Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHENG Xinqing, LI Yuanchao, LIANG Jilin, LIN Rongcheng, WANG Daoru
- Performance of ecological restoration in an impaired coral reef in the Wuzhizhou Island, Sanya, China
- Journal of Oceanology and Limnology, 39(1): 135-147
- http://dx.doi.org/10.1007/s00343-020-9253-z
Article History
- Received Oct. 17, 2019
- accepted in principle Jan. 7, 2020
- accepted for publication Mar. 9, 2020
2 Hainan Academy of Ocean and Fisheries Science, Haikou 570125, China;
3 Fujian Provincial Station for Field Observation and Research of Island and Costal Zone in Zhangzhou, Xiamen 361005, China;
4 Fujian Provincial Key Laboratory of Marine Ecological Conservation and Restoration, Xiamen 361005, China
Coral reefs are one of the most complex and diverse ecosystems in ocean. They offer habitats that sustain a high biodiversity, food, ecological services, and livelihoods for coastal communities (Moberg and Folke, 1999). However, coral reefs are increasingly threatened by natural and anthropogenic stresses. Many reefs have been severely degraded in recent years (Pandolfi et al., 2003; Zhao et al., 2010; Hughes et al., 2013). The coastal waters of China are rich in coral reefs, occupying about 30 000 km2 on China's coastal fringing reefs as well as offshore atolls and islands in the South China Sea (SCS) (Wu and Zhang, 2012; Hughes et al., 2013). Unfortunately, nearly 80% of the corals cover on fringing reefs along mainland China and around the Hainan Island (Zhao et al., 2010; Hughes et al., 2013). Corals covering on offshore atolls in the SCS has declined from an average of >60% to around 20% in the past 15 years (Hughes et al., 2013).
Faced with degradation of coral reefs, coral restoration becomes a feasible way to help restoring impaired reefs (Harriott, 1998; Rinkevich, 2005; Yeemin et al., 2006; Edwards and Gomez, 2007; Shaish et al., 2008; Muko and Iwasa, 2011; Ng et al., 2016; Tortolero-Langarica et al., 2019). Many coral reefs fail to recover naturally without human intervention (Young et al., 2012). Various methods have been proposed to accelerate the recovery of natural reefs, including amelioration or removal of the stressors, coral gardening, artificial reefs, coral transplantation, and the introduction of fish or zoobenthos with key ecological functions (Edwards and Clark, 1999; Shaish et al., 2008, 2010a, b; Omori, 2011; Kheawwongjan and Kim, 2012; Ng et al., 2016; Richmond et al., 2019). However, coral restoration via rehabilitation remains a challenge due to poor understanding of the factors affecting posttransplantation survivorship.
Natural disturbances such as typhoon (Hongo et al., 2012; White et al., 2017), outbreak of Acanthaster planci (Lourey et al., 2000; Baird et al., 2013), and poor management (Rudianto and Zainul, 2019) would result in the failure of reef recovery. Typhoons are common natural disturbances on coral reefs and modify the community structure of reef corals via mechanical destruction, change in sedimentation and in turbidity, and decreasing the salinity by heavy precipitation (Herbeck et al., 2011; Hongo et al., 2012; Li et al., 2013; White et al., 2017). China is subjected to at least 10 typhoon attacks every year, which can lead to severe damages and huge economic loss (Wu et al., 2007; Liu et al., 2009). Typhoons may greatly influence the performance of coral restoration in vulnerable regions (Shaish et al., 2010a; Wells et al., 2010). Typhoons increase coral mortality, detachment of transplanted corals, and speciesspecific coral bleaching (Shaish et al., 2010a). Meanwhile, typhoons result in reef-scale failure of coral settlement (Doropoulos et al., 2014). Sediment accumulation caused by rain and typhoons also has a strong impact on coral communities (Li et al., 2013). Artificial reef (AR) techniques are widely used to coral reef restoration in the southeastern Asia by offering the substrate for transplanted corals and the settlement of coral larvae. However, most countries in this area rarely have typhoons, where ARs are usually made from PVC tubes, metal shelf, tubular concretes, or reef balls (Edwards, 2010). AR techniques used in some regions showed good restoration performance. Few studies focused on the influence of typhoon to coral reef restoration (Shaish et al., 2010a). Therefore, efforts are needed to evaluate the impact of these storms on reef recovery (Shaish et al., 2010a; Wells et al., 2010).
Coral restoration in China begun in 1990s (Chen et al., 1995). The majority of coral reef restoration was completed in the past 30 years in the waters around the SCS, Sanya, the Daya Bay, and the Weizhou Island, despite that few papers were published on this topic (Chen et al., 1995; Zhang et al., 2016). Surprisingly, few studies have tried to monitor the long-term recovery of coral reefs. It is difficult to assess the outcome of the restoration in China where typhoons are common (Wu et al., 2007; Liu et al., 2009). These data are useful to improve restoration strategies.
This study is a long-term monitoring project to evaluate the outcome of coral restoration for an impaired coral reef in China. We carried out a 4-year monitoring survey on the growth of three transplanted Acropora corals and naturally-attached Pocillopora damicornis on ARs from October 2014 to September 2018. During this period, several catastrophic typhoons struck this region. Specifically, the objectives of the paper were to: 1) monitor the longterm recovery of coral reefs to guide the coral reef in China; 2) assess if AR techniques widely used in southeastern countries are suitable to China where frequently suffers from typhoons.
