Institute of Oceanology, Chinese Academy of Sciences
Article Information
- EL HEDENY Magdy, RASHWAN Mohamed, RICHIANO Sebastián, AL FARRAJ Saleh, AL BASHER Ghada
- Epi- and endobiont faunal communities on an Egyptian Mediterranean rocky shore: species composition and their competition for space
- Journal of Oceanology and Limnology, 39(1): 198-207
- http://dx.doi.org/10.1007/s00343-020-9281-8
Article History
- Received Oct. 22, 2019
- accepted in principle Feb. 7, 2020
- accepted for publication Feb. 23, 2020
2 Department of Biological and Geological Sciences, Faculty of Education, Alexandria University, Alexandria 14037, Egypt;
3 Instituto Patagónico de Geología y Paleontología (CONICET-CENPAT), Puerto Madryn 9120, Argentina;
4 Department of Zoology, College of Sciences, King Saud University, P.O. Box 2455, Riyadh 11451, Saudi Arabia
Rocky shores are common intertidal environments in coastal areas, making up approximately 33% of the coasts worldwide (Reading and Collinson, 1996). Owing to the reduced or null sedimentation rates in this transitional environment, rocky shores may be colonized by a diverse array of endobionts, in addition to suspension feeding epibionts (among others; Taylor and Wilson, 2003; Santos and Mayoral, 2008; de Gibert et al., 2012).
Modern rocky shore environments provide good information on the ecology and habitat of epi- and endolithic communities (Devescovi, 2009; Furlong et al., 2016), and can provide analogues for ancient hard ground substrates, as well as information about biotic assemblages and environmental conditions (Bromley and Asgaard, 1993; Santos et al., 2008; Baarli et al., 2013; Furlong et al., 2016; Bagur et al., 2019).
Rocky shores are a common feature in the Egyptian Mediterranean coast. They appear as strongly contorted and broken and sometimes flat or horizontal platforms. Among these rocky shores, the Abu Qir Headland is considered one of the most important natural habitats for numerous endolithic and epilithic marine organisms (Figs. 1-2). Despite most of the Mediterranean coast (about 63%) being rocky (Johnson, 2006; Furlani et al., 2014), studies on encrusting and endolithic bionts (sclerobionts sensu Taylor and Wilson, 2002) on these rocks are rare (Benedetti-Cecchi et al., 2003; Devescovi, 2009; Wilson, 2013; Furlong et al., 2016).
|
| Fig.1 Simplified location map showing the five sampling sites of the study area |

|
| Fig.2 View of the rocky shore sampling area in the Abu Qir, east of Alexandria, Egypt |
The aim of this contribution is threefold: 1) to study the sclerobiont (epi- and endolithic) communities on the rocky shore of the Abu Qir Headland, eastern coast of Alexandria, Egypt; 2) demonstrate and explain the successive stages of their infestation and settlements; 3) and discuss the ecological significance of epi- and endobionts recorded in the studied area.
2 MATERIAL AND METHODThe Abu Qir area is located at the eastern tip of the Mediterranean coast of Alexandria (Fig. 1). The shoreline there has a characteristic landform called the Abu Qir Headland which consists of solid rocks, rocky pools, variably sized boulders, and many erosional features (Fig. 2). The Abu Qir Headland is situated between Abu Qir Bay to the east and Abu Qir open sea to the west (Fig. 1).
Rocks in the studied area consist primarily of limestone and, because of the continuous action of the tides and waves, are characterized by many erosional features (e.g., pitted and broken surfaces, deep gutters and channels; Fig. 2).
The present work is based on field and laboratory examinations of the limestone that primarily forms the headland of the Abu Qir. Samples were collected from five sites to cover most of the Abu Qir Headland area (Fig. 1). In the field, the morphological features, and encrusting and bioeroding fauna, were recorded, described, and photographed. In the laboratory, the epi- and endolithic organisms were carefully examined using a hand lens and binocular microscope. All specimens examined are housed in the Department of Geology, Faculty of Science, Alexandria University, Egypt. Specimen numbers are prefixed with EpAQ or EnAQ.
3 RESULT 3.1 Epilithic faunaEpilithic organisms in the calcareous rocky shore of the Abu Qir are low in biodiversity, but abundant and complex, including among the most important taxa, serpulid and spirorbid worms, bryozoans, and balanoid barnacles.
3.1.1 Serpulid and spirorbid tubewormsTubeworms are common on the studied rocky shore, represented by serpulids and spirorbids. These worms dwell in calcium carbonate tubes made of aragonite, calcite, or a mixture of aragonite and calcite (Bornhold and Milliman 1973; Vinn et al., 2008). Tubeworms represented the most abundant encrusters in terms of area occupied, and serpulids are usually more abundant than spirorbids.
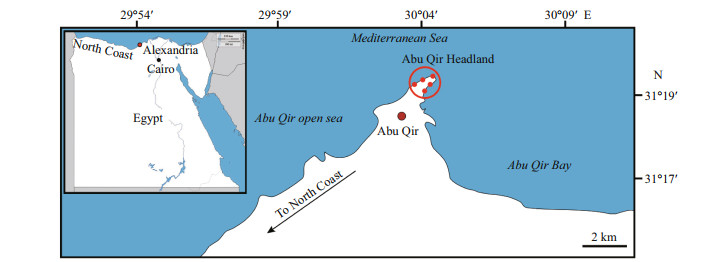
Serpulids are represented by Spirobranchus tetraceros (Schmarda, 1861), Protula and/or Apomatus sp., Vermiliopsis striaticeps (Grube, 1862) and Hydroides elegans (Haswell, 1883) (Figs. 3a-c, 4a, 5a). Among these, Hydroides elegans was the most common species, usually appearing in dense clusters filling rock cavities (Fig. 5a), or accumulated in the eroded or concave parts of the rocky shore (Figs. 3b & 5a, 6a-c). In some cases, serpulids appeared as post-mortem encrusters over other sclerobionts, e.g. balanoid barnacles and bryozoan sheets (Figs. 3b-c & 4a). On the other hand, spirorbid worms (identified as Spirorbis) were less common (Fig. 5b). These tubes range from 1 mm to 3 mm in diameter. They are most commonly found attached to the basement rock and rarely overgrow the other sclerobionts.
|
| Fig.3 Spatial competition of epibionts (a); well-developed calcified scars (arrowheads), appeared when balanoid barnacles dislodged from the basement rock, together with other sclerobionts (balanoid barnacle and serpulid polychaetes) (b); hierarchical sequence of serpulid polychaetes (black arrowhead), encrusting Balanus sp., and the latter encrusting bivalve shell of Brachiodontes pharaonis (white arrowhead) (c); cheilostome bryozoan Biflustra savartii overgrowing Balanus sp. (black arrowhead), and small sized serpulids overgrowing bryozoan colony (black arrows), note the presence of serpulid polychaetes (white arrowhead) occupying the aperture of the balanoid barnacles indicating post-mortem encrustation (d); cheilostome bryozoan Biflustra savartii overgrowing Perforatus perforatus (white arrowheads) and the bivalve Brachiodontes pharaonis (black arrowhead); note the intraspecific stand-off between two individuals of Perforatus perforatus (white arrow) |

|
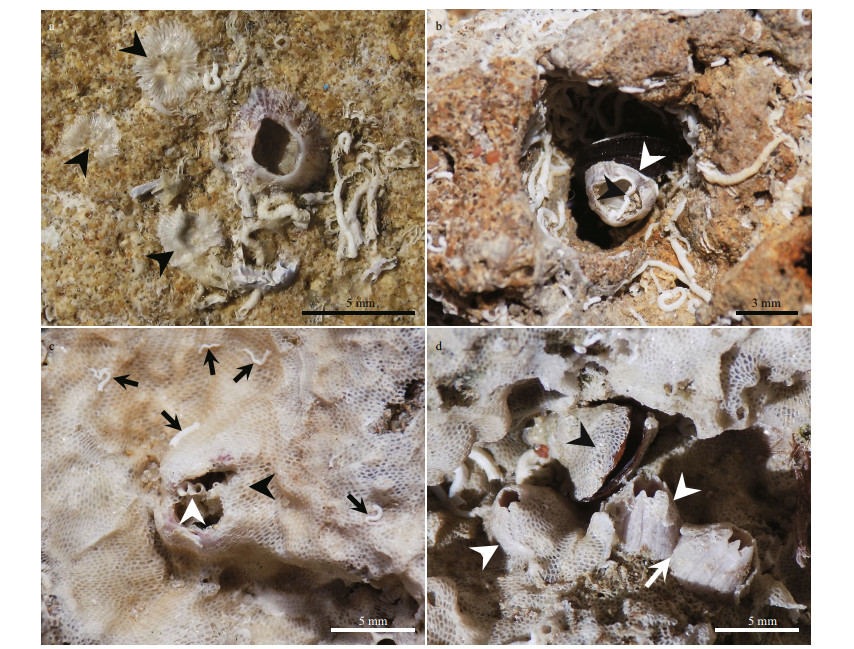
| Fig.4 Spatial competition of epibionts (a); cheilostome bryozoan Biflustra savartii overgrowing Balanus sp. (black arrowhead). Note the small sized serpulid worm tubes (black arrow) and Balanus sp. (white arrow) overgrowing bryozoan (b); cheilostome bryozoan Biflustra savartii overgrowing two Balanus individuals (black and white arrows). Note the aperture coverage the left balanoid barnacle (black arrow), which suggests post-mortem overgrowth (c); fatal overgrowth of Cheilostome bryozoan Biflustra savartii over serpulid polychaetes (note the xenomorphic shape of bryozoan surface (black arrows), and bryozoan sheet overgrowing Balanus trigonus (black arrowhead) (d); multilayered colony of Cheilostome bryozoan Biflustra savartii. Note encrustation of serpulid worm over the bivalve shell Brachiodontes pharaonis (white arrowhead) |
|
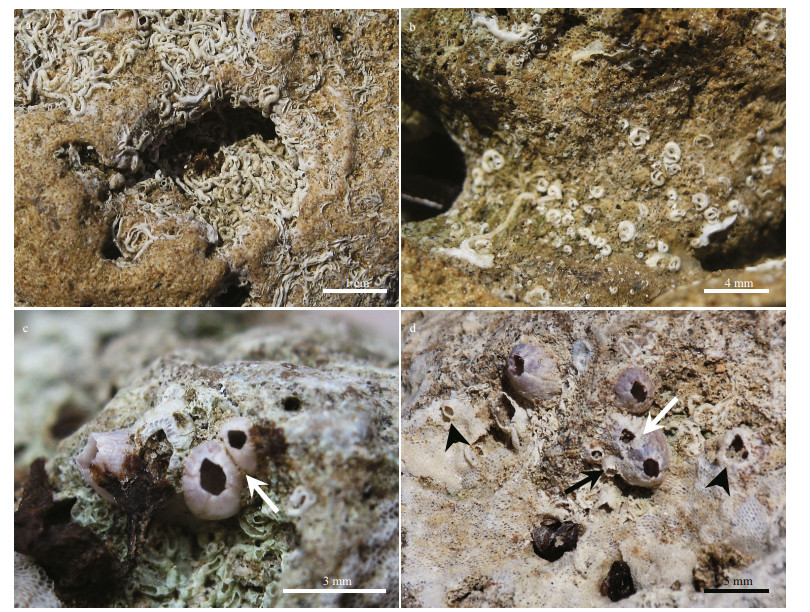
| Fig.5 Spatial competition of epibionts (a); dense cluster of serpulid polychaetes occupying rock cavity (b); concave rock surface encrusted by the spirorbid worms (Spirorbis) (c); intraspecific stand-off between two Balanus sp. (white arrow) (d); different cases of overgrowth, bryozoans overgrowing Balanus sp. (black arrowheads), and small sized Balanus overgrowing other balanoid barnacles (black arrow). Note the under-cut encounter pattern between balanoids (white arrow) |
Balanoid barnacles represented the second most abundant encrusters in terms of area occupied. Three main species were observed in the study area: the striped barnacle Amphibalanus amphitrite (Darwin, 1854) (Figs. 3a, 5d, 6b & 6d), the triangle barnacle Balanus trigonus Darwin, 1854 (Figs. 4c & 5d), and the volcano-shaped Perforatus perforatus (Bruguière, 1789-1792) (Fig. 3d). Single individuals or aggregations of some of these species can be observed. In this study, balanoid barnacles are commonly attached directly to the basement rock and overgrown by bryozoans (Figs. 4a-c) or serpulids (Fig. 3b) or sometimes by other balanoid species. When individuals are dislodged from the basement rock, they leave a well-developed calcified scar behind (Fig. 3a).

|
| Fig.6 Endolithic fauna (a); bivalves traces of Gastrochaenolites containing trace-makers preserved in situ (black arrowheads). Note the dense cluster of serpulid polychaetes occupying rock cavity in the middle (b); balanoid barnacle occupied by bivalves (white arrowhead), the nestling clam invade the borehole at a later stage (post-mortem) (c); cluster serpulid polychaetes occupying a large rock cavity (d, e, f); traces of annelids (Maeandropoldora; white and black arrowheads) (g); clean sector of the rocky shore showing different traces of Gastrochaenolites (black arrowheads) and Maeandropolydora? (white arrowheads) (h); small etching pits (Finichnus) after detaching of bryozoan colony from the basement rock (white circle) |
Cheilostome bryozoan colonies appeared as moderately to well-preserved extensive sheets, either encrusting the bioeroded basement directly (Fig. 5d), or encrusting other epilithic taxa, e.g. balanoid barnacles (Figs. 3c-d & 4a-d) and serpulid worms (Fig. 4c). Generally, bryozoans are capable of winning and retaining space in competitive interactions. They are frequently attributed to different varieties of Biflustra savartii (Audouin, 1826; Taylor, personal communication).
3.2 Endolithic faunaThe biological community recorded in this study generated bioerosional structures on the rocky shore, as shown in our analyses. All the organisms identified (i.e. polychaetes, barnacles, bivalves and bryozoans) modified their respective inhabited substrates, by both attaching and boring. There were three types of bioerosive structures identified, those generated by bivalves, by annelids, and by bryozoans. The traces of bivalves included dwelling structures (Figs. 6a-c), the shape of which resembled a bottle or a tear of up to 3 cm long and 1 cm wide (Fig. 6c). In general, the traces presented circular to subcircular in cross-sections, and sometimes the trace-maker was preserved (Fig. 6a). These characteristic features are typical of the ichnogenus Gastrochaenolites (Kelly and Bromley, 1984). In other cases, after the abandonment of the dwelling structure by the bivalve, the surface could be eroded, leaving the substrate available for other organisms like barnacles and polychaetes (Fig. 6c). Annelids, generally left several circular apertures (Fig. 6d) and tortuous galleries (Fig. 6e & f) in the rock. The apertures had a maximum diameter of 3-4 mm, and the galleries were of similar size, being visible only in some eroded areas of the rocks (Fig. 6e & f). These characteristic traces were identified to belong to the ichnogenus Maeandropolydora (Voigt, 1965), and were interpreted as dwelling structure of several polychaete families, especially Spionidae (Bromley and D'Alessandro, 1983). Usually, a higher degree of erosion enabled identification of the relationship between different traces (Fig. 6g). The traces of bryozoan ichnogenus Finichnus were observed as small etching pits (less than 1 mm), left by cheilostome bryozoans on the rocky shore (Fig. 6h), and were only visible when the bryozoan colonies were detached from substrates (Taylor et al., 1999, 2013). The empty borings in the rocky shore of the Abu Qir were often inhabited by nestling bivalve species [e.g. Brachidontes pharaonic (Fischer, 1870), Figs. 4d & 6b].
4 DISCUSSIONThe rocky shore of the Abu Qir Headland represents an important natural laboratory to observe the distribution of different taxa (and associated traces) in marine warm-water environments. The area under study here showed a low diversity of inhabitants, but excellent examples of biotic interactions.
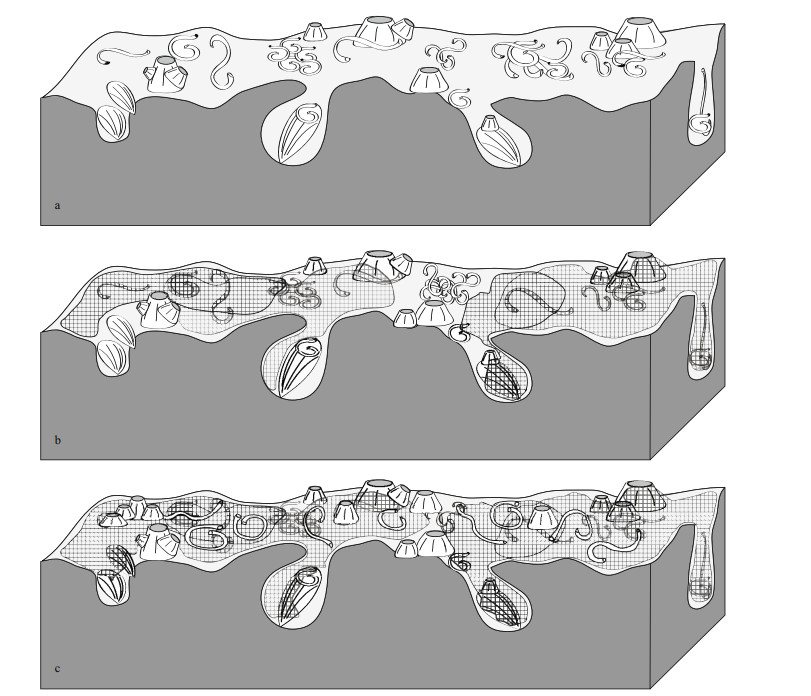
4.1 Stages of epibiont and endobiont emplacementsIn the rocky shore studied here, it is possible to identify several stages of encrusters and bioeroders, as not all of the organisms recorded were coeval. In this sense, we observed three successive stages of organism activities (Fig. 7).
|
| Fig.7 Diagrammatic succession of epilithic communities in the Abu Qir rocky shore area a. the first stage of encrustation dominated by serpulid polychaetes and Balanus sp., with bivalve shells nestling the rock cavities; b. the second stage characterized by multilayered bryozoans overgrowing the previous epilithic community; c. the last stage showing continuous overgrowth of bryozoans with the appearance of new individuals of serpulids and balanoid barnacles overgrowing bryozoans. |
During the first stage (Fig. 7a) the lower levels of the rocky shore were intensively bioeroded with Gastrochaenolites isp. and Maendropolydora isp. (Fig. 6f & g). Most of the large borings, comprising Gastrochaenolites isp., contained evidence of their producers (Fig. 6a & b). Concerning epibionts, they were mostly represented by dense accumulations of serpulids and spirorbids, which generally inhabited the borings, grooves, and balanoids apertural margins to evade the strong currents (Fig. 6c). Balanoid barnacles, ranging from juvenile to adults, frequently appear in clusters and usually competed with each other for space (see below). Bryozoans were very rare in this phase.
During the second stage (Fig. 7b), bryozoans formed flat sheets that spread out over most areas of the rocky shore as well as the epibionts (serpulids, Balanus spp. and even boring bivalves) of the first stage (Figs. 3c-d & 4a-c). The bryozoan colonies were identified as the Anascan-grade cheilostome Biflustra savartii. It was apparent that the balanoid barnacles were not completely covered, especially on their apertures (Figs. 3c-d, 4a-c, 6e), possibly indicative of a pre-mortem condition (i.e. Balanus sp. were still alive during the spreading sheets formation).
Finally, the third stage (Fig. 7c) was characterized by a new layer of epibionts that encrusted over the bryozoan sheets formed during the second stage. These epibionts were restricted to rare occurrences of Balanus spp. and small isolated patches of serpulids (Figs. 3c & 4a).
4.2 Spatial competition of epibionts of the Abu Qir rocky shoreHard substrates communities in rocky shore areas mainly consist of sessile sclerobionts. During their growth, these sclerobionts compete for consuming microplankton and oxygen, or to dominate the living space. The latter is the main reason for their competition because it is the principal limiting resource (Knight-Jones and Moyse, 1961; Buss and Jackson, 1979; Ferguson et al., 2013; Taylor, 2016).
In the study area, the hard substrate was highly variable, comprising shells and skeletons (biotic substrate), variable sized grains (pebbles to boulders), and rocky platforms. Sclerobionts mainly consist of three major types: bryozoans, serpulid polychaetes, and balanoid barnacles. In some cases, some bivalve shells were observed to nest in the rocks. Their exact spatial relationships were studied in order to determine the type of competition between adjacent individuals and their hierarchical sequence.
Two types of competitive interactions were recorded: 1) Fouling, including larval settling of balanoid barnacles on an empty hard surface (Fig. 3a). Post-mortem fouling of encrusting sclerobionts is not considered a case of space competition (Fig. 3b & c; McCook et al., 2001); 2) marginal, which describes the relationship between two organisms when they come in contact during their growth. Two possible outcomes were recorded: overgrowth and stand-off (Fig. 3d).
In communities containing more than two species, the vertical succession of individuals can be described as a competitive hierarchy (Fig. 3b-d; Centurión and López Gappa, 2011).
The studied sclerobionts displayed overgrowth in many cases, with some of them being fatal, depending on the shape and size of the encrusting organisms. Bryozoans are colonial species; their plasticity in shape and size allow them to survive from multiple partial overgrowth (Fig. 4a). In contrast, overgrowth of solitary species (such as serpulids and Balanus) was often more fatal, especially when their functional feeding opening was covered (Figs. 3d, 4b, c; Taylor, 2016). Several multilayered growth sheets of bryozoans were recorded in the studied specimens (Fig. 4d). This type of growth strategy was used to overgrow the organisms that had encrusted the colony surface (Fig. 4b & c), and to increase the height of the colony and consequently prevent overgrowth by these organisms (Liow et al., 2019). When a growing bryozoan colony meets another encrusting organism (e.g., serpulids, balanoid barnacles, or another bryozoan), it may overgrow or be overgrown and finally the survival and reproduction of the overgrown will be affected (Liow et al., 2016, 2017, 2019).
The studied serpulids are generally found as clusters filling rock cavities (Fig. 5a) or accumulated in concave areas (Fig. 5b). This living strategy may be a type of symbiotic overgrowth in order to obtain possible trophic benefits, or to escape complete overgrowth by other competitors, or even to avoid transportation by currents to unfavorable habitats.
On the other hand, balanoid barnacles are commonly observed as being solitary individuals, generally overgrown by bryozoans and serpulids (Figs. 3b-d & 4a-c). In some cases, balanoids show a stand-off relationship with other barnacles (Figs. 3d & 5c) or appear as overgrowth on bryozoans' colonies and other balanoids (Figs. 4a & 5d). In rare cases, barnacles have the ability to undercut competitors and lift them off the substrate (Fig. 5d).
According to these observations, the ability to win space is clearly dependent upon taxon type (bryozoans vs. serpulids vs. balanoids), form (sheets vs. tubular), and the growth rate (fast vs. slow) (Jackson, 1977; Russ, 1982; Keough, 1984; Nandakumar et al., 1993). Bryozoans are considered a superior competitor for winning the substrate space.
Apart from the competitive point of view, another interesting idea to explore is that empty bioerosion structures help increase the biodiversity in rocky shores (Bagur et al., 2019). In the case under study, although it is not possible to confirm, this idea could be applied, as it was demonstrated that several empty Gastrochaenolites acted as refuges for serpulids, balanids or bivalves (Figs. 3b & 4d).
The findings documented herein act as a significant contribution to our knowledge of sclerobiont composition, sequence of their attachments and/or bioerosion, and their mutual relationships in the intertidal rocky shore of the Abu Qir Headland.
5 CONCLUSIONThe exposed calcareous rocky area of the Abu Qir Headland was significantly colonized by sclerobionts (epibionts and endobionts).
The epilithic community, with little boring organisms, was predominant and characterized by the balanoid barnacles, bryozoans and polychaete worms. These organisms displayed a remarkable overgrowth relationship.
Encrusting bryozoans were the most common epibionts attempting to cover everything (basement, epibionts and bioerosion structures) on the rocky shore.
Morphological analysis of the recorded bioerosion structures revealed three ichnogenera. These structures were produced by bivalves (Gastrochaenolites isp.), polychaete annelids (Maeandropolydora isp.) and bryozoans (Finichnus isp.).
In the present study, encrusting sclerobionts (bryozoans) competed actively for living space in the form of skeletal overgrowths.
6 DATA AVAILABILITY STATEMENTThe datasets generated during the current study are available from the corresponding author on reasonable request.
7 ACKNOWLEDGMENTWe thank the reviewers for the positive evaluation of our manuscript, and the constructive comments and suggestions, which significantly improved the manuscript. The authors thank Harry ten Hove (Naturalis Biodiversity Center, Leiden) and Paul Taylor (Natural History Museum, London) for their help in polychaete and bryozoan identification, respectively.
Audouin J V. 1826. Explication sommaire des Planches De Polypes de l'Egypte et de la Syrie, publiées par Jule-César Savignyi. In: Audouin J V ed. Description de l'Egypte, Ou Recueil Des Observations et Des Recherches Qui Ont Été Faites en Egypte Pendant L'expédition de L'armée Française. Histoire Naturelle. Imprimerie Impériale, Paris.
|
Baarli B G, Santos A G, Mayoral E J, Ledesma-Vazquez J, Johnson M E, Da Silva C M, Cachão M. 2013. What Darwin did not see: Pleistocene fossil assemblages on a high-energy coast at Ponta das Bicudas, Santiago, Cape Verde Islands. Geological Magazine, 150(1): 183-189.
DOI:10.1017/S001675681200074X |
Bagur M, Gutiérrez J L, Arribas L P, Gabriela Palomo M. 2019. Vacant bivalve boreholes increase invertebrate species richness in a physically harsh, low intertidal platform. Diversity, 11(3): 39.
DOI:10.3390/d11030039 |
Benedetti-Cecchi L, Bertocci I, Micheli F, Maggi E, Fosella T, Vaselli S. 2003. Implications of spatial heterogeneity for management of marine protected areas (MPAs): examples from assemblages of rocky coasts in the northwest Mediterranean. Marine Environmental Research, 55(5): 429-458.
DOI:10.1016/S0141-1136(02)00310 |
Bornhold B D, Milliman J D. 1973. Generic and environmental control of carbonate mineralogy in serpulid (polychaete) tubes. The Journal of Geology, 81(3): 363-373.
DOI:10.1086/627876 |
Bromley R G, Asgaard U. 1993. Endolithic community replacement on a Pliocene rocky coast. Ichnos, 2(2): 93-116.
DOI:10.1080/10420949309380081 |
Bromley R G, D'Alessandro A. 1983. Bioerosion in the Pleistocene of southern Italy: ichnogenera Caulostrepsis and Maeandropolydora. Rivista Italiana di Paleontologia e Stratigrafia, 89: 283-309.
|
Bruguière J. 1789-1792. Encyclopedie méthodique ou par ordre de matières. Histoire naturelle des vers. Vol. 1. Part 1. Panckoucke, Paris, 344p.
|
Buss L W, Jackson J B C. 1979. Competitive networks: nontransitive competitive relationships in cryptic coral reef environments. The American Naturalist, 113(2): 223-234.
DOI:10.1086/283381 |
Centurión R, López Gappa J. 2011. Bryozoan assemblages on hard substrata: species abundance distribution and competition for space. Hydrobiologia, 658(1): 329-341.
DOI:10.1007/s10750-010-0503-5 |
Darwin C. 1854. A Monograph on the Sub-Class Cirripedia with Figures of All the Species. The Ray Society, London. p.1-30.
|
de Gibert J M, Domènech R, Martinell J. 2012. Rocky shorelines. Developments in Sedimentology, 64: 441-462.
|
Devescovi M. 2009. Biometric differences between date mussels Lithophaga lithophaga colonizing artificial and natural structures. Acta Adriatica, 50(2): 129-138.
|
Ferguson N, White C R, Marshall D J. 2013. Competition in benthic marine invertebrates: the unrecognized role of exploitative competition for oxygen. Ecology, 94(1): 126-135.
DOI:10.1890/12-0795.1 |
Fischer P. 1870. Sur la faune conchyliologique marine des baies de Suez et de l'Akabah. Journal de Conchyliologie, 18: 161-179.
|
Furlani S, Pappalardo M, Gómez-Pujol L, Chelli A. 2014. The rock coast of the Mediterranean and black seas. In: Kennedy D M, Stephenson W J, Naylor L A eds. Rock Coast Geomorphology: A Global Synthesis. Geological Society, London. p.89-123.
|
Furlong C M, Schultz S K, Gingras M K, Zonneveld J P. 2016. Oregon Sea stack: ecological diversity of a modern Trypanites ichnofacies. Ichnos, 23(1-2): 77-98.
DOI:10.1080/10420940.2015.1136512 |
Grube A E. 1862. Mittheilungen über die Serpulen, mit besonderer Berücksichtigung ihrer Deckel. Jahresbericht und Abhandlungen der Schlesischen Gesellschaft in Breslau, 39: 53-69.
|
Haswell W A. 1883. On some new Australian tubicolous Annelids. Proceedings of the Linnean Society of New South Wales, 7: 633-638.
|
Jackson J B C. 1977. Competition on marine hard substrata: the adaptive significance of solitary and colonial strategies. The American Naturalist, 111(980): 743-767.
DOI:10.1086/283203 |
Johnson M E. 2006. Uniformitarianism as a guide to rocky-shore ecosystems in the geological record. Canadian Journal of Earth Sciences, 43(8): 1 119-1 147.
DOI:10.1139/e06-045 |
Kelly S R A, Bromley R G. 1984. Ichnological nomenclature of clavate borings. Palaeontology, 27(4): 793-807.
|
Keough M J. 1984. Dynamics of the epifauna of the bivalve Pinna bicolor: interactions among recruitment, predation, and competition. Ecology, 65(3): 677-688.
DOI:10.2307/1938040 |
Knight-Jones E W, Moyse J. 1961. Intraspecific competition in sedentary marine animals. Symposium of the Society of Experimental Biology, 15: 72-95.
|
Liow L H, Di Martino E, Krzeminska M, Ramsfjell M, Rust S, Taylor P D, Voje K L. 2017. Relative size predicts competitive outcome through 2 million years. Ecology Letters, 20(8): 981-988.
DOI:10.1111/ele.12795 |
Liow L H, Di Martino E, Voje K L, Rust S, Taylor P D. 1937. Interspecific interactions through 2 million years: are competitive outcomes predictable?. Proceedings of the Royal Society B: Biological Sciences, 283: 20161645.
DOI:10.1098/rspb.2016.0981 |
Liow L H, Reitan T, Voje K L, Taylor P D, Di Martino E. 2019. Size, weapons, and armor as predictors of competitive outcomes in fossil and contemporary marine communities. Ecological Monographs, 89(2): e01354.
DOI:10.1002/ecm.1354 |
McCook L, Jompa J, Diaz-Pulido G. 2001. Competition between corals and algae on coral reefs: a review of evidence and mechanisms. Coral Reefs, 19(4): 400-417.
DOI:10.1007/s003380000129 |
Nandakumar K, Tanaka M, Kikuchi T. 1993. Interspecific competition among fouling organisms in Tomioka Bay, Japan. Marine Ecology Progress Series, 94: 43-50.
DOI:10.3354/meps094043 |
Reading H G, Collinson J D. 1996. Clastic coasts. In: Reading H G ed. Sedimentary Environments: Processes, Facies and Stratigraphy. Blackwell, Oxford. p.154-231.
|
Russ G R. 1982. Overgrowth in a marine epifaumal community: competitive hierarchies and competitive networks. Oecologia, 53(1): 12-19.
DOI:10.1007/BF00377130 |
Santos A, Mayoral E, da Silva C M, Cachão M, Domènech R, Martinell J. 2008. Trace fossil assemblages on Miocene rocky shores of southern Iberia. In: Wisshak M, Tapanila L eds. Current Developments in Bioerosion. Springer, Berