Institute of Oceanology, Chinese Academy of Sciences
Article Information
- LOW Kyleyoung, CHAI Layching, LEE Choonweng, ZHANG Gan, ZHANG Ruijie, VAHAB Vaezzadeh, BONG Chuiwei
- Prevalence and risk assessment of antibiotics in riverine estuarine waters of Larut and Sangga Besar River, Perak
- Journal of Oceanology and Limnology, 39(1): 122-134
- http://dx.doi.org/10.1007/s00343-020-9246-y
Article History
- Received Sep. 24, 2019
- accepted in principle Dec. 18, 2019
- accepted for publication Mar. 11, 2020
2 Institute of Graduate Studies, University of Malaya, Kuala Lumpur 50603, Malaysia;
3 Institute of Biological Sciences, Faculty of Science, University of Malaya, Kuala Lumpur 50603, Malaysia;
4 Guangzhou Institute of Geochemistry, Chinese Academy of Sciences, Guangzhou 510640, China;
5 School of Marine Sciences, Guangxi University, Nanning 530004, China
Antibiotic residues as an emerging contaminant generated several environmental risk implications in recent years due to its increased consumption rate in human and animal sectors (Cabello, 2006; Zhang et al., 2012; van Boeckel et al., 2014). Deaths related to antimicrobial resistance (AMR) is currently estimated at 700 000 annually but could rise to approximately 10 million annually and potentially cost US$ 100 trillion in lost economic output by the year 2050 (O'Neill, 2016). According to the National Surveillance on Antibiotic Utilization (NSAU) program of Malaysia, the mean defined daily doses' (DDD) per 1 000 patient days for antibiotic utilization in hospital wards showed an overall upward trend (Ministry of Health Malaysia, 2017b).
The concern arising from this emerging contaminant lies in the considerable amount of antibiotics and its partially metabolized products being expelled through pharmaceutical, healthcare, agriculture, aquaculture and poultry industries (Iglesias et al., 2013; Lundborg and Tamhankar, 2017). Constant input paired with multiple contamination sources had led to pseudopersistent antibiotics in an environment (Houtman et al., 2004). The primary risk arising from misuse and overuse of antibiotics is its ability to culminate the development of AMR in both pathogenic and nonpathogenic bacteria (Cabello et al., 2016; Zarfel et al., 2017; Pérez Gaudio et al., 2018; Divya and Hatha, 2019) while the secondary risk involves altering the structure of natural bacterial communities involved in key ecosystem functions (Allen et al., 2010; Grenni et al., 2018).
The aquatic environment is vulnerable to pollution from different anthropogenic activities. The presence of antibiotics in the environment may occur through several channels, e.g. untreated municipal wastewater (Xu et al., 2009), wastewater treatment plant effluents (Miao et al., 2004), animal waste discharge (Marni et al., 2010), and agricultural runoffs (Davis et al., 2006). Once in an aquatic environment, antibiotics and/or their metabolized products will persist in vast quantities within environmental compartments (Yao et al., 2017). This is a cause for concern because under antibiotic selective pressure, the aquatic environments could promote antibiotic resistance gene acquisition and dissemination among environmental bacterial communities and pathogenic bacteria either via horizontal gene transfer or lateral gene transfer, also known as chromosomal mutations (Martinez, 2009; Garcillán-Barcia et al., 2011; Partridge, 2011; Berendonk et al., 2015). Several studies have demonstrated the direct antibiotic resistance gene exchange between environmental bacteria and clinically significant bacteria (Humeniuk et al., 2002; Poirel et al., 2005; Baquero et al., 2008).
Past studies generally focused on antibiotic occurrences or specific antibiotic concentrations in Malaysian freshwater surface waters (Sakai et al., 2016; Al-Qaim et al., 2018; Praveena et al., 2018). Studies on the levels of antibiotic residues in river water and their ecological risks remain scarce. As such, this study aims to determine the levels of antibiotic residues and their ecological risks in the riverine estuarine water continuum of Larut and Sangga Besar River that received wastewater effluents from zoo, hospital, and poultry slaughterhouse. Both rivers belong in part to two distinct major river basin systems located within the Larut, Matang and Selama District in Perak, Malaysia. The climate of the area was characterized by uniform temperature of average 32 ℃, high humidity (80%–90%), and high average annual rainfall (4 000 mm/year) (Samuding et al., 2009). A total of twenty-two antibiotics comprising of six antibiotic groups (Sulfonamides, fluoroquinolones, macrolides, tetracyclines, amphenicols, and diaminopyrimidine) were screened using highperformance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS). An environmental risk assessment was performed on detected antibiotic residues to evaluate the risks involved.
2 MATERIAL AND METHOD 2.1 Standard and chemicalAll 22 target antibiotics were obtained from SigmaAldrich (Saint Louis, Missouri, USA). The list consists of six major antibiotic classes: macrolides (roxithromycin, RTM; clarithromycin, CTM; azithromycin, AZM; erythromycin-H2O, ETM-H2O), fluoroquinolones (enoxacin, ENX; enrofloxacin, ENRO; norfloxacin, NOX; ofloxacin, OFX; ciprofloxacin, CIX), tetracyclines (chlortetracycline, CTC; oxytetracycline, OTC; tetracycline, TC), amphenicols (florfenicol, FF; chloramphenicol, CAP), diaminopyrimidine (trimethoprim, TMP), sulfonamides (sulfacetamide, SAAM; sulfathiazole, STZ; sulfadimethoxine, SDM; sulfadimidine, SMA; sulfapyridine, SPD; sulfadiazine, SDZ; sulfamethoxazole, SMX). The surrogate standard, 13C3-caffeine solution (1 mg/mL with methanol), from Sigma-Aldrich was dissolved in methanol before cold storage at -20 ℃.
All solvents used in this work were in HPLC grade. Erythromycin-H2O standard stock was prepared by acidifying erythromycin with 3 mol/L H2SO4 in accordance to McArdell et al. (2003). As such, erythromycin in its dehydration product was detected, ETM-H2O, due to it being readily dehydrated by the loss of one water molecule (Göbel et al., 2005). Methanol, acetonitrile, and Ethylenediamine tetraacetic acid disodium (Na2EDTA) were obtained from Merck (Darmstadt, Germany). Formic acid was obtained from CNW (Germany). Water was deionized using the Milli-Q water purification system (Millipore, Bedford, Massachusetts, USA).
2.2 SamplingWater samples were taken from six sites located downstream and upstream of Larut River, and three sites from Sangga Besar River. Larut River receives wastewater effluents from hospital, zoo, and poultry slaughterhouse whereas Sangga Besar River is less polluted. (Fig. 1). The rivers each belongs in part to their respective independent major river basin systems, moving westward into the Strait of Malacca (Ahmad and Hasan, 2011). The settlements along Larut River had a population size of 334 073 (Department of Statistics Malaysia, 2011) and is approximately 20.9 km long, discharging exclusively from Larut Hill (elevation: 1 250 m) (S1a), moving through the town area while carrying point-source discharges from zoo, hospital, and slaughterhouse before passing through downstream Larut (S1b) and finally reaching Larut Estuary (S1c). For Sangga Besar River, its length runs a shorter distance of approximately 9.9 km from the fishing village of Kuala Sepetang (S2a), passing through a small-scale cage aquaculture (S2b), before reaching the river mouth of Sepetang Estuary (S2c). The settlements along Sangga Besar River had a smaller population size of about 31 800 (Forestry Department of Perak, 2010) due to it being nestled within the 40 466 hm2 protected Matang Mangrove Forest Reserve (MMFR). Apart from serving as a waterway for fishing boats and as a cage aquaculture site (Annual Fisheries of Perak, 2000), other anthropogenic activities were kept minimal by the exemplary forestry management system that was put in place (Muda et al., 2005). Duplicate water samples were collected about 0.3 m below the water surface using autoclaved amber glass bottles (2.0 L) from the nine sampling sites between April and May 2015. The wastewater effluents were collected from the main outlet that flows into the river. The samples were then cooled in iceboxes before being transported to the laboratory for further analysis.
