; Tukey's test). |
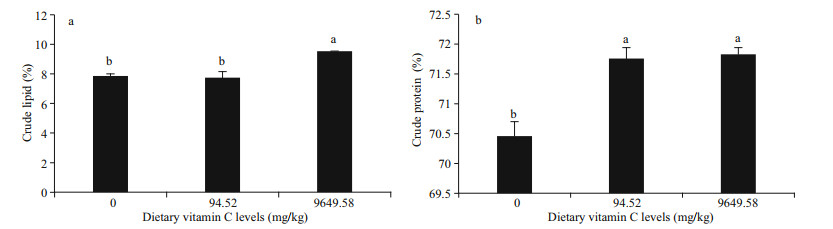
The contents of ascorbic acid in the soft body of abalone are shown in Fig. 3. Ascorbic acid contents had the highest value in viscera (449.78 μg/g) and muscle (253.33 μg/g) in the group with 9 649.58 mg/kg of dietary vitamin C (P < 0.05). There was no significant difference between the treatments with 0 mg/kg and 94.52 mg/kg of dietary vitamin C (P > 0.05).
|
| Fig.3 Effects of dietary vitamin C on the contents of ascorbic acid in viscera (a) and muscle (b) of abalone Haliotis discus hannai Ino after a 240-day feeding trial Data were presented in mean±SE (n=3). Bars bearing different letters are significantly different (P < 0.05; Tukey's test). |
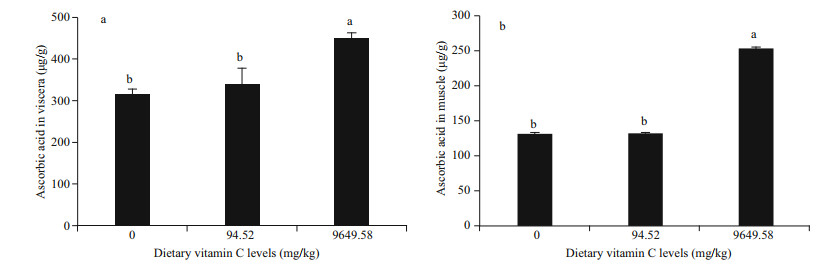
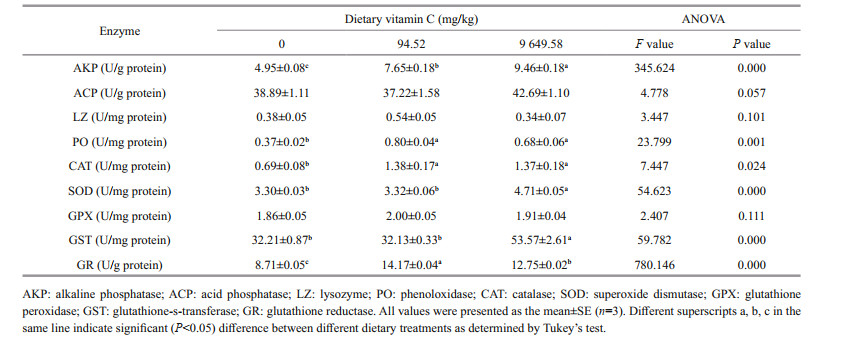
The activities of AKP, ACP, LZ, and PO in viscera and muscle are shown in Tables 3 & 4, respectively. The highest value of AKP activity in viscera was found to be 23.67 U/g protein in the 9 649.58 mg/kg group (P < 0.05). In muscle, the activity of AKP significantly increased with the increasing of dietary vitamin C levels (P < 0.05). However there was no significant difference in ACP activity in viscera and muscle among the three treatments (P > 0.05).
|
|
With the increasing of dietary vitamin C levels, the activity of LZ in viscera significantly increased (P < 0.05). In muscle, there was no significant differences in LZ activity among all the groups (P > 0.05). The addition of dietary vitamin C (94.52 and 9 649.58 mg/kg) significantly increased the activity of PO in muscle (P < 0.05). However, in viscera, the highest value of PO activity was 4.96 U/ mg protein in the 0 mg/kg group.
The activities of CAT, SOD, GPX, GST, and GR in viscera and muscle are shown in Tables 3 & 4, respectively. There was no significant difference in CAT activity in viscera among the three treatments (P > 0.05). Supplementation of dietary vitamin C (94.52 and 9 649.58 mg/kg) significantly increased the activity of CAT in muscle (P < 0.05).