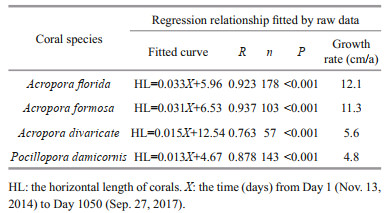
2 MATERIAL AND METHOD 2.1 Study siteThe Wuzhizhou Island is located in the northeast of Sanya City in Hainan Province (Fig. 1), which has an area of 1.48 km2 with the coastline of 5.70 km long. It is 1.5 km long from east to west, and 1.1 km wide from south to north. There were abundant coral reefs before 2007 dominated by branching Acropora species, such as Acropora hyacithus, A. formosa, and A. florida in shallow waters around the island (Wu et al., 2012). However, in the past decades, the coral coverage in the Wuzhizhou showed a significant reduction from around 80% in 2007 to 21.5% in 2019 largely due to over-exploitation. The number of people visiting the Wuzhizhou Island kept increasing year by year, with an annual growth rate of more than 25%, from 1.02 million in 2012 to 3 million in 2017. The number of visitors to the diving program also increased from 119 000 in 2012 to 400 000 in 2017, with an annual increase of nearly 28%. The increasing trend of small-scale changes was negatively correlated to the number of people on the island. The dominant coral species shifted from an Acorpora-dominated communities before 2007 to one dominated by multiple scleractinian corals, such as A. hyacinthus, Montipora foliosa, Galaxea fascicularis, Porites lutea, and Pocillopora meandrina.
|
| Fig.1 Restoration site in the Wuzhizhou Island, Sanya City, Hainan Province, China Black circle represents the restoration areas. |
Considering continuous degradation of coral reefs in the Island, the coral restoration is gaining increasing attraction by local governments and stakeholders. The restoration was carried out in 2014 in the north of the Wuzhizhou Island (Fig. 1). The shallow water (3 m) is the diving area for tourism in winter and spring. The depth of the restoration area is about 7 m, where Acropora communities have ever distributed but there are only small patches of live corals. On the contrary, massive dead Acropora skeleton were found. However, the water quality here is suitable for scleractinian corals (Supplementary Table S1). A few juvenile scleractinian corals (< 1 year old) grow on dead coral skeleton, but have high mortality rates under strong current due to a lack of fixed substrates.
2.2 Descriptions of artificial reefsArtificial reefs (ARs) were employed to stabilize the substrate and attract the settlement of coral larvae. The structure of the ARs is showed in Fig. 2a, which has been authorized by Patent Office of the People's Republic of China (authorization number: ZL 201610013044.7). The ARs with a two-layer structure (Fig. 2b) were mainly composed of supporting shelves made of stainless steel and magnesium (Mg)- aluminum (Al) alloy mesh covering the supporting shelf. The site contained a square mesh grid with 5 cm edge lengths and Mg content of 2%–5% in the alloy.

|
| Fig.2 Structure of artificial reefs a. the structural design of artificial reefs; b. the picture of real product; c. the artificial reefs deployed in the field; d. example for measuring horizontal length of corals (Pocillopora damicornis). |
The restoration area (RA) was about 5 hectares. About 100 ARs were deployed into the restoration area. ARs were fixed by divers and tightly packed together in order to enhance the resistance from typhoons and strong currents (Fig. 2c). In this study, three Acropora species including A. florida, A. formosa, and A. divaricata were transplanted on the ARs (Fig. 3), which were widely distributed in Sanya in the past. The corals collected around the Weizhou Island are about 6 cm in length for A. florida and A. formosa, and about 10 cm in length for A. divaricata (seen figures in Section 3).

|
| Fig.3 Scleractinian corals on artificial reefs Transplanted (a. Acropora florida; b. A. formosa; c. A. divaricate) and naturally-attached corals (d. Pocillopora damicornis) on artificial reefs. |
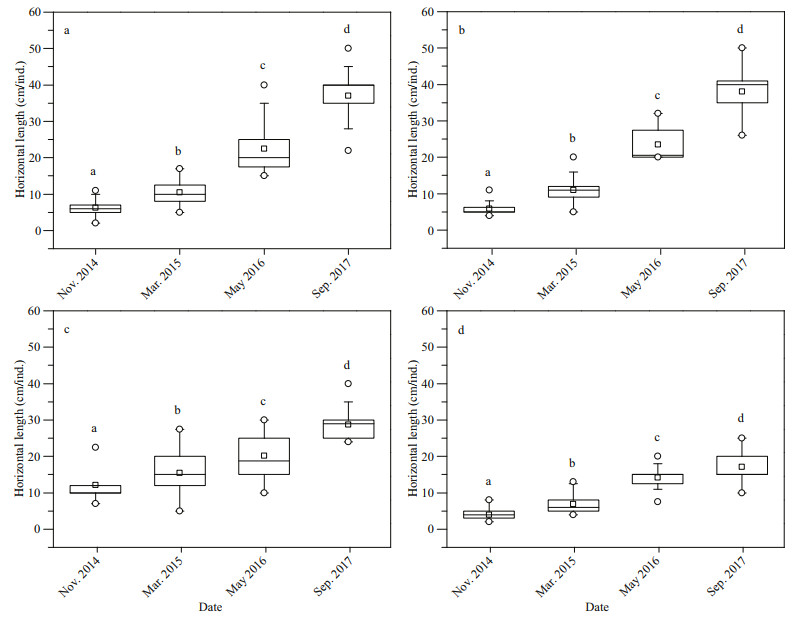
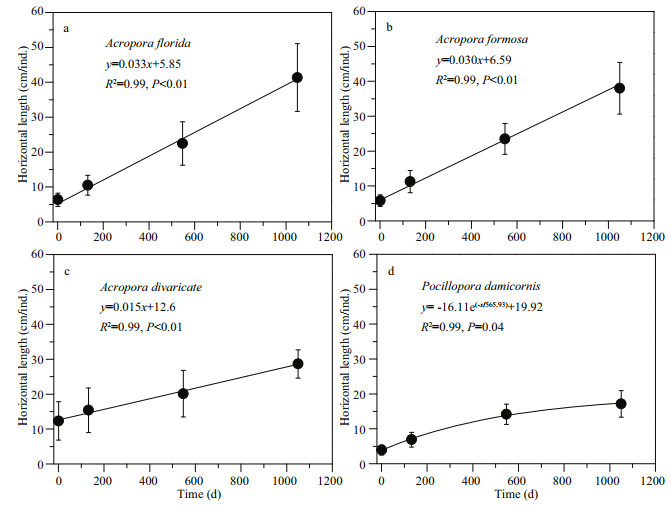
Growth of corals on ARs were calculated according to the changes of horizontal length (HL) of corals (Fig. 2d). Corals were fixed by nylon cable ties on the surface of the ARs. The HL of corals on ARs was calculated by comparing the difference between HL and the side length of aluminium-alloy mesh (5 cm, Fig. 2b & d). The HL information was recorded by videos and photos. The linear regression or power function model was fitted for four coral species. Here, the raw data were used to fit the linear regression and the slope was the growth rate of corals, and the average values of HL for each coral species were used to fit the regression. The linear functions were fitted for three Acropora species, while power function was fitted for P. damicornis.