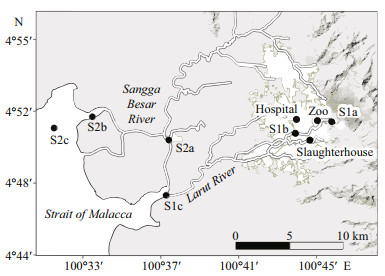
|
| Fig.1 Surface water sampling sites of Larut, Matang and Selama District, Perak, Malaysia |
A concurrent study measured the in situ physical parameters (temperature, salinity, pH, and dissolved oxygen) and dissolved inorganic nutrients [ammonium (NH4), nitrite (NO2), nitrate (NO3), and phosphate (PO4)] concentrations have been carried out and the results are already published in (Lye et al., 2019).
2.4 Detection and quantification of antibiotic residuesFor antibiotic detection and quantification, two liters of water samples from each sampling site were filtered using precombusted 0.7-μm glass-fiber filters (GF/F, Sartorius, Gӧttingen, Germany). Filtrates were adjusted to pH 3 using 3.0 mol/L sulphuric acid to avoid oxidation of antibiotics with the addition of 0.2 g of Na2EDTA as a chelating agent before 100 ng of 13C3-caffeine was used as a surrogate standard to monitor the recovery rate as described by Zhang et al.(2013). Briefly, Oasis Hydrophilic-lipophilic balance (HLB) cartridges (500 mg, Waters, UK) were pretreated with 6 mL of deionized water, 6 mL of 10 mmol/L acidified Na2EDTA buffer and 6 mL of methanol. Target antibiotics were then concentrated by solid-phase extraction (SPE) by the Visiprep SPE system (Bellefonte, Pennsylvania, USA). The loading rate for each filtrate that passed through a cartridge was set at 10 min/mL. Cartridges loaded with filtrate were washed using 10 mL of acidified deionized water before vacuum dried for 10 min. The analytes were then eluted three times using 2 mL of methanol, concentrated to a volume of about 20 μL using nitrogen gas and finally dissolved to a final volume of 1.0 mL with 90% aqueous methanol.
Analysis of the twenty-two extracted antibiotics was completed using Agilent 1200 series (Palo Alto, California, USA) connected to a Thermo Scientific Hypersil GOLD columns (2.1 mm×100 mm, 1.9 μm). Binary mobile phase was made by combining mobile phase A, which included 5 mmol/L ammonium acetate aqueous solution and 0.2% (v/v) formic acid, with mobile phase B that contained methanol. The following mobile phase gradient program was applied: 10% to 60% in 15 min, 60% to 95% within the following 5 min and held for 5 min, then returned to 10% in 1 min and held for 1 min. The flow rate maintained at 0.3 mL/min, column temperature was held at 40 ℃ and the injection volume of samples was at 5 μL. Parameters for MS/MS conditions were summarized in Supplementary Table S1. All the analyses were carried out in duplicates.
2.5 Quality analysis and quality controlThe internal standard curve was applied to calculate the concentrations of the twenty-two antibiotics, i.e. concentration of the analytes were plotted against peak area ratio. For each compound, concentrations with a signal to noise (S/N) ratio of 10 were set to be the limits of quantification (LQ). The range of LQs for the selected antibiotic was between 0.02–36.50 ng/L, while limits of detection (LOD) were between 0.01– 10.95 ng/L. The recoveries of 13C3-caffeine was in the range of 0–176.7% in all the water samples.
2.6 Risk assessmentEcological risk quotients (RQs) was calculated based on the European technical guidance document on risk assessment (European Commission Joint Research Centre, 2003) for the evaluation of potential ecological effect for antibiotic presence in the environment. RQs were calculated by the equation:
RQ=MEC/PNEC,
where MEC was the "measured environmental concentration" and PNEC was the "predicted noeffect concentration" for the respective antibiotics. PNEC is the division of lethal concentration or effective concentration for 50% of exposed population (EC50/LC50) against assessment factor (AF) based on toxicity data value on non-target aquatic organisms: algae, invertebrate Daphnia magna, and fish (Supplementary Table S2). RQs for all the above formulas were defined according to Xue et al. (2013): low risk (0.01 < RQs < 0.1), medium risk (0.1 < RQs < 1), and high risk (RQs > 1).
2.7 Statistical analysisCluster analysis for sampling sites was performed based on the antibiotic residue concentrations using PAST Statistics version 3.22 (Hammer et al., 2001) through Bray-Curtis similarity index. Correlation and linear regression analyses were performed to measure the effect between water quality (Lye et al., 2019) and antibiotic concentrations.
3 RESULT 3.1 Antibiotics concentration in river waters and wastewater effluentsFrom the twenty-two antibiotics screened, sixteen types of antibiotic residues were positively detected among the sites except for aquaculture farm in Sangga Besar River (Table 1). The total antibiotic concentration ranged from LOD–1 262.30 ng/L with a mean concentration of 13.05 ng/L. The total antibiotic detection frequency was 88.89%, whereby ETM-H2O (77.78%) and CIX (55.56%) were the most prevalent antibiotics detected. The detection frequency of antibiotic residues in Larut River was higher in comparison to Sangga Besar with concentrations ranged from LOD–18.28 ng/L.
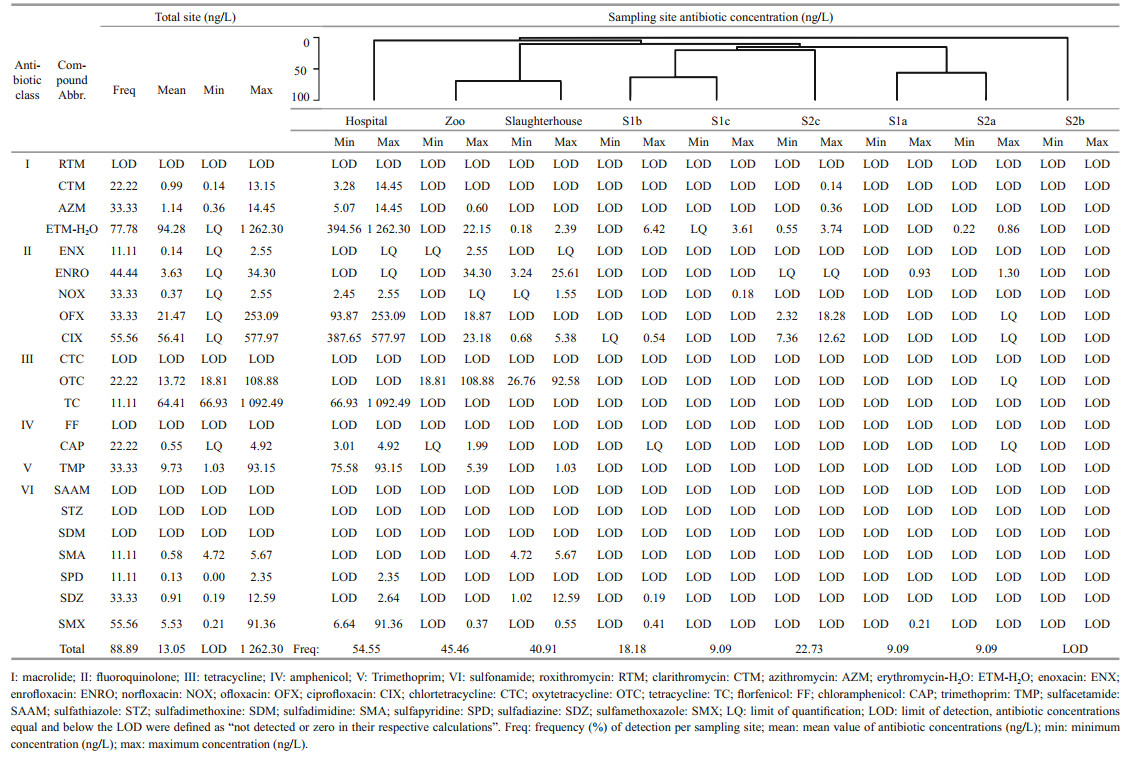
|