With the increasing of dietary vitamin C levels, the activity of SOD significantly increased in viscera (P < 0.05). In muscle, the 9 649.58 mg/kg treatment group significantly increased the activity of SOD (P < 0.05). There was no significant difference between the groups of 0 and 94.52 mg/kg (P > 0.05).
The highest value of GPX activity in viscera was 2.94 U/mg protein in 9 649.58 mg/kg group. There was no significant difference in GPX activity in muscle among all the groups (P > 0.05). The activity of GST in viscera and muscle was significantly higher in the 9 649.58 mg/kg group than those of 0 and 94.52 mg/kg groups (P < 0.05). The highest value of GR activity in viscera was 8.65 U/mg protein in the 9 649.58 mg/kg group (P < 0.05). In muscle, the activity of GR significantly increased in the 94.52 mg/kg group (P < 0.05).
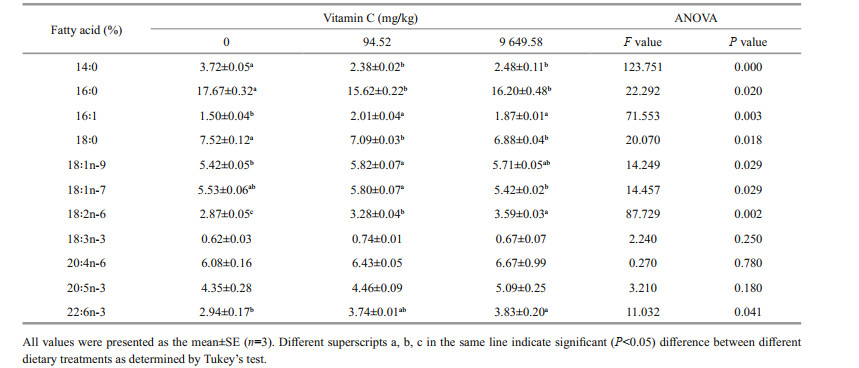
3.5 Fatty acid compositionsThe fatty acid compositions in soft body of abalone are shown in Table 5. The addition of dietary vitamin C (groups of 94.52 and 9 649.58 mg/kg) significantly decreased the contents of saturated fatty acid (SFA) 14:0, 16:0, and 18:0 in soft body of abalone (P < 0.05). With the increasing of dietary vitamin C levels, the contents of monounsaturated fatty acid (16:1 and 18:1n-9) and PUFA (18:2n-6 and 22:6n-3) were increased in soft body of abalone (P < 0.05). There was no significant difference in 18:3n-3, 20:4n-6, and 20:5n-3 in soft body of abalone among the three treatment groups (P > 0.05).
|
As shown in the last section, different levels of dietary vitamin C had no significant effects on the growth performance of abalone, which is consistent with a previous study on abalone (Mai, 1998). Dietary vitamin C supplementation significantly increased the content of total lipid in the soft body of abalone (Fig. 2), which was consistent with the finding in freshwater prawn Macrobrachium malcolmsoni (Asaikkutti et al., 2016). The possible reason is that vitamin C can activate the lipid soluble system, so that triacylglycerol could decrease and the lipid peroxidation could be prevented (Kosutarak et al., 1995; Wang and Huang, 2015). Moreover, dietary vitamin C supplementation significantly increased the content of crude protein in the soft body of abalone, which is consistent with the findings in Japanese eel Anguilla japonica (Shahkar et al., 2015). Researches showed that high levels of vitamin C could stimulate protein production in fish, since diets supplemented with higher vitamin C can increase protein synthesis (Chagas and Val, 2003).
When foreign bodies are phagocytized into cells and fused with lysosome, they are finally digested by various hydrolases to decompose (Roosta et al., 2014). The AKP is a hydrolytic enzyme that can enhance the recognition and phagocytosis ability of the invasive organism (Zhou et al., 2012). In present study, the activity of AKP in viscera and muscle reached the maximum value when excessive dietary vitamin C supplemented. In previous studies, dietary vitamin C supplementation could effectively increase the serum AKP level in Wuchang bream M. amblycephala under pH stress (Wan et al., 2014). Zhou et al. (2012) found that dietary vitamin C supplementation enhanced serum AKP activity in cobia R. canadum. Our results show that vitamin C could improve the non-specific immunity of abalone, and play vital roles in defending the pathogen.
It is generally believed that LZ has bactericidal effect and is one of the main defense mechanisms of mollusc (Andersen et al., 1998; Ordás et al., 2000). In present study, dietary vitamin C significantly increased the activity of LZ in viscera, which is consistent with the findings in grass carp Ctenopharyngodon idella and yellow drum N. albiflora (Xu et al., 2016; Wang et al., 2017). The PO is a key enzyme in the immune system of invertebrates (Gollas-Galván et al., 1997; Moreau et al., 2000). It is closely related to the identification of foreign and host defense. In present study, in the 94.52 mg/kg group, the activity of PO in muscle was significantly increased, which is consistent with the findings in grass shrimp Penaeus monodon and white shrimp L. vannamei (Lee and Shiau, 2002; Qiao et al., 2011).
The anti-oxidative enzymes, such as CAT, SOD, GPX, and GST, can remove excessive damaging ROS, and reduce the damage by lipid peroxidation. The GR could prevent the oxidative decomposition of hemoglobin, and maintain the activity of mercapto group protein to ensure the integrity of cells (Freeman and Crapo, 1982; Yang and He, 2007; Asaikkutti et al., 2016; Liang et al., 2017). The present results showed that compared with vitamin C deficiency, dietary vitamin C supplementation significantly increased the activity of anti-oxidative enzymes in viscera and muscle of abalone. Although the activity of enzymes in different tissues was slightly different, the basic trend was identical. These findings were in agreement with those in yellow catfish Pelteobagrus fulvidraco (Liang et al., 2017) and black carp Mylopharyngodon piceus (Hu et al., 2013). These data indicate that vitamin C can enhance the anti-oxidative ability of abalone. In addition, it is speculated that the high anti-oxidative response are associated with the accumulation of ascorbic acid in abalone. In this study, with the increasing of dietary vitamin C levels, the content of ascorbic acid in the soft body of abalone was also increased, and the anti-oxidative responses of abalone were improved.
Vitamin C is known affecting the fatty acid composition of Terapon jarbua (Chien and Hwang, 2001). Researches showed that the SFA were represented by C14:0, C16:0, and C18:0. The monounsaturated fatty acid (MUFA) were dominated by C18:1n-7, C18:1n-9, and C20:1n-9 in soft body of abalone (Lou et al., 2013). In this study, we found that dietary vitamin C could significantly reduce the content of SFA 14:0 and 16:0 in abalone, while the content of MUFA (16:1 and 18:1n-9) and PUFA (18:2n-6 and 22:6n-3) were increased. A previous study shows that with the increasing of dietary vitamin C levels, the percentages of EPA, DHA, 22:5n-3, and total n-3 fatty acids significantly increased and the percentages of 14:0 and 18:0 decreased in red sea bream Pagrus major (Gao et al., 2013), which was consistent with the present study to some extent. However, we found that increasing the levels of dietary vitamin C could decrease the contents of EPA, 22:5n-3, DHA, and n-3 highly unsaturated fatty acid (HUFA) in liver of Japanese flounder Paralichthys olivaceus (Gao et al., 2014). A similar result was found by Chien and Hwang (2001) who reported that vitamin C significantly reduced the % of PUFA and increased the % of SFA in the liver lipid of thornfish T. jarbua. The mechanism behind the effect vitamin C on fatty acid compositions in abalone needs further research.
5 CONCLUSIONAlthough there were no significant effects on the growth performance, dietary vitamin C supplementation improved the anti-oxidation and immune responses, significantly increased specific MUFA (i.e., 16:1, 18:1n-7, and 18:1n-9), and PUFA (i.e., 18:2n-6 and 22:6n-3) contents, and decreased the contents of specific SFA (i.e., 14:0, 16:0, and 18:0) in the soft body of abalone. These data suggest that the addition of dietary vitamin C could improve the anti-oxidative response and fatty acid composition of abalone.
6 DATA AVAILABILITY STATEMENTThe data that support the findings of this study are available from the corresponding author upon reasonable request.
Ames B N, Shigenaga M K, Hagen T M. 1993. Oxidants, antioxidants, and the degenerative diseases of aging. Proceedings of the National Academy of Sciences of the United States of America, 90(17): 7 915-7 922.