Ten ARs were randomly chosen every year to semi-qualitatively evaluate the performance of the type of ARs in coral reef restoration. Coral cover in the ARs was estimated by the formula: CC=Scoral/SARs×100, where Scoral represents the area occupied by live corals on ARs, and SARs represents the surface area of ARs. The number of four corals on ARs (A. florida, A. formosa, A. divaricate, and P. damicornis) was counted to calculate occurrence frequency and mortality of corals on ARs.
Additionally, to quantitatively compare the restoration performance in restoration area (RA), the areas without ARs nearby RA (109°46′02.5″E, 18°19′06.9″N) and on the west side of the island (109°45′29.9″E, 18°18′28.8″N) was chosen as the controls, and the coverage of live corals in control area and RA was surveyed based on the method of line intercept transect (LIT) from 2014 to 2018. The detailed procedure of LIT was described in methods for ecological monitoring of coral reefs by Hill and Wilkinson (2004).
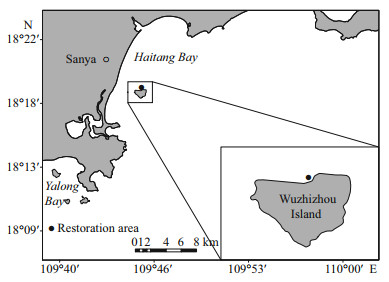
2.4 Statistical analysisTukey test of one-way ANOVA was employed to compare the difference of the coral HL over the four periods (November 2014, March 2015, May 2016, and September 2017). The significant difference is P < 0.05.
3 RESULT 3.1 Growth of corals on artificial reefsThe growth of corals on ARs is shown in Figs. 4 & 5. Four reef corals showed significant growth (Fig. 4, Supplementary Table S2, P < 0.01), with a mean HL increasing from 6.3±1.9 cm/ind. in November 2014 to 41.4±9.7 cm/ind. in September 2017 for A. florida; from 5.8±1.6 cm/ind. to 38.0±7.4 cm/ind. for A. formosa; from 12.3±5.5 cm/ind. to 28.7±4.1 cm/ind. for A. divaricate; and from 3.9±1.4 cm/ind. to 17.2±3.8 cm/ind. for P. damicornis.
|
| Fig.4 Variation of horizontal length of transplanted scleractinian corals a. Acropora florida; b. A. formosa; and c. A. divaricate) and corals naturally attached in artificial reefs (d. Pocillopora damicornis) with the date. The boxes span the 75th (top) to the 25th (bottom) percentiles with the central lines indicating the 50th percentile. The vertical lines outside the boxes indicate the maximum (top) and minimum (bottom) percentiles. The letters above the bar represents the differences in horizontal length of corals among dates. The significant difference is P<0.05. |
|
| Fig.5 Growth pattern of scleractinian corals transplanted and naturally attached on artificial reefs Error bar stands for the standard error (SE). a. Acropora florida; b. A. formosa; c. A. divaricate; d. Pocillopora damicornis. |
Our results show that the four coral species had different growth rates (Table 1). The highest was for A. florida (12.1 cm/a) and A. formosa (11.3 cm/a), followed by A. formosa (5.6 cm/a) and P. damicornis (4.8 cm/a). Linear growth patterns were observed in the three Acropora species, but power-exponential growth was observed for P. damicornis (Fig. 5 and Table 1) with growth rates from 6.7 cm/a in October 2014 to 3.2 cm/a in September 2017. The slopes, which are the Acropora growth rates, fitted by raw data and basically agree with those based on the data after average processing (Table 1, Fig. 5).
The corals that grew on ARs in Wuzhizhou showed good performance. The Acropora corals covered all over the surface in some unbroken ARs (Fig. 6c–d) after two years with a variation of coral cover in some ARs increased from less than 5% in November 2014 to approximately 100% in September 2017 (Fig. 6a–d). P. darmicornis grew well as well, but being far smaller than Acropora species (Figs. 4 & 5). The number of P. darmicornis colonies declined (Fig. 4d). Continued monitoring the abundance of four corals on ARs showed that occurrence of P. damicornis on ARs reduced quickly in 2016, with occurrence from 34% in 2014 to 17% in 2017 (Fig. 7), indicating the reduced number of P. damicornis with intense intercompetition.

|
| Fig.6 Performance of coral restoration from October in 2014 to September in 2017 a. November 2014; b. March 2015; c. May 2016; d. September 2017. P.d: Pocillopora damicornis (red arrow); A.fl: Acropora florida (black arrow); A.fo: Acropora formosa (purple arrow); M.sp: Montipora sp. (blue arrow). |

|
| Fig.7 Temporal variation of occurrence for four corals (Acropora florida, A. formosa, A. divaricate and Pocillopora damicornis) on artificial reefs n: the total number of corals recorded on artificial reefs. |
Compared with two control areas where the coverage of coral reefs slightly decreased from 2014 to 2018 (Table 2), the mean coral coverage on ARs in RA sharply increased from < 2% in 2014 to 48.6% in 2017, and then sharply declined to less than 10% in 2018 due to the attack of typhoon (Table 2). Typhoons were a severe problem for coral reef restoration at the Wuzhizhou Island. Many ARs collapsed, overturned, and were even completely destroyed by typhoons in 2018, resulting in massive death of many colonies (Fig. 8a); Mortality of corals on ARs sharply increased (Table 2), though some strong coral individuals were still thriving (Fig. 8b–d).

|

|
| Fig.8 Artificial reefs destroyed by typhoons in 2018 in the Wuzhizhou Islands |
Acropora and P. damicornis had different growth rates and pattern. Staghorn Acropora species had the highest growth rates, and annual growth rates for A. formosa reaching approximately 11 cm. It fell in between 4.7–13.8 cm as previously reported (Supplementary Table S3, Charuchinda and Hylleberg 1984; Okubo et al., 2005; Shaish et al., 2008). It was also close to that grown in land-based aquaculture systems (10.8 cm/a, Zheng et al., unpublished data), but higher than that (6.4 cm/a) in a suspended nursery near Silaqui Island, Bolinao, Philippines (Shaish et al., 2008) and that (about 8 cm/a) at a fringing reef in front of Phuket Marine Biological Center in the Andaman Sea (Charuchinda and Hylleberg, 1984). P. damicornis growth rates were similar with previous studies (Supplementary Table S3, 3.4–6.5 cm/a) by Shaish et al. (2008) and Tortolero-Langarica et al.(2014), but was lower than that (6.7–7.2 cm/a) for in situ coral farming in a highly degraded coral reef in Singapore (Bongiorni et al., 2011). The regional differences in coral growth may be explained by varied environmental conditions, such as temperature, pH, nutrients, and suspended particle matter as potential food sources. These variables affected the growth of corals both in laboratory and in situ environments (Harriott, 1998; Fabricius, 2005; Tortolero-Langarica et al., 2014; Cohen et al., 2016; Saptarini et al., 2017; Zheng et al., 2018, 2019). These environment factors potentially limiting calcification were likely to vary greatly between locations (Saptarini et al., 2017). Additionally, some coral species have been found to have different coral genotype and clade of symbiotic zooxanthellae (Ayre et al., 1991; Gutner-Hoch and Fine, 2011), which also determined the coral growth (Gold and Palumbi, 2018).
Four-year monitoring of coral growth on ARs showed that all Acropora species had linear growth pattern. Theoretically, branching corals exhibit linear extension when there is no or low space competition with other colonies or organisms: this has been widely observed in Acropora species or other branching corals, such as Montipora digitata (Charuchinda and Hylleberg, 1984; Shaish et al., 2008; TortoleroLangarica et al., 2014; Saptarini et al., 2017). However, seasonal variations in growth rates were observed for conspecifics in previous studies, perhaps due to seasonal fluctuations of temperature (Shaish et al., 2008; Tortolero-Langarica et al., 2014; Saptarini et al., 2017), which would strongly affect the growth of corals in situ (Saptarini et al., 2017). In our study, we monitored coral horizontal growth for more than one year; therefore, the growth rates calculated over such a long-time span would eliminate the temporal variation of growth, perhaps affected by different environmental conditions.
Pocillopora damicornis gradually decreased in growth rates with time in our study. This may be due to the competition among corals. Interspecific competition would reduce the growth of P. damicornis (Tanner, 1997), due to more energetic cost caused by fighting among corals (Romano, 1990). Bongiorni et al. (2011) also reported linear growth for conspecific coral in 420 days in coral farming (R2=0.996 in St. John Island and R2=1 in Lazarus-Fish Farm) when the size of P. damicornis colonies were small. Here, P. damicornis growth on the ARs should not be limited by space in the first year; thus, the coral may grow linearly with time in a short time when growth rates were expressed as monthly growth rates, as reported in P. damicornis and two other Pocillopora species in Islas Marietas National Park, Nayarit, Mexico (Tortolero-Langarica et al., 2014). However, fierce competition for space among corals could slow down the P. damicornis growth and fitness when free space for colonization was limited for corals on ARs.
4.2 Interspecific competition among transplanted acroporids and P. damicornisHere, we found that the larvae of P. damicornis quickly settled down on ARs compared to other acroporids, which could be attributable to its brooding reproductive mode. P. damicornis can periodically produce larvae every month (Richmond, 1987) and asexually breed from isolated colonies via parthenogenesis (Ayre and Miller, 2004). Therefore, P. damicornis has the strong colonization ability (Tanner et al., 1994), allowing for successful reproduction at low population densities. However, associated with the quick growth of acroporids after the transplantation, a strong competition between acroporids and P. damicornis was observed and P. damicornis was seemingly the loser of competition, which was also revealed by the dramatically reduced number and gradually slow growth of P. damicornis colonies over time (Figs. 5d & 7). It might be related to the different life cycle strategies adopted by both corals. In reef corals, the growth rate is an important mechanism of space competition (Lang and Chornesky, 1990). Most acroporids adopted a competitively dominant life-history strategy by fast growth (Darling et al., 2012). They can create large, arborescent colonies canopies to shade out competitors for space and light as in our case (Fig. 6), making them effective competitors in ideal environments (Baird and Hughes, 2000). Differently, P. damicornis adopts weedy or ruderal strategies, which theoretically reproduces faster and survive in low competition (Darling et al., 2012). P. damicornis is usually an opportunistic or pioneer species to colonize recently disturbed habitats (Tanner et al., 1994; Grime and Pierce, 2012). These early colonists persist when free space is present (Tanner and Hughes, 1994). It may be a response of reproductive mode of corals to the trade-offbetween colonization ability and competition, as findings in our case that a number of P. damicornis naturally attached on ARs survived and grew well in the first years after ARs were deployed (Table 2, Figs. 6b & 7).
Moreover, interspecific competition incurred a decrease in growth and fitness for weak corals (Romano, 1990; Tanner, 1997), and it might further enhance the competition capacity of acorporids on ARs. This agreed with the succession of reef-coral communities in a coral reef reaching a climax assemblage, that is, slow-growing coral species lose the competition and fast-growing Acropora corals dominated in some mature coral reefs (Tanner et al., 1994). It is common to observe that on the shallow fore-reef slopes of the Great Barrier Reef, which are dominated by Acropora and other tabular corals that have crowded out most other species in other morphologies (Done, 1982). In our study, a mass of P. damicornis attached on ARs in March 2015 but dramatically decreased over time (Fig. 7), which should be the outcome of interspecific competition among fast-growing transplanted acroporids and P. damicornis.
4.3 Performance of coral reef restorationIn the Wuzhizhou Island, the ARs combined with coral transplantation quickly increased coverage of corals in RA within two years and greatly increased AR complexity (Fig. 6), demonstrating excellent performance of coral recovery. It was firstly attributed to good water quality and high coral recruitment in waters around the Wuzhizhou Island (Supplementary Table S1; Wu et al., 2012; Li et al., 2019). In spite of high suspended particle matter (SPM, 7–19.2 mg/L) reported in a previous study (Wu et al., 2012), SPM seemingly varied temporally (only 4.2–6.0 mg/L in our case), and remained below the stress threshold of hard corals (10 mg/L, Rogers, 1990; Li et al., 2013; Jones et al., 2016). Except for the ammonium (0.59– 2.72 μmol/L), the inorganic nutrient concentrations (Supplementary Table S1) is close to the levels in the pristine area growing coral reefs (0.2–0.5 μmol/L ammonium, 0.1–0.5 μmol/L nitrate, and < 0.3 μmol/L phosphate, Furnas 1991). The concentration of the ammonium is beyond the range, but still suitable for coral survival and growth (Bucher and Harrison, 2000; Bongiorni et al., 2003; Browne et al., 2015; Zheng et al., unpublished data). The DIN concentration also approached the values in Hongkong water, where the highest coral species richness and coral cover occurred at DIN < 2 μmol/L (Duprey et al. 2016). This ideal environment benefits the colonization of some weedy corals as pioneer species, such as P. damicornis, and crusting Montipora species on ARs (Fig. 6b). It is also suitable for the growth of competitive corals, such as Acropora species. In spite of the strong competition between transplanted acroporids and P. damicornis, the results suggested that coral restoration was feasible in this island by stabilizing substrates or offering artificial substrates for the transplanted corals and for the settlement of coral larvae.
However, typhoons brought severe threat to coral reef recovery in the Wuzhizhou Island. Catastrophic typhoons generally cause mechanical damages to coral communities (Rogers et al., 1982; Herbeck et al., 2011; Hongo et al., 2012; Li et al., 2013; White et al., 2017). They overturned massive corals, dislodged tabular and branching corals, and led to reef-scale failure of coral settlement (Highsmith et al., 1980; Doropoulos et al., 2014). Some southeastern countries rarely have typhoons, where ARs are usually made from PVC tubes, small tubular concretes, or reef balls (Shaish et al., 2008; Edwards, 2010; Damayanti et al., 2011; Xin et al., 2016). This may only be applicable in naturally protected lagoons or deeper waters (Ogg and Koslow, 1978; Hongo et al., 2012), but not in shallow coral reefs that are distributed in relatively open waters with potential typhoon disturbance. Several typhoons directly hit Hainan every year (Li et al., 2013). Catastrophic typhoon Son-Tinh crossed Hainan in 2018, and overturned and severely damaged many ARs deployed in Wuzhizhou waters. It caused severe damage to ARs (Fig. 7) and high mortality of corals on ARs (Table 2). Although the ARs made of metal frames in our study attracted the larvae settlements and stimulated coral growth (Li et al., 2019), it was also not strong enough and was damaged by strong typhoons.
Besides mechanical damages to ARs structure (Rogers et al., 1982), SPM and sedimentation resulting from the typhoons (Herbeck et al., 2011; Li et al., 2013) are also detrimental to many hard corals (Jones et al., 2016). SPM more than ca. 10 mg/L and sedimentation ca. 10 mg/(cm2∙d) can produce a severe physiological stress to hard corals (Rogers, 1990; Li et al., 2013), even resulting in the coral bleaching, suffocation and death, especially for branching Acropora corals with small polyps (Jones et al., 2016). Although the concentrations of SPM (4.2– 6.0 mg/L, Supplementary Table S1) did not exceed the stress threshold for many hard corals sensitive to environment stress (10 mg/L, Rogers, 1990; Li et al., 2013), typhoon dramatically increases SPM concentrations several to even more than a dozen times during and after the typhoon (Wang et al., 2009; Herbeck et al., 2011; Li et al., 2013). It could far exceed the threshold (He et al., 2013; Li and Li, 2016), resulting in the death of many transplanted Acropora corals on ARs after a strong disturbance (Fig. 8).
In conclusion, this is the first report on the longterm monitoring and performance of coral restoration in China, although coral reef restoration has been ongoing since 1990s (Chen et al., 1995). Our results show that the shallow water around the Wuzhizhou Island was suitable for coral restoration via artificial reefs and coral transplantation. However, we must consider the potential impacts of typhoons. Future ARs in waters frequently affected by typhoon must adopt more sturdy and environmental-friendly materials. They should avoid secondary pollution and to resist strong current from typhoons. Therefore, how to combine the more concrete materials and active metal material should be a future feasible direction for coral reef restoration in the coastal waters of China
5 DATA AVAILABILITY STATEMENTAll data generated and/or analyzed during this study are available from the corresponding author upon request.
Electronic supplementary materialSupplementary material (Supplementary Tables S1–S3) is available in the online version of this article at https://doi.org/10.1007/s00343-020-9253-z.
Ayre D J, Veron J E N, Dufty S L. 1991. The corals Acropora palifera and Acropora cuneata are genetically and ecologically distinct. Coral Reefs, 10(1): 13-18.
DOI:10.1007/BF00301901 |
Baird A H, Pratchett M S, Hoey A S, Herdiana Y, Campbell S J. 2013. Acanthaster planci is a major cause of coral mortality in Indonesia. Coral Reefs, 32(3): 803-812.
DOI:10.1007/s00338-013-1025-1 |
Baird A H, Hughes T P. 2000. Competitive dominance by tabular corals:an experimental analysis of recruitment and survival of understorey assemblages. Journal of Experimental Marine Biology and Ecology, 251(1): 117-132.
DOI:10.1016/S0022-0981(00)00209-4 |
Bongiorni L, Giovanelli D, Rinkevich B, Pusceddu A, Chou L M, Danovaro R. 2011. First step in the restoration of a highly degraded coral reef (Singapore) by in situ coral intensive farming. Aquaculture, 322-323: 191-200.
DOI:10.1016/j.aquaculture.2011.09.024 |
Bongiorni L, Shafir S, Angel D, Rinkevich B. 2003. Survival, growth and gonad development of two hermatypic corals subjected to in situ fish-farm nutrient enrichment. Marine Ecology Progress Series, 253: 137-144.
DOI:10.3354/meps253137 |
Browne N K, Tay J K L, Low J, Larson O, Todd P A. 2015. Fluctuations in coral health of four common inshore reef corals in response to seasonal and anthropogenic changes in water quality. Marine Environmental Research, 105: 39-52.
DOI:10.1016/j.marenvres.2015.02.002 |
Bucher D J, Harrison P L. 2002. Growth response of the reef coral Acropora longicyathus to elevated inorganic nutrients: do responses to nutrients vary among coral taxa? In: Proceedings of the 9th International Coral Reef Symposium. State Ministry for the Environment, Bali.p.443-448.
|
Charuchinda M, Hylleberg J. 1984. Skeletal extension of Acropora formosa at a fringing reef in the Andaman Sea. Coral Reefs, 3(4): 215-219.
DOI:10.1007/BF00288257 |
Chen G, Xiong S L, Xie J N, Zou X P, Cui Y C. 1995. A study on the transplantation of reef-building corals in Sanya waters, Hainan Province. Tropic Oceanology, 14(3): 51-57.
(in Chinese with English abstract) |
Cohen I, Dubinsky Z, Erez J. 2016. Light enhanced calcification in hermatypic corals:new insights from light spectral responses. Frontiers in Marine Science, 2: 122.
DOI:10.3389/fmars.2015.00122 |
Damayanti L P A, Ahyadi H, Candri D A, Sabil A. 2011. Growth rate of Acropora formosa and Montipora digitata transplanted on biorock in Gili Trawangan. Journal of Indonesia Coral Reefs, 1(2): 114-119.
|
Darling E S, Alvarez-Filip L, Oliver T A, McClanahan T R, Côté I M. 2012. Evaluating life-history strategies of reef corals from species traits. Ecology Letters, 15(12): 1 378-1 386.
DOI:10.1111/j.1461-0248.2012.01861.x |
Done T J. 1982. Patterns in the distribution of coral communities across the central Great Barrier Reef. Coral Reefs, 1(2): 95-107.
DOI:10.1007/BF00301691 |
Doropoulos C, Roff G, Zupan M, Nestor V, Isechal A L, Mumby P J. 2014. Reef-scale failure of coral settlement following typhoon disturbance and macroalgal bloom in Palau, Western Pacific. Coral Reefs, 33(3): 613-623.
DOI:10.1007/s00338-014-1149-y |
Duprey N N, Moriaki Y, David M. 2016. Reefs of tomorrow:eutrophication reduces coral biodiversity in an urbanized seascape. Global Change Biology, 22(11): 3 550-3 565.
DOI:10.1111/gcb.13432 |
Edwards A J, Clark S. 1999. Coral Transplantation:a useful management tool or misguided meddling?. Marine Pollution Bulletin, 37(8-12): 474-487.
DOI:10.1016/S0025-326X(99)00145-9 |
Edwards A J. 2010. Reef rehabilitation manual. Coral reef targeted research & capacity. St Lucia, Australia.
|
Edwards A J, Gomez E D. 2007. Reef restoration concepts & guidelines: making sensible management choices in the face of uncertainty. Capacity Building for Management Programme. Coral Reef Targeted Research: St Lucia, Australia.
|
Fabricius K E. 2005. Effects of terrestrial runoff on the ecology of corals and coral reefs:review and synthesis. Marine Pollution Bulletin, 50(2): 125-146.
DOI:10.1016/j.marpolbul.2004.11.028 |
Furnas M J. 1991. The nutrient status of Great Barrier Reef waters. In: Yellowlees D ed. Land Uses, Patterns and Nutrient Loading of the Great Barrier Reef Region. James Cook University, Townsville. p.162-179.
|
Gold Z, Palumbi S R. 2018. Long-term growth rates and effects of bleaching in Acropora hyacinthus. Coral Reefs, 37(1): 267-277.
DOI:10.1007/s00338-018-1656-3 |
Grime J P, Pierce S. 2012. The Evolutionary Strategies that Shape Ecosystems, Wiley-Blackwell, Oxford, UK.
|
Gutner-Hoch E, Fine M. 2011. Genotypic diversity and distribution of Ostreobium quekettii within scleractinian corals. Coral Reefs, 30(3): 643-650.
DOI:10.1007/s00338-011-0750-6 |
Harriott V J. 1998. Growth of the staghorn coral Acropora formosa at Houtman Abrolhos, Western Australia. Marine Biology, 132(2): 319-325.
DOI:10.1007/s002270050397 |
He X Q, Bai Y, Pan D L, Huang N L, Dong X, Chen J S, Chen C T A, Cui Q F. 2013. Using geostationary satellite ocean color data to map the diurnal dynamics of suspended particulate matter in coastal waters. Remote Sensing of Environment, 133: 225-239.
DOI:10.1016/j.rse.2013.01.023 |
Herbeck L S, Unger D, Krumme U, Liu S M, Jennerjahn T C. 2011. Typhoon-induced precipitation impact on nutrient and suspended matter dynamics of a tropical estuary affected by human activities in Hainan, China. Estuarine, Coastal and Shelf Science, 93(4): 375-388.
DOI:10.1016/j.ecss.2011.05.004 |
Highsmith R C, Riggs A C, D'Antonio C M. 1980. Survival of hurricane-generated coral fragments and a disturbance model of reef calcification/growth rates. Oecologia, 46(3): 322-329.
DOI:10.1007/BF00346259 |
Hill J J, Wilkinson C R. 2004. Methods for ecological monitoring of coral reefs: a resource for managers.Australian Institute of Marine Science, Australian.
|
Hongo C, Kawamata H, Goto K. 2012. Catastrophic impact of typhoon waves on coral communities in the Ryukyu Islands under global warming. Journal of Geophysical Research:Biogeosciences, 117(G2): G02029.
DOI:10.1029/2011JG001902 |
Hughes T P, Huang H, Young M A L. 2013. The wicked problem of China's disappearing coral reefs. Conservation Biology, 27(2): 261-269.
DOI:10.1111/j.1523-1739.2012.01957.x |
Jones R, Bessell-Browne P, Fisher R, Klonowski W, Slivkoff M. 2016. Assessing the impacts of sediments from dredging on corals. Marine Pollution Bulletin, 102(1): 9-29.
DOI:10.1016/j.marpolbul.2015.10.049 |
Kheawwongjan A, Kim D S. 2012. Present status and prospects of artificial reefs in Thailand. Ocean & Coastal Management, 57: 21-33.
DOI:10.1016/j.ocecoaman.2011.11.001 |
Lang J C, Chornesky E A. 1990. Competition between scleractinian reef corals-a review of mechanisms and effects. In: Dubinsky Z ed. Ecosystems of the World.Elsevier, Amsterdam. p.209-252.
|
Li X B, Huang H, Lian J S, Liu S, Huang L M, Yang J H. 2013. Spatial and temporal variations in sediment accumulation and their impacts on coral communities in the Sanya Coral Reef Reserve, Hainan, China. Deep Sea Research Part Ⅱ:Topical Studies in Oceanography, 96: 88-96.
DOI:10.1016/j.dsr2.2013.04.015 |
Li Y D, Li X F. 2016. Remote sensing observations and numerical studies of a super typhoon-induced suspended sediment concentration variation in the East China Sea. Ocean Modelling, 104: 187-202.
DOI:10.1016/j.ocemod.2016.06.010 |
Li X B, Li Y C, Xu Q. 2019. Status. ecological restoration and protection strategies of Wuzhizhou Island in Sanya.Ocean Press, Beijing.
|
Liu D F, Pang L, Xie B T. 2009. Typhoon disaster in China:prediction, prevention, and mitigation. Natural Hazards, 49(3): 421-436.
DOI:10.1007/s11069-008-9262-2 |
Lourey M J, Ryan D A J, Miller I R. 2000. Rates of decline and recovery of coral cover on reefs impacted by, recovering from and unaffected by crown-of-thorns starfish Acanthaster planci:a regional perspective of the Great Barrier Reef. Marine Ecology Progress Series, 196: 179-186.
DOI:10.3354/meps196179 |
Moberg F, Folke C. 1999. Ecological goods and services of coral reef ecosystems. Ecological Economics, 29(2): 215-233.
DOI:10.1016/S0921-8009(99)00009-9 |
Muko S, Iwasa Y. 2011. Long-term effect of coral transplantation:restoration goals and the choice of species. Journal of Theoretical Biology, 280(1): 127-138.
DOI:10.1016/j.jtbi.2011.04.012 |
Ng C S L, Toh T C, Chou L M. 2016. Coral restoration in Singapore's sediment-challenged sea. Regional Studies in Marine Science, 8: 422-429.
DOI:10.1016/j.rsma.2016.05.005 |
Ogg J G, Koslow J A. 1978. The impact of typhoon Pamela(1976) on Guam's coral reefs and beaches. Pacific Science, 32(2): 105-118.
|
Okubo N, Taniguchi H, Motokawa T. 2005. Successful methods for transplanting fragments of Acropora formosa and Acropora hyacinthus. Coral Reefs, 24(2): 333-342.
DOI:10.1007/s00338-005-0496-0 |
Omori M. 2011. Degradation and restoration of coral reefs:experience in Okinawa, Japan. Marine Biology Research, 7(1): 3-12.
DOI:10.1080/17451001003642317 |
Pandolfi J M, Bradbury R H, Sala E, Hughes T P, Bjorndal K A, Cooke R G, McArdle D, McClenachan L, Newman M J H, Paredes G, Warner R R, Jackson J B C. 2003. Global trajectories of the long-term decline of coral reef ecosystems. Science, 301(5635): 955-958.
DOI:10.1126/science.1085706 |
Richmond R H. 1987. Energetic relationships and biogeographical differences among fecundity, growth and reproduction in the reef coral Pocillopora damicornis. Bulletin of Marine Science, 41(2): 594-604.
|
Richmond R H, Golbuu Y, Shelton Ⅲ A J. 2019. Successful management of coral reef-watershed networks. In: Wolanski E, Day J W, Elliott M, Ramachandran R eds.Coasts and Estuaries. Elsevier, Amsterdam. p.445-459, https://doi.org/10.1016/B978-0-12-814003-1.00026-5.
|
Rinkevich B. 2005. Conservation of coral reefs through active restoration measures:recent approaches and last decade progress. Environmental Science & Technology, 39(12): 4 333-4 342.
DOI:10.1021/es0482583 |
Rogers C S, Suchanek T H, Pecora F A. 1982. Effects of hurricanes David and Frederic (1979) on shallow Acropora palmata reef communities:St. Croix, U.S.Virgin Islands. Bulletin of Marine Science, 32(2): 532-548.
|
Rogers C S. 1990. Responses of coral reefs and reef organisms to sedimentation. Marine Ecology Progress Series, 62: 185-202.
DOI:10.3354/meps062185 |
Romano S L. 1990. Long-term effects of interspecific aggression on growth of the reef-building corals Cyphastrea ocellina (Dana) and Pocillopora damicomis(Linnaeus). Journal of Experimental Marine Biology and Ecology, 140(1-2): 135-146.
DOI:10.1016/0022-0981(90)90087-S |
Saptarini D, Rumengan, Rumengan I F M. 2017. Growth rate of two species branched Acropora in the area of discharged power plant cooling water. Indian Journal of Geo Marine Sciences, 46(7): 1 327-1 332.
|
Shaish L, Levy G, Gomez E, Rinkevich B. 2008. Fixed and suspended coral nurseries in the Philippines:establishing the first step in the "gardening concept" of reef restoration. Journal of Experimental Marine Biology and Ecology, 358(1): 86-97.
DOI:10.1016/j.jembe.2008.01.024 |
Shaish L, Levy G, Katzir G, Rinkevich B. 2010a. Coral reef restoration (Bolinao, Philippines) in the face of frequent natural catastrophes. Restoration Ecology, 18(3): 285-299.
DOI:10.1111/j.1526-100X.2009.00647.x |
Shaish L, Levy G, Katzir G, Rinkevich B. 2010b. Employing a highly fragmented, weedy coral species in reef restoration. Ecological Engineering, 36(10): 1 424-1 432.
DOI:10.1016/j.ecoleng.2010.06.022 |
Tanner J E, Hughes T P, Connell J H. 1994. Species coexistence, keystone species, and succession:a sensitivity analysis. Ecology, 75(8): 2 204-2 219.
DOI:10.2307/1940877 |
Tanner J E. 1997. Interspecific competition reduces fitness in scleractinian corals. Journal of Experimental Marine Biology and Ecology, 214(1-2): 19-34.
DOI:10.1016/S0022-0981(97)00024-5 |
Tortolero-Langarica J J A, Cupul-Magaña A L, RodríguezTroncoso A P. 2014. Restoration of a degraded coral reef using a natural remediation process:a case study from a Central Mexican Pacific National Park. Ocean & Coastal Management, 96: 12-19.
DOI:10.1016/j.ocecoaman.2014.04.020 |
Tortolero-Langarica J J A, Rodríguez-Troncoso A P, CupulMagaña A L, Alarcón-Ortega L C, Santiago-Valentín J D. 2019. Accelerated recovery of calcium carbonate production in coral reefs using low-tech ecological restoration. Ecological Engineering, 128: 89-97.
DOI:10.1016/j.ecoleng.2019.01.002 |
Wang A J, Gao S, Chen J, Li D Y. 2009. Sediment dynamic responses of coastal salt marsh to typhoon "KAEMI" in Quanzhou Bay, Fujian Province, China. Chinese Science Bulletin, 54(1): 120-130.
DOI:10.1007/s11434-008-0365-7 |
Wells L, Perez F, Hibbert M, Clerveaux L, Johnson J, Goreau T J. 2010. Effect of severe hurricanes on Biorock coral reef restoration projects in Grand Turk, Turks and Caicos Islands. Revista de Biología Tropical, 58(S3): 141-149.
|
White K N, Weinstein D K, Ohara T, Denis V, Montenegro J, Reimer J D. 2017. Shifting communities after typhoon damage on an upper mesophotic reef in Okinawa, Japan. PeerJ, 5: e3573.
DOI:10.7717/peerj.3573 |
Wu S H, Zhang W J. 2012. Current status, crisis and conservation of coral reef ecosystems in China. Proceedings of the International Academy of Ecology and Environmental Sciences, 2(1): 1-11.
|
Wu Y J, Wu S G, Zhai P M. 2007. The impact of tropical cyclones on Hainan Island's extreme and total precipitation. International Journal of Climatology, 27(8): 1 059-1 064.
DOI:10.1002/joc.1464 |
Wu Z J, Wang D R, Ye C X, Li Y C, Chen M, Chen C H. 2012. Variation tendency and analysis of cause of coral in Sanya. Marine Environmental Science, 31(5): 682-685.
(in Chinese with English abstract) |
Xin L H, Adzis K A A, Hyde J, Cob Z C. 2016. Growth performance of Acropora formosa in natural reefs and coral nurseries for reef restoration. Aquaculture, Aquarium, Conservation & Legislation, 9(5): 1 090-1 100.
|
Yeemin T, Sutthacheep M, Pettongma R. 2006. Coral reef restoration projects in Thailand. Ocean & Coastal Management, 49(9-10): 562-575.
DOI:10.1016/j.ocecoaman.2006.06.002 |
Young C N, Schopmeyer S A, Lirman D. 2012. A review of reef restoration and coral propagation using the threatened genus Acropora in the Caribbean and Western Atlantic. Bulletin of Marine Science, 88(4): 1 075-1 098.
DOI:10.5343/bms.2011.1143 |
Zainul A. 2019. Management options for restoring artificial coral reefs in Indonesia:strengthening in institutional approach. IOP Conference Series:Earth and Environmental Science, 236: 012049.
DOI:10.1088/1755-1315/236/1/012049 |
Zhang Y Y, Huang H, Huang J Y, You F, Lian J S, Yang J H, Wen C K C. 2016. The effects of four transplantation methods on five coral species at the Sanya Bay. Acta Oceanologica Sinica, 35(10): 88-95.
DOI:10.1007/s13131-016-0916-8 |
Zhao M X, Yu K F, Zhang Q M, Shi Q. 2010. Long-term change in coral cover in Luhuitou fringing reef, Sanya. Oceanologia et Limnologia Sinica, 41(3): 440-447.
(in Chinese with English abstract) |
Zheng X Q, Kuo F W, Pan K, Huang H N, Lin R C. 2019. Different calcification responses of two hermatypic corals to CO2-driven ocean acidification. Environmental Science and Pollution Research, 26(30): 30 596-30 602.
DOI:10.1007/s11356-018-1376-9 |
Zheng X Q, Li Y, Chen S Q, Lin R C. 2018. Effects of calcium ion concentration on calcification rates of six stony corals:a mesocosm study. Aquaculture, 497: 246-252.
DOI:10.1016/j.aquaculture.2018.07.041 |
 2021, Vol. 39
2021, Vol. 39