The upstream site (S1a) of Larut River generally had lower antibiotic detection frequency (9.09%) compared to other positively detected sampling sites. Of the antibiotics examined, only ENRO (0.93 ng/L) and SMX (0.21 ng/L) were detected. Among the wastewater effluents, hospital wastewater effluent was found to have the highest antibiotic detection frequency along with the highest total concentration of antibiotics (54.55%; 2 227.94 ng/L) followed by the wastewater effluent from the zoo (45.46%; 160.10 ng/L) and slaughterhouse (40.91%; 93.34 ng/L). Twelve antibiotics belonging to macrolides (CTM, AZM, ETM-H2O; 3.28–1 262.30 ng/L), fluoroquinolones (NOX, OFX, CIX; LOD–577.97 ng/L), sulfonamides (SPD, SDZ, SMX; 6.64–91.36 ng/L), tetracycline (TC; 66.93–1 092.49 ng/L), chloramphenicol (CAP; 3.01– 4.92 ng/L) and trimethoprim (TMP; 75.58–93.15 ng/L) had positive detections in hospital wastewater effluent. In contrast, antibiotics detected from zoo wastewater effluent was mainly from fluoroquinolones class (4/5, ENX, ENRO, OFX, CIX) in the range of LOD– 34.30 ng/L followed by macrolides (AZM, ETM-H2O, 0.60–22.15 ng/L), tetracyclines (OTC, 18.81– 108.88 ng/L), amphenicol (CAP, LOD–1.99 ng/L), trimethoprim (TMP, 5.39 ng/L) and sulfonamides (SMX, 0.37 ng/L). For slaughterhouse wastewater effluent, fluoroquinolones (ENRO, NOX, CIX, 0.68– 25.61 ng/L) and sulfonamides (SPD, SDZ, SMX, 0.55–12.59 ng/L) were the major class detected whereas for macrolides, tetracyclines and trimethoprim, only ETM-H2O (0.18–2.39 ng/L), OTC (26.7– 92.58 ng/L) and TMP (1.03 ng/L) were detected. For the downstream sites (S1b and S1c) fewer antibiotics were detected, ETM-H2O was detected for both sides with 6.42 ng/L and LOD–3.61 ng/L respectively. Sulfonamides were only detected in immediate downstream, S1b (2/7, SDZ, SMX) with the range of 0.19–0.41 ng/L but not in S1c. The same trend was observed for CIX with the range of LOD–0.54 ng/L whereas NOX was only detected in S1c with 0.18 ng/L.
For Sangga Besar River sites, antibiotic residues were only detected for S2a and S2c with a total concentration of 5.14 ng/L and 23.16 ng/L, respectively. For ENRO, it was only detected at S2a with 1.30 ng/L. The detected antibiotics mainly come from fluoroquinolones, OFX and CIX, which contributed to the range of 2.32–18.28 ng/L in S2c whereas the site at S2a the concentration was < LQ. Macrolides were detected in low concentrations in S2c except for RTM whereas for S2a only ETM-H2O was detected.
3.2 Ecological risk assessment for antibiotics (RQs)For this work, PNEC of algae, invertebrate Daphnia magna, and fish were analyzed to assess the RQs. RQ for ENX was not calculated, as the toxicology data were unavailable for these aquatic organisms. The degree of sensitivity of the aquatic organisms towards antibiotics in surface waters of Larut and Sangga Besar River was in the following descending order: Algae > Daphnia magna > fish.
In Table 2, the risk assessment showed that among the macrolides detected, ETM-H2O posed low to high ecological risk to algae among the study sites except for S1a and S2b, conversely, low risk was detected for invertebrate, whereas AZM posed a low to medium risk to algae. CTM detected in hospital was found to have a high risk for algae, contrastingly, algae in S2c of Sangga River was exposed to low ecological risk. For fluoroquinolones, OFX detected at zoo, hospital, and S2c posed a high ecological risk to algae whereas at S2c only medium risk was found. CIX detected in hospital had a low risk for algae. Among tetracyclines, OTC detected in hospital, slaughterhouse and S2a posed low to medium risk for algae whereas TC detected in hospital posed a medium risk for algae, medium risk for invertebrates and low risk for fish. Among the sulfonamides compounds tested, only SMX and SMA detected in hospital and slaughterhouse posed medium and low risk respectively to algae.
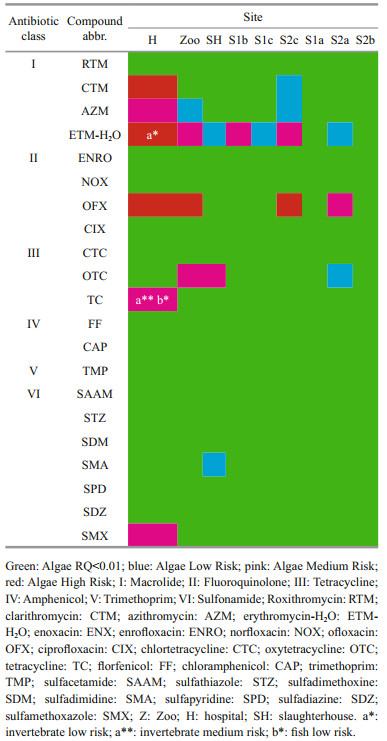
|
In this present study, our data showed that hospital, zoo, and slaughterhouse effluents from midstream of Larut River were important contributors to antibiotic contamination along Larut River, which was consistent with Lye et al. (2019). However, cluster analysis showed that, the antibiotic contamination in effluents from the zoo and the slaughterhouse were more similar (69.00%) than effluent from hospital (Table 1). The effluent from both zoo and slaughterhouse could have contained partially metabolized antibiotics from veterinary sources, in comparison to hospital effluent that was from human sources. The high prevalence of antibiotic residues in hospital concurred with other studies (Verlicchi et al., 2012; Ory et al., 2016). In the wastewater effluent of hospital, ETM-H2O was found to be the most prevalent antibiotic, as it was the secondhighest antibiotics prescribed 22.4%, (Ministry of Health Malaysia, 2017) in Malaysia to treat susceptible bacterial infection and/or as a motility agent in critically ill patients (Siti et al., 2014; Shamsuddin et al., 2016). Furthermore, ETM has good stability in the aquatic environment (Li et al., 2018). Tan et al. (2017) revealed that ETM-ethylsuccinate was one of the antibiotics that were inappropriately prescribed in a Malaysia hospital for upper respiratory tract infections, as most of the prescribers were unaware of the removal of this drug from Malaysia's National Antibiotic Guideline due to the resistance developed by Streptococci. Besides, ETM usage is legally permitted in Malaysia for poultry and cattle farming (Hassali et al., 2018), the total amount used in 2015 was 218 290 kg/year (Marzuki, 2017). The detected concentrations in this study were comparable to the levels from Pearl River (not detected–1 540 ng/L, Li et al., 2018) but higher than Tamagawa River, Japan (21.0–120.0 ng/L, Managaki et al., 2007), Lake Taihu (not detected–624.80 ng/L, Xu et al., 2014), and the South Yellow Sea (not detected–138.90 ng/L, Du et al., 2017), however, lower than wastewater treatment plants (WWTP) in Brazil (not detected–1 586.0 ng/L, Jank et al., 2014) and hospital effluents from Romania Hospital (not detected–7 520.00 ng/L, Szekeres et al., 2017) (Fig. 2).
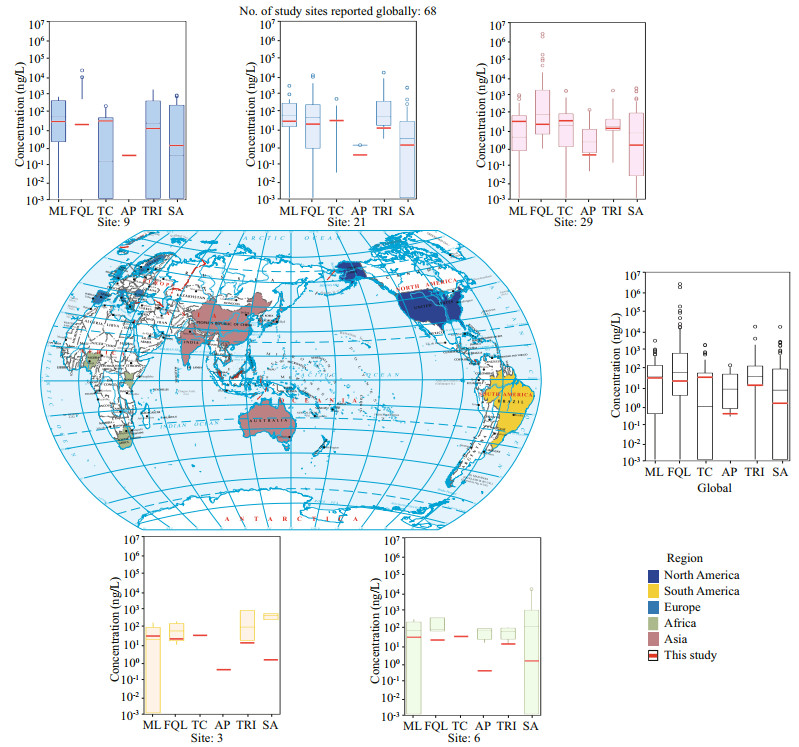
|
| Fig.2 Countries with antibiotic residue detected in surface waters over this work and reported literature totaling 68 sites Box plots show the concentrations (ng/L) of six antibiotic classes: macrolides (ML); fluoroquinolones (FQL); tetracylines (TC); amphenicols (AP); trimethoprim (TRI); sulfonamides (SA). Red lines show concentrations detected in Larut River and Sangga Besar River, Malaysia in this study. Map review No.GS(2016)2964. |
The use of fluoroquinolones had increased substantially globally due to its broad-spectrum antimicrobial properties against Gram-negative pathogens, especially those resistant to other antimicrobial classes (Pham et al., 2019). Among the fluoroquinolone compounds examined, secondgeneration CIX and OFX were the most prevalent. CIX was the second highest expenditure in Malaysian public hospitals between 2009 and 2010 and are commonly used as systemic medication and to treat bacterial eye infection with utilization of 0.365 DDD/ 1 000 inhabitants/day and 0.022 DDD/1 000 inhabitants/day respectively, whereas the usage in livestock was 4 615 kg/year (Siti et al., 2014; Marzuki, 2017). OFX is for systemic use and to treat outer ear infections; according to the Malaysian Statistic Medicines 2011–2014, the increasing trend was observed for the usage of anti-infective OFX ear drop from 0.015 DDD/1 000 inhabitants/day in 2011 to 0.018 DDD/1 000 inhabitants/day in 2014 (Ministry of Health Malaysia, 2017a). Generally, NOX is less potent than CIX, therefore, their usage rate as systemic medication (0.024 DDD/1 000 inhabitants/day) and bacterial eye infection treatment (0.001 DDD/1 000 inhabitants/day) was much lower. For ENRO, it was positively detected in S1a, zoo, slaughterhouse, and S1a whereas ENX was only detected in the zoo. As the usage of these two antibiotics were legally permitted in animal feed for preventive measures in Malaysia (Marzuki, 2017). Overall, the fluoroquinolone concentrations detected in this work were below the global mean (Fig. 2). However, CIX showed an increasing systemic utilization and was the drug with the second highest expenditure in Malaysian public hospitals between 2009 and 2010 (Siti et al., 2014). CIX levels in this study (not detected– 577.97 ng/L) were higher if not similar to rivers and basins from Lui, Gombak, and Selangor rivers, Malaysia (52.50–299.88 ng/L, Praveena et al., 2018), Dongjiang River, China (not detected–442.10 ng/L, Chen et al., 2018), Chongqing, China (not detected–458.00 ng/L, Chang et al., 2010), and WWTP near a hospital in Lake Victoria Basin, Kenya (not detected–420.00 ng/L, Kimosop et al., 2016), but were several orders lower compared to WWTPs from Ter River, Spain (4.7–13 779.70 ng/L, Rodriguez-Mozaz et al., 2015), New York, USA (not detected–5 600.00 ng/L, Batt et al., 2006), and STPs from Okhla, India (2 900.00–45 400.00 ng/L, Mutiyar and Mittal, 2014), hospitals from Ujjain, India (not detected–236 600 ng/L, Diwan et al., 2010) (Fig. 2).
In this work, TC and OTC were the tetracycline residues detected in the wastewater effluents of hospital, zoo, and slaughterhouse in Larut River. Tetracyclines were one of the considerably cheaper classes of antibiotics available, making it attractive to be used for human and veterinary drugs in developing countries like Malaysia (Michalova et al., 2004; Roberts et al., 2012; Siti et al., 2014). The concentrations of tetracyclines detected in this work (LOD–1 092.49 ng/L) were higher than the global mean concentrations for surface waters (1.01– 254 820 ng/L, Fig. 2), rivers in Huangpu River, China (not detected–135.50 ng/L, Jiang et al., 2011) and Cache La Poudre River, USA (not detected–1 210.00 ng/L, Kim and Carlson, 2006), lakes in Taihu Lake, China (not detected–142.50 ng/L, Xu et al., 2014), and Choptank Basin, USA (not detected–388.00 ng/L, Arikan et al., 2008) but lower than hospital effluents from Xinxiang, China (1 147.83–1 727.05 ng/L, Wang et al., 2018) and Romania (not detected–1 340.00 ng/L, Szekeres et al., 2017) (Fig. 2).
In Malaysia, TMP was used in combination with sulfonamides such as SMX and SDZ due to their synergistic antibacterial properties (Siti et al., 2014; Ministry of Health Malaysia, 2017a). As such, TMP was detected in locations shared by sulfonamides including hospital, zoo, and slaughterhouse. For sulfonamides, SMX was the most abundant and frequently detected residue type in this work, which is in agreement with Lye et al. (2019). Sulfonamides were reported as ubiquitous in tropical Asian countries (Shimizu et al., 2013), yet the concentrations in this study were below the mean antibiotic concentration in surface waters in the Asia region. Sulfonamides in this study (not detected–93.15 ng/L) had low levels comparable to Choptank watershed (not detected– 9.00 ng/L, Arikan et al., 2008) and the Bohai Sea (not detected–96.00 ng/L, Zhang et al., 2013). Specifically, SMX in this study had levels similar to Taihu Lake (not detected–114.70 ng/L, Xu et al., 2014).
In this study, CAP was the only amphenicol compound detected in hospital and zoo. CAP was used as a systemic medicine and a topical ear drop to treat ear infections in Malaysia (Mohamad et al., 2014) while commonly used in veterinary for horses (McElligott et al., 2017). Malaysia had banned the usage of CAP for food use in food-producing animals since 1998 (Malaysia Food Act 1983 (Act 281) and Regulations) (Ministry of Health Malaysia, 2014). The concentration levels of CAP (LOD–4.92 ng/L) in the current work was lower than Huangpu River, China (4.18–2.36 ng/L, Jiang et al., 2011), Taff and Ely River, South Wales (not detected–40.00 ng/L, Kasprzyk-Hordern et al., 2008), Owo River, Nigeria (not detected–360.00 ng/L, Olarinmoye et al., 2016), WWTP in Lake Victoria Basin, Nigeria (not detected–60.00 ng/L, Kimosop et al., 2016), South Yellow Sea (not detected–73.20 ng/L., Du et al., 2017) and hospitals in Lake Victoria Basin, Nigeria (70.00–80.00 ng/L, Kimosop et al., 2016) (Fig. 2).
In comparison to the number and range of antibiotics detected from Larut River, sites from Sangga Besar River generally had low antibiotic detections and concentrations. This could be attributed to the lower population density of Sangga Besar compared to Larut River, which population was ten times larger (Ghaderpour et al., 2015), as studies had shown significant correlations between population density and antibiotic compounds in the surface waters of river (Osorio et al., 2016). Thus, Sangga Besar received less anthropogenic pollution. Besides that, antibiotic practices and doses applied in both humans and animals varied between regions and countries, which may greatly influence the type, distribution and variation of antibiotic residues in the aquatic environments (Managaki et al., 2007; Shimizu et al., 2013). We did not find any correlation between antibiotic concentrations and water parameters measured (data from Lye et al., 2019), even though some studies have shown that physicochemical properties of the antibiotics are affected by the local environment parameters (e.g. temperature, pH, salinity, moisture, oxygen level, etc.) (Luo et al., 2011; Lu et al., 2015; Yang et al., 2015). Hydrodynamics and microbiological degradation by bacteria will also affect the degradation and persistence of antibiotic in the environment (Gauthier et al., 2010; Garcáa-Galán et al., 2011; Tappe et al., 2013; Topp et al., 2013).
4.2 Risks posed by antibiotic residues in riverine estuarine environmentCalculated RQs revealed that individual antibiotic residue could be a risk to aquatic organisms. Individually, the antibiotics, ETM-H2O, CTM, and OFX posed high risks to algae ecology in several sites from the current study where hospital and zoo had been identified as a risk site. Although algae's sensitivity towards antibiotics in the aquatic environment had been verified by numerous studies (Halling-Sørensen, 2000; Ando et al., 2007; Magdaleno et al., 2015; Li et al., 2018), the ecological risks posed in this study is still important as it affects 78% of the studied sites. Apart from ETM-H2O, TC, CTM, and OFX, environmental toxicology data for antibiotics and the results compiled in this study indicates that most concentration of each of these antibiotics in river waters were not high enough to cause acute effects on more complex aquatic organisms. This study showed similar high risks for OFX present in sites from Laizhou Bay, China (Zhang et al., 2012), Korean aquatic environment (Lee et al., 2008), and Hong Kong sewage (Deng et al., 2016).
Furthermore, the assessment indicates that ETMH2O, CTM, and OFX from hospital effluent could be at risk of promoting antibiotic resistance selection (Kemper, 2008) in the environment. Antibiotic resistance selection is based on the assumptions from Bengtsson-Palme and Larsson (2016) that selective concentrations need to be lower than those completely inhibiting growth. Given time and exposure, antibiotics in the environment could increase the prevalence of resistance by selecting resistant phenotypes via inhibition of sensitive strains (Ågerstrand et al., 2015). Thus, usage of the above antibiotics should be minimized and monitored to curb the development of antibiotic resistance.
5 CONCLUSIONSixteen antibiotics residues with concentrations ranging from LOD to 1 262.3 ng/L were detected in Larut River and Sangga Besar River. The results showed a wide prevalence of antibiotics in the sampling area where fluoroquinolones and macrolides were frequently detected in the water samples. RQs showed that ETM-H2O, CTM, and OFX detected from hospital and zoo posed the high risk to algae, while TC had low to medium ecological risk towards all tested aquatic organisms: algae, invertebrate Daphnia magna, and fish. Therefore, the wastewater effluents from hospital, zoo, and slaughterhouse introduced into the Larut River should be closely monitored.
6 DATA AVAILABILITY STATEMENTThe raw data produced and/or analyzed in the current study are not publicly available, because they will be used by the first author for Master's degree thesis preparation and so requires secure protection before thesis submission and graduation. However, data will be provided by the corresponding author upon request given valid reasons.
Electronic supplementary materialSupplementary material (Supplementary Tables S1–S2) is available in the online version of this article at https://doi.org/10.1007/s00343-020-9246-y.
Ågerstrand M, Berg C, Björlenius B, Breitholtz M, Brunström B, Fick J, Gunnarsson L, Larsson D G J, Sumpter J P, Tysklind M, Rudén C. 2015. Improving environmental risk assessment of human pharmaceuticals. Environmental Science & Technology, 49(9): 5 336-5 345.
DOI:10.1021/acs.est.5b00302 |
Ahmad B, Hasan Z A. 2011. Flood map of Tupai River using combined 1D and 2D modeling. In: Proceedings of the 3rd International Conference on Managing Rivers in the 21st Century: Sustainable Solutions for Global Crisis of Flooding, Pollution and Water Scarcity. Penang, Malaysia.p.491-496.
|
Allen H K, Donato J, Wang H H, Cloud-Hansen K A, Davies J, Handelsman J. 2010. Call of the wild:antibiotic resistance genes in natural environments. Nature Reviews Microbiology, 8(4): 251-259.
DOI:10.1038/nrmicro2312 |
Al-Qaim F F, Mussa Z H, Yuzir A, Tahrim N A, Hashim N, Azman S. 2018. Transportation of different therapeutic classes of pharmaceuticals to the surface water, sewage treatment plant, and hospital samples, Malaysia. Water, 10(7): 916.
DOI:10.3390/w10070916 |
Ando T, Nagase H, Eguchi K, Hirooka T, Nakamura T, Miyamoto K, Hirata K. 2007. A novel method using cyanobacteria for ecotoxicity test of veterinary antimicrobial agents. Environmental Toxicology and Chemistry, 26(4): 601-606.
DOI:10.1897/06-195R.1 |
Annual Fisheries of Perak. 2000. Annual Fisheries Statistics 2000-2004. p.24-40.
|
Arikan O A, Rice C, Codling E. 2008. Occurrence of antibiotics and hormones in a major agricultural watershed. Desalination, 226(1-3): 121-133.
DOI:10.1016/j.desal.2007.01.238 |
Baquero F, Martínez J L, Cantón R. 2008. Antibiotics and antibiotic resistance in water environments. Current Opinion in Biotechnology, 19(3): 260-265.
DOI:10.1016/j.copbio.2008.05.006 |
Batt A L, Bruce I B, Aga D S. 2006. Evaluating the vulnerability of surface waters to antibiotic contamination from varying wastewater treatment plant discharges. Environmental Pollution, 142(2): 295-302.
DOI:10.1016/j.envpol.2005.10.010 |
Bengtsson-Palme J, Larsson D G J. 2016. Concentrations of antibiotics predicted to select for resistant bacteria:proposed limits for environmental regulation. Environment International, 86: 140-149.
DOI:10.1016/j.envint.2015.10.015 |
Berendonk T U, Manaia C M, Merlin C, Fatta-Kassinos D, Cytryn E, Walsh F, Bürgmann H, Sørum H, Norström M, Pons M N, Kreuzinger N, Huovinen P, Stefani S, Schwartz T, Kisand V, Baquero F, Martinez J L. 2015. Tackling antibiotic resistance:the environmental framework. Nature Reviews Microbiology, 13(5): 310-317.
DOI:10.1038/nrmicro3439 |
Cabello F C, Godfrey H P, Buschmann A H, Dölz H J. 2016. Aquaculture as yet another environmental gateway to the development and globalisation of antimicrobial resistance. The Lancet Infectious Diseases, 16(7): E127-E133.
DOI:10.1016/S1473-3099(16)00100-6 |
Cabello F C. 2006. Heavy use of prophylactic antibiotics in aquaculture:a growing problem for human and animal health and for the environment. Environmental Microbiology, 8(7): 1 137-1 144.
DOI:10.1111/j.1462-2920.2006.01054.x |
Chang X S, Meyer M T, Liu X Y, Zhao Q, Chen H, Chen J A, Qiu Z Q, Yang L, Cao J, Shu W Q. 2010. Determination of antibiotics in sewage from hospitals, nursery and slaughter house, wastewater treatment plant and source water in Chongqing region of Three Gorge Reservoir in China. Environmental Pollution, 158(5): 1 444-1 450.
DOI:10.1016/j.envpol.2009.12.034 |
Chen Y H, Chen H J, Zhang L, Jiang Y, Gin K Y H, He Y L. 2018. Occurrence, distribution, and risk assessment of antibiotics in a subtropical river-reservoir system. Water, 10(2): 104.
DOI:10.3390/w10020104 |
Davis J G, Truman C C, Kim S C, Ascough Ⅱ J C, Carlson K. 2006. Antibiotic transport via runoff and soil loss. Journal of Environmental Quality, 35(6): 2 250-2 260.
DOI:10.2134/jeq2005.0348 |
Deng W J, Li N, Zheng H L, Lin H Y. 2016. Occurrence and risk assessment of antibiotics in river water in Hong Kong. Ecotoxicology and Environmental Safety, 125: 121-127.
DOI:10.1016/j.ecoenv.2015.12.002 |
Department of Statistics Malaysia. 2011. Population Distribution and Basic Demographic Characteristics.Department of Statistics Malaysia. p.67-68.
|
Divya S P, Hatha A A M. 2019. Screening of tropical estuarine water in south-west coast of India reveals emergence of ARGs-harboring hypervirulent Escherichia coli of global significance. International Journal of Hygiene and Environmental Health, 222(2): 235-248.
DOI:10.1016/j.ijheh.2018.11.002 |
Diwan V, Tamhankar A J, Khandal R K, Sen S, Aggarwal M, Marothi Y, Iyer R V, Sundblad-Tonderski K, StålsbyLundborg C. 2010. Antibiotics and antibiotic-resistant bacteria in waters associated with a hospital in Ujjain, India. BMC Public Health, 10: 414.
DOI:10.1186/1471-2458-10-414 |
Du J, Zhao H X, Liu S S, Xie H J, Wang Y, Chen J W. 2017. Antibiotics in the coastal water of the South Yellow Sea in China:occurrence, distribution and ecological risks. Science of the Total Environment, 595: 521-527.
DOI:10.1016/j.scitotenv.2017.03.281 |
European Commission Joint Research Centre. 2003. Technical Guidance Document on Risk Assessment. Part Ⅱ. EUR 20418 EN/2. European Commission Joint Research Centre.
|
Forestry Department of Perak. 2010. The Management of Matang Mangrove Forest, Perak, Malaysia. http://www.unepscs.org/Mangrove-Training/20-Matang-Management.pdf. Accessed on 2018-12-13.
|
García-Galán M J, Díaz-Cruz M S, Barceló D. 2011. Occurrence of sulfonamide residues along the Ebro river basin:Removal in wastewater treatment plants and environmental impact assessment. Environment International, 37(2): 462-473.
DOI:10.1016/j.envint.2010.11.011 |
Garcillán-Barcia M P, Alvarado A, de la Cruz F. 2011. Identification of bacterial plasmids based on mobility and plasmid population biology. FEMS Microbiology Reviews, 35(5): 936-956.
DOI:10.1111/j.1574-6976.2011.00291.x |
Gauthier H, Yargeau V, Cooper D G. 2010. Biodegradation of pharmaceuticals by Rhodococcus rhodochrous and Aspergillus niger by co-metabolism. Science of the Total Environment, 408(7): 1 701-1 706.
DOI:10.1016/j.scitotenv.2009.12.012 |
Ghaderpour A, Ho W S, Chew L L, Bong C W, Chong V C, Thong K L, Chai L C. 2015. Diverse and abundant multidrug resistant E. coli in Matang mangrove estuaries, Malaysia. Frontiers in Microbiology, 6: 977.
DOI:10.3389/fmicb.2015.00977 |
Göbel A, Thomsen A, McArdell C S, Joss A, Giger W. 2005. Occurrence and sorption behavior of sulfonamides, macrolides, and trimethoprim in activated sludge treatment. Environmental Science & Technology, 39(11): 3 981-3 989.
DOI:10.1021/es048550a |
Grenni P, Ancona V, Caracciolo A B. 2018. Ecological effects of antibiotics on natural ecosystems:a review. Microchemical Journal, 136: 25-39.
DOI:10.1016/j.microc.2017.02.006 |
Halling-Sørensen B. 2000. Algal toxicity of antibacterial agents used in intensive farming. Chemosphere, 40(7): 731-739.
DOI:10.1016/S0045-6535(99)00445-2 |
Hammer Ø, Harper D A T, Ryan P D. 2001. PAST:paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4(1): 1-9.
|
Hassali M A A, Yann H R, Verma A K, Hussain R, Sivaraman S. 2018. Antibiotic Use in Food Animals: Malaysia Overview. Discipline of Social & Administrative Pharmacy, School of Pharmaceutical Sciences, Universiti Sains Malaysia, Penang, Malaysia. 29p.
|
Houtman C J, van Oostveen A M, Brouwer A, Lamoree M H, Legler J. 2004. Identification of estrogenic compounds in fish bile using bioassay-directed fractionation. Environmental Science & Technology, 38(23): 6 415-6 423.
DOI:10.1021/es049750p |
Humeniuk C, Arlet G, Gautier V, Grimont P, Labia R, Philippon A. 2002. β-lactamases of Kluyvera ascorbata, probable progenitors of some plasmid-encoded CTX-M types. Antimicrobial Agents and Chemotherapy, 46(9): 3 045-3 049.
DOI:10.1128/AAC.46.9.3045-3049.2002 |
Iglesias A, Nebot C, Miranda J M, Vázquez B I, Abuín C M F, Cepeda A. 2013. Determination of the presence of three antimicrobials in surface water collected from urban and rural areas. Antibiotics, 2(1): 46-57.
DOI:10.3390/antibiotics2010046 |
Jank L, Hoff R B, da Costa F J, Pizzolato T M. 2014. Simultaneous determination of eight antibiotics from distinct classes in surface and wastewater samples by solid-phase extraction and high-performance liquid chromatography-electrospray ionisation mass spectrometry. International Journal of Environmental Analytical Chemistry, 94(10): 1 013-1 037.
DOI:10.1080/03067319.2014.914184 |
Jiang L, Hu X L, Yin D Q, Zhang H C, Yu Z Y. 2011. Occurrence, distribution and seasonal variation of antibiotics in the Huangpu River, Shanghai, China. Chemosphere, 82(6): 822-828.
DOI:10.1016/j.chemosphere.2010.11.028 |
Kasprzyk-Hordern B, Dinsdale R M, Guwy A J. 2008. The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water Research, 42(13): 3 498-3 518.
DOI:10.1016/j.watres.2008.04.026 |
Kemper N. 2008. Veterinary antibiotics in the aquatic and terrestrial environment. Ecological Indicators, 8(1): 1-13.
DOI:10.1016/j.ecolind.2007.06.002 |
Kim S C, Carlson K. 2006. Occurrence of ionophore antibiotics in water and sediments of a mixed-landscape watershed. Water Research, 40(13): 2 549-2 560.
DOI:10.1016/j.watres.2006.04.036 |
Kimosop S J, Getenga Z M, Okello V A, Cheruiyot J K. 2016. Residue levels and discharge loads of antibiotics in wastewater treatment plants (WWTPs), hospital lagoons, and rivers within Lake Victoria Basin, Kenya. Environmental Monitoring and Assessment, 188(9): 532.
DOI:10.1007/s10661-016-5534-6 |
Lee Y J, Lee S E, Lee D S, Kim Y H. 2008. Risk assessment of human antibiotics in Korean aquatic environment. Environmental Toxicology and Pharmacology, 26(2): 216-221.
DOI:10.1016/j.etap.2008.03.014 |
Li S, Shi W Z, Liu W, Li H M, Zhang W, Hu J R, Ke Y C, Sun W L, Ni J R. 2018. A duodecennial national synthesis of antibiotics in China's major rivers and seas (2005-2016). Science of the Total Environment, 615: 906-917.
DOI:10.1016/j.scitotenv.2017.09.328 |
Lu Z H, Na G S, Gao H, Wang L J, Bao C G, Yao Z W. 2015. Fate of sulfonamide resistance genes in estuary environment and effect of anthropogenic activities. Science of the Total Environment, 527-528: 429-438.
DOI:10.1016/j.scitotenv.2015.04.101 |
Lundborg C S, Tamhankar A J. 2017. Antibiotic residues in the environment of South East Asia. BMJ, 358: j2440.
DOI:10.1136/BMJ.J2440 |
Luo Y, Xu L, Rysz M, Wang Y Q, Zhang H, Alvarez P J J. 2011. Occurrence and transport of tetracycline, sulfonamide, quinolone, and macrolide antibiotics in the Haihe River Basin, China. Environmental Science & Technology, 45(5): 1 827-1 833.
DOI:10.1021/es104009s |
Lye Y L, Bong C W, Lee C W, Zhang R J, Zhang G, Suzuki S, Chai L C. 2019. Anthropogenic impacts on sulfonamide residues and sulfonamide resistant bacteria and genes in Larut and Sangga Besar River, Perak. Science of the Total Environment, 688: 1 335-1 347.
DOI:10.1016/j.scitotenv.2019.06.304 |
Magdaleno A, Saenz M E, Juárez A B, Moretton J. 2015. Effects of six antibiotics and their binary mixtures on growth of Pseudokirchneriella subcapitata. Ecotoxicology and Environmental Safety, 113: 72-78.
DOI:10.1016/j.ecoenv.2014.11.021 |
Managaki S, Murata A, Takada H, Tuyen B C, Chiem N H. 2007. Distribution of macrolides, sulfonamides, and trimethoprim in tropical waters:ubiquitous occurrence of veterinary antibiotics in the Mekong Delta. Environmental Science & Technology, 41(23): 8 004-8 010.
DOI:10.1021/es0709021 |
Marni S, Malintan N T, Faridah I, Mustafa A M. 2010. Chloramphenicol in Malaysia waste water and its residues in animal husbandaries products. Health and the Environment Journal, 1(1): 41-45.
|
Martinez J L. 2009. Environmental pollution by antibiotics and by antibiotic resistance determinants. Environmental Pollution, 157(11): 2 893-2 902.
DOI:10.1016/j.envpol.2009.05.051 |
Marzuki Z. 2017. Use of antimicrobial agents in veterinary medicine in Malaysia. 2nd OIE Information Seminar for Praticing Veterinatians: combating AMR. Kuala Lumpur, Malaysia. http://vam.org.my/home/wp-content/uploads/2017/11/Mazuki_AMU-in-Vet-Med.pdf. Accessed on 2019-08-29.
|
McArdell C S, Molnar E, Suter M J F, Giger W. 2003. Occurrence and Fate of Macrolide Antibiotics in Wastewater Treatment Plants and in the Glatt Valley Watershed, Switzerland. Environmental Science & Technology, 37(24): 5 479-5 486.
DOI:10.1021/es034368i |
McElligott E M, Sommardahl C S, Cox S K. 2017. Pharmacokinetics of chloramphenicol base after oral administration in adult horses. Journal of the American Veterinary Medical Association, 251(1): 90-94.
DOI:10.2460/javma.251.1.90 |
Miao X S, Bishay F, Chen M, Metcalfe C D. 2004. Occurrence of antimicrobials in the final effluents of wastewater treatment plants in Canada. Environmental Science & Technology, 38(13): 3 533-3 541.
DOI:10.1021/es030653q |
Michalova E, Novotna P, Schlegelova J. 2004. Tetracyclines in veterinary medicine and bacterial resistance to them. Veterinarni Medicina, 49(3): 79-100.
DOI:10.17221/5681-VETMED |
Ministry of Health Malaysia. 2014. Food Act 1983 Food(Amendment) (No. 3) Regulations 2014. Ministry of Health, Malaysia. 45p.
|
Ministry of Health Malaysia. 2017a. Malaysian Statistics on Medicines 2011-2014. Ministry of Health Malaysia. p.67-219.
|
Ministry of Health Malaysia. 2017b. Malaysian Action Plan on Antimicrobial Resistance (MyAP-AMR) 2017-2021.
|
Ministry of Health, Malaysia, Ministry of Agriculture & Agro-Based Industry Malaysia, Malaysia. 51p.
|
Mohamad I, Johan K B, Hashim H Z, Othman N N. 2014. Otitis externa complicated with chloramphenicol ear drops-induced perichondritis. Malaysian Family Physician, 9(1): 28-29.
|
Muda A, Ahmad Z M A, Lim K L. 2005. Sustainable management and conservation of the matang mangroves. Forestry Department Peninsular Malaysia: 39-52.
|
Mutiyar P K, Mittal A K. 2014. Occurrences and fate of selected human antibiotics in influents and effluents of sewage treatment plant and effluent-receiving river Yamuna in Delhi (India). Environmental Monitoring and Assessment, 186(1): 541-557.
DOI:10.1007/s10661-013-3398-6 |
O'Neill J. 2016. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. The Review on Antimicrobial Resistance, London. 1-76.
|
Olarinmoye O, Bakare A, Ugwumba O, Hein A. 2016. Quantification of pharmaceutical residues in wastewater impacted surface waters and sewage sludge from Lagos, Nigeria. Journal of Environmental Chemistry and Ecotoxicology, 8(3): 14-24.
DOI:10.5897/JECE2015.0364 |
Ory J, Bricheux G, Togola A, Bonnet J L, Donnadieu-Bernard F, Nakusi L, Forestier C, Traore O. 2016. Ciprofloxacin residue and antibiotic-resistant biofilm bacteria in hospital effluent. Environmental Pollution, 214: 635-645.
DOI:10.1016/j.envpol.2016.04.033 |
Osorio V, Larrañaga A, Aceña J, Pérez S, Barceló D. 2016. Concentration and risk of pharmaceuticals in freshwater systems are related to the population density and the livestock units in Iberian Rivers. Science of the Total Environment, 540: 267-277.
DOI:10.1016/j.scitotenv.2015.06.143 |
Partridge S R. 2011. Analysis of antibiotic resistance regions in Gram-negative bacteria. FEMS Microbiology Reviews, 35(5): 820-855.
DOI:10.1111/j.1574-6976.2011.00277.x |
Pérez Gaudio D S, Colello R, Fernández D, Mozo J, Martínez G, Fernández Paggi M B, Decundo J M, Romanelli A, Dieguez S, Etcheverría A, Padola N L, Soraci A L. 2018. Horizontal transference of antimicrobial resistance genes between a non pathogenic Escherichia coli and a pathogenic shiga toxin-producing E. coli strain. EC Veterinary Science, 3(2): 293-299.
|
Pham T D M, Ziora Z M, Blaskovich M A T. 2019. Quinolone antibiotics. MedChemComm, 10(10): 1 719-1 739.
DOI:10.1039/C9MD00120D |
Poirel L, Liard A, Rodriguez-Martinez J M, Nordmann P. 2005. Vibrionaceae as a possible source of Qnr-like quinolone resistance determinants. Journal of Antimicrobial Chemotherapy, 56(6): 1 118-1 121.
DOI:10.1093/jac/dki371 |
Praveena S M, Shaifuddin S N M, Sukiman S, Nasir F A M, Hanafi Z, Kamarudin N, Ismail T H T, Aris A Z. 2018. Pharmaceuticals residues in selected tropical surface water bodies from Selangor (Malaysia):occurrence and potential risk assessments. Science of the Total Environment, 642: 230-240.
DOI:10.1016/j.scitotenv.2018.06.058 |
Roberts J A, Norris R, Paterson D L, Martin J M. 2012. Therapeutic drug monitoring of antimicrobials. British Journal of Clinical Pharmacology, 73(1): 27-36.
DOI:10.1111/j.1365-2125.2011.04080.x |
Rodriguez-Mozaz S, Chamorro S, Marti E, Huerta B, Gros M, Sànchez-Melsió A, Borrego C M, Barceló D, Balcázar J L. 2015. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Research, 69: 234-242.
DOI:10.1016/j.watres.2014.11.021 |
Sakai N, Yusof M R, Sapar M, Yoneda M, Mohd M A. 2016. Spatial analysis and source profiling of beta-agonists and sulfonamides in Langat River basin, Malaysia. Science of the Total Environment, 548-549: 43-50.
DOI:10.1016/j.scitotenv.2016.01.040 |
Samuding K, Tadza M, Rahman A, Abustan I, Mejus L, Mostapa R. 2009. Integrated study on the distribution of contamination flow path at a waste disposal site in Malaysia.In: Yu X Y ed. Municipal and Industrial Waste Disposal.IntechOpen. p.55-70, https://doi.org/10.5772/30766.
|
Shamsuddin S, Akkawi M E, Zaidi S T R, Ming L C. 2016. Antimicrobial drug use in primary healthcare clinics:a retrospective evaluation. International Journal of Infectious Diseases, 52: 16-22.
DOI:10.1016/j.ijid.2016.09.013 |
Shimizu A, Takada H, Koike T, Takeshita A, Saha M, Rinawati, Nakada N, Murata A, Suzuki T, Suzuki S, Chiem N H, Tuyen B C, Viet P H, Siringan M A, Kwan C, Zakaria M P, Reungsang A. 2013. Ubiquitous occurrence of sulfonamides in tropical Asian waters. Science of the Total Environment, 452-453: 108-115.
DOI:10.1016/j.scitotenv.2013.02.027 |
Siti F A, Kamarudin A, Nik N A N O. 2014. Malaysian Statistics on Medicines 2009 & 2010. Pharmaceutical Services Division and Clinical Research Centre, Malaysia. 206p.
|
Szekeres E, Baricz A, Chiriac C M, Farkas A, Opris O, Soran M L, Andrei A S, Rudi K, Balcázar J L, Dragos N, Coman C. 2017. Abundance of antibiotics, antibiotic resistance genes and bacterial community composition in wastewater effluents from different Romanian hospitals. Environmental Pollution, 225: 304-315.
DOI:10.1016/j.envpol.2017.01.054 |
Tan G H, Low Q Q, Lim H C, Seah H K, Chan H K. 2017. Inappropriate antibiotic utilization:outpatient prescription review of a regional secondary hospital in Kedah, Malaysia. Journal of Pharmacy Practice and Community Medicine, 3(4): 215-219.
DOI:10.5530/jppcm.2017.4.62 |
Tappe W, Herbst M, Hofmann D, Koeppchen S, Kummer S, Thiele B, Groeneweg J. 2013. Degradation of sulfadiazine by Microbacterium lacus strain SDZm4, isolated from lysimeters previously manured with slurry from sulfadiazine-medicated pigs. Applied and Environmental Microbiology, 79(8): 2 572-2 577.
DOI:10.1128/AEM.03636-12 |
Topp E, Chapman R, Devers-Lamrani M, Hartmann A, Marti R, Martin-Laurent F, Sabourin L, Scott A, Sumarah M. 2013. Accelerated biodegradation of veterinary antibiotics in agricultural soil following long-term exposure, and isolation of a sulfamethazine-degrading microbacterium sp. Journal of Environmental Quality, 42(1): 173-178.
DOI:10.2134/jeq2012.0162 |
van Boeckel T P, Gandra S, Ashok A, Caudron Q, Grenfell B T, Levin S A, Laxminarayan R. 2014. Global antibiotic consumption 2000 to 2010:an analysis of national pharmaceutical sales data. The Lancet Infectious Diseases, 14(8): 742-750.
DOI:10.1016/S1473-3099(14)70780-7 |
Verlicchi P, Al Aukidy M, Galletti A, Petrovic M, Barceló D. 2012. Hospital effluent:investigation of the concentrations and distribution of pharmaceuticals and environmental risk assessment. Science of the Total Environment, 430: 109-118.
DOI:10.1016/j.scitotenv.2012.04.055 |
Wang Q, Wang P L, Yang Q X. 2018. Occurrence and diversity of antibiotic resistance in untreated hospital wastewater. Science of the Total Environment, 621: 990-999.
DOI:10.1016/j.scitotenv.2017.10.128 |
Xu J, Zhang Y, Zhou C B, Guo C S, Wang D M, Du P, Luo Y, Wan J, Meng W. 2014. Distribution, sources and composition of antibiotics in sediment, overlying water and pore water from Taihu Lake, China. Science of the Total Environment, 497-498: 267-273.
DOI:10.1016/j.scitotenv.2014.07.114 |
Xu W H, Zhang G, Zou S C, Ling Z H, Wang G L, Yan W. 2009. A Preliminary Investigation on the Occurrence and Distribution of Antibiotics in the Yellow River and its Tributaries, China. Water Environment Research, 81(3): 248-254.
DOI:10.2175/106143008X325719 |
Xue B M, Zhang R J, Wang Y H, Liu X, Li J, Zhang G. 2013. Antibiotic contamination in a typical developing city in south China:occurrence and ecological risks in the Yongjiang River impacted by tributary discharge and anthropogenic activities. Ecotoxicology and Environmental Safety, 92: 229-236.
DOI:10.1016/j.ecoenv.2013.02.009 |
Yang C C, Huang C L, Cheng T C, Lai H T. 2015. Inhibitory effect of salinity on the photocatalytic degradation of three sulfonamide antibiotics. International Biodeterioration & Biodegradation, 102: 116-125.
DOI:10.1016/j.ibiod.2015.01.015 |
Yao L L, Wang Y X, Tong L, Deng Y M, Li Y G, Gan Y Q, Guo W, Dong C J, Duan Y H, Zhao K. 2017. Occurrence and risk assessment of antibiotics in surface water and groundwater from different depths of aquifers:a case study at Jianghan Plain, central China. Ecotoxicology and Environmental Safety, 135: 236-242.
DOI:10.1016/j.ecoenv.2016.10.006 |
Zarfel G, Lipp M, Gürtl E, Folli B, Baumert R, Kittinger C. 2017. Troubled water under the bridge:screening of River Mur water reveals dominance of CTX-M harboring Escherichia coli and for the first time an environmental VIM-1 producer in Austria. Science of the Total Environment, 593-594: 399-405.
DOI:10.1016/j.scitotenv.2017.03.138 |
Zhang R J, Tang J H, Li J, Zheng Q, Liu D, Chen Y J, Zou Y D, Chen X X, Luo C L, Zhang G. 2013. Antibiotics in the offshore waters of the Bohai Sea and the Yellow Sea in China:occurrence, distribution and ecological risks. Environmental Pollution, 174: 71-77.
DOI:10.1016/j.envpol.2012.11.008 |
Zhang R J, Zhang G, Zheng Q, Tang J H, Chen Y J, Xu W H, Zou Y D, Chen X X. 2012. Occurrence and risks of antibiotics in the Laizhou Bay, China:impacts of river discharge. Ecotoxicology and Environmental Safety, 80: 208-215.
DOI:10.1016/j.ecoenv.2012.03.002 |
 2021, Vol. 39
2021, Vol. 39