DOI:10.1073/pnas.90.17.7915 |
Andersen F, Lygren B, Maage A, Waagbø R. 1998. Interaction between two dietary levels of iron and two forms of ascorbic acid and the effect on growth, antioxidant status and some non-specific immune parameters in Atlantic salmon (Salmo salar) smolts. Aquaculture, 161(1-4): 437-451.
DOI:10.1016/S0044-8486(97)00291-3 |
AOAC. 1995. Official Methods of Analysis. 16th edn. AOAC International Publishers, Arlington VA.
|
Asaikkutti A, Bhavan P S, Vimala K, Karthik M, Cheruparambath P. 2016. Effect of different levels dietary vitamin C on growth performance, muscle composition, antioxidant and enzyme activity of freshwater prawn, Macrobrachium malcolmsonii. Aquaculture Reports, 3: 229-236.
DOI:10.1016/j.aqrep.2016.04.002 |
Bendich A, Machlin L J, Scandurra O, Burton G W, Wayner D D M. 1986. The antioxidant role of vitamin C. Advances in Free Radical Biology & Medicine, 2(2): 419-444.
|
Berger T M, Polidor M C, Dabbagh A, Evans P J, Halliwell B, Morrow J D, Roberts L J, Frei B. 1997. Antioxidant activity of vitamin C in iron-overloaded human plasma. The Journal of Biological Chemistry, 272(25): 15 656-15 660.
DOI:10.1074/jbc.272.25.15656 |
Chagas E C, Val A L. 2003. Efeito da vitamina C no ganho de peso e em parametros hematológicos de tambaqui. Pesquisa Agropecuária Brasileira, 38(3): 397-402.
DOI:10.1590/S0100-204X2003000300009 |
Chen Y J, Yuan R M, Liu Y J, Yang H J, Liang G Y, Tian L X. 2015. Dietary vitamin C requirement and its effects on tissue antioxidant capacity of juvenile largemouth bass, Micropterus salmoides. Aquaculture, 435: 431-436.
DOI:10.1016/j.aquaculture.2014.10.013 |
Chew B P. 1996. Importance of antioxidant vitamins in immunity and health in animals. Animal Feed Science and Technology, 59(1-3): 103-114.
DOI:10.1016/0377-8401(95)00891-8 |
Chien L T, Hwang D F. 2001. Effects of thermal stress and vitamin C on lipid peroxidation and fatty acid composition in the liver of thornfish Terapon jarbua. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 128(1): 91-97.
DOI:10.1016/S1096-4959(00)00299-2 |
Fishery Bureau, Ministry of Agriculture, People's Republic of China. 2018. China Fishery Statistical Yearbook 2018. China Agriculture Press, Beijing, China.
(in Chinese)
|
Freeman B A, Crapo J D. 1982. Biology of disease: free radicals and tissue injury. Laboratory Investigation, 47(5): 412-426.
|
Gao J, Koshio S, Ishikawa M, Yokoyama S, Mamauag R E P. 2014. Interactive effects of vitamin C and E supplementation on growth performance, fatty acid composition and reduction of oxidative stress in juvenile Japanese flounder Paralichthys olivaceus fed dietary oxidized fish oil. Aquaculture, 422-423: 84-90.
DOI:10.1016/j.aquaculture.2013.11.031 |
Gao J, Koshio S, Ishikawa M, Yokoyama S, Nguyen B T, Mamauag R E. 2013. Effect of dietary oxidized fish oil and vitamin C supplementation on growth performance and reduction of oxidative stress in Red Sea Bream Pagrus major. Aquaculture Nutrition, 19(1): 35-44.
|
Gollas-Galván T, Hernández-Lόpez J, Vargas-Albores F. 1997. Effect of Calcium on the prophenoloxidase system activation of the brown shrimp (Penaeus californiensis, Holmes). Comparative Biochemistry and Physiology Part A: Physiology, 117(3): 419-425.
DOI:10.1016/S0300-9629(96)00363-5 |
Halver J E. 1995. Vitamin requirement study techniques. Journal of Applied Ichthyology, 11(3-4): 215-224.
DOI:10.1111/j.1439-0426.1995.tb00021.x |
Hu Y, Huang Y, Wen H, Huan Z L, Zhong L, Mao X W, Li J L, Xiao T Y. 2013. Effect of vitamin C on growth, immunity and anti-ammonia-nitrite stress ability in juvenile black carp (Mylopharyngodon piceus). Journal of Fisheries of China, 37(4): 565-573.
(in Chinese with English abstract) DOI:10.3724/SP.J.1231.2013.38322 |
Hwang D F, Lin T K. 2002. Effect of temperature on dietary vitamin c requirement and lipid in common carp. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 131(1): 1-7.
DOI:10.1016/S1096-4959(01)00449-3 |
Kosutarak P, Kanazawa A, Teshima S I, Koshio S. 1995. Interactions of L-ascorbyl-2-phosphate Mg and oxidized fish oil on red sea bream juveniles. Fisheries Science, 61(4): 696-702.
DOI:10.2331/fishsci.61.696 |
Lee M H, Shiau S Y. 2002. Dietary vitamin C and its derivatives affect immune responses in grass shrimp, Penaeus monodon. Fish & Shellfish Immunology, 12(2): 119-129.
|
Liang X P, Li Y, Hou Y M, Qiu H, Zhou Q C. 2017. Effect of dietary vitamin C on the growth performance, antioxidant ability and innate immunity of juvenile yellow catfish (Pelteobagrus fulvidraco Richardson). Aquaculture Research, 48(1): 149-160.
DOI:10.1111/are.12869 |
López N, Cuzon G, Gaxiola G, Taboada G, Valenzuela M, Pascual C, Sánchez A, Rosas C. 2003. Physiological, nutritional, and immunological role of dietary β 1-3 glucan and ascorbic acid 2-monophosphate in Litopenaeus vannamei juveniles. Aquaculture, 224(1-4): 223-243.
DOI:10.1016/S0044-8486(03)00214-X |
Lou Q M, Wang Y M, Xue C H. 2013. Lipid and fatty acid composition of two species of abalone, Haliotis discus hannai Ino and Haliotis diversicolor Reeve. Journal of Food Biochemistry, 37(3): 296-301.
DOI:10.1111/j.1745-4514.2011.00631.x |
Mai K S. 1998. Comparative studies on the nutrition of two species of abalone, Haliotis tuberculata L. and Haliotis discus hannai Ino.: VII. Effects of dietary vitamin C on survival, growth and tissue concentration of ascorbic acid. Aquaculture, 161(1-4): 383-392.
DOI:10.1016/S0044-8486(97)00286-X |
Metcalfe L D, Schmitz A A, Pelka J R. 1966. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Analytical Chemistry, 38(3): 514-515.
DOI:10.1021/ac60235a044 |
Ming J H, Xie J, Xu P, Ge X P, Liu W B, Ye J Y. 2012. Effects of emodin and vitamin C on growth performance, biochemical parameters and two HSP70s mRNA expression of Wuchang bream (Megalobrama amblycephala Yih) under high temperature stress. Fish & Shellfish Immunology, 32(5): 651-661.
|
Moreau S J M, Doury G, Giordanengo P. 2000. Intraspecific variation in the effects of parasitism by Asobara tabida on phenoloxidase activity of Drosophila melanogaster larvae. Journal of Invertebrate Pathology, 76(2): 151-153.
DOI:10.1006/jipa.2000.4956 |
NRC. 2011. Nutrient Requirements of Fish and Shrimp. National Academy Press, Washington, DC, USA. p.207-209.
|
Ordás M C, Ordás A, Beloso C, Figueras A. 2000. Immune parameters in carpet shell clams naturally infected with Perkinsus atlanticus. Fish & Shellfish Immunology, 10(7): 597-609.
|
Qiao J, Du Z H, Zhang Y L, Du H, Guo L L, Zhong M Q, Cao J S, Wang X Y. 2011. Proteomic identification of the related immune-enhancing proteins in shrimp Litopenaeus vannamei stimulated with vitamin C and Chinese herbs. Fish & Shellfish Immunology, 31(6): 736-745.
|
Ren T J, Koshio S, Uyan O, Komilus C F, Yokoyama S, Ishikawa M, Abdul K. 2008. Effects of dietary vitamin C on blood chemistry and nonspecific immune response of juvenile red sea bream, Pagrus major. Journal of the World Aquaculture Society, 39(6): 797-803.
DOI:10.1111/j.1749-7345.2008.00216.x |
Roosta Z, Hajimoradloo A, Ghorbani R, Hoseinifar S H. 2014. The effects of dietary vitamin C on mucosal immune responses and growth performance in Caspian roach (Rutilus rutilus caspicus) fry. Fish Physiology and Biochemistry, 40(5): 1 601-1 607.
DOI:10.1007/s10695-014-9951-6 |
Shahkar E, Yun H, Kim D J, Kim S K, Lee B I, Bai S C. 2015. Effects of dietary vitamin C levels on tissue ascorbic acid concentration, hematology, non-specific immune response and gonad histology in broodstock Japanese eel, Anguilla japonica. Aquaculture, 438: 115-121.
DOI:10.1016/j.aquaculture.2015.01.001 |
Shao L Y, Han D, Yang Y X, Jin J Y, Liu H K, Zhu X M, Xie S Q. 2018. Effects of dietary vitamin C on growth, gonad development and antioxidant ability of on-growing gibel carp (Carassius auratus gibelio var. CAS III). Aquaculture Research, 49(3): 1 242-1 249.
DOI:10.1111/are.13578 |
Wan J J, Ge X P, Liu B, Xie J, Cui S L, Zhou M, Xia S L, Chen R L. 2014. Effect of dietary vitamin C on non-specific immunity and mRNA expression of three heat shock proteins (HSPs) in juvenile Megalobrama amblycephala under pH stress. Aquaculture, 434: 325-333.
DOI:10.1016/j.aquaculture.2014.08.043 |
Wang C C, Huang C H. 2015. Effects of dietary vitamin C on growth, lipid oxidation, and carapace strength of soft-shelled turtle, Pelodiscus sinensis. Aquaculture, 445: 1-4.
DOI:10.1016/j.aquaculture.2015.04.009 |
Wang L G, Chen D X, Lou B, Zhan W, Chen Y Q, Liu F, Mao G M. 2017. The effects of dietary vitamin C on growth performance, serum enzymes activities and resistance to Vibrio alginolyticus challenge of yellow drum Nibea albiflora. Aquaculture Research, 48(9): 4 684-4 695.
DOI:10.1111/are.13290 |
Wu C L, Zhang W B, Mai K S, Liang X F, Xu W, Wang J, Ma H M. 2010. Molecular cloning, characterization and mRNA expression of selenium-binding protein in abalone (Haliotis discus hannai Ino): response to dietary selenium, iron and zinc. Fish & Shellfish Immunology, 29(1): 117-125.
|
Wu C L, Zhang W B, Mai K S, Xu W, Zhong X L. 2011. Effects of dietary zinc on gene expression of antioxidant enzymes and heat shock proteins in hepatopancreas of abalone Haliotis discus hannai. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 154(1): 1-6.
|
Wu Y S, Liau S Y, Huang C T, Nan F H. 2016. Beta 1, 3/1, 6-glucan and vitamin C immunostimulate the non-specific immune response of white shrimp (Litopenaeus vannamei). Fish & Shellfish Immunology, 57: 269-277.
|
Xu H J, Jiang W D, Feng L, Liu Y, Wu P, Jiang J, Kuang S Y, Tang L, Tang W N, Zhang Y A, Zhou X Q. 2016. Dietary vitamin C deficiency depresses the growth, head kidney and spleen immunity and structural integrity by regulating NF-κB, TOR, Nrf2, apoptosis and MLCK signaling in young grass carp (Ctenopharyngodon idella). Fish & Shellfish Immunology, 52: 111-138.
|
Zhang W B, Mai K S, Xu W, Ma H M. 2003. Metabolic responses to dietary cholecalciferol and phosphorus in abalone Haliotis discus hannai ino. Journal of Experimental Zoology Part A: Comparative Experimental Biology, 299A(2): 110-117.
DOI:10.1002/jez.a.10295 |
Zhao Y, Zhao J X, Zhang Y, Gao J. 2017. Effects of different dietary vitamin C supplementations on growth performance, mucus immune responses and antioxidant status of loach (Misgurnus anguillicaudatus Cantor) juveniles. Aquaculture Research, 48(8): 4 112-4 123.
DOI:10.1111/are.13231 |
Zhou Q C, Wang L G, Wang H L, Xie F J, Wang T. 2012. Effect of dietary vitamin C on the growth performance and innate immunity of juvenile cobia (Rachycentron canadum). Fish & Shellfish Immunology, 32(6): 969-975.
|
 2020, Vol. 38
2020, Vol. 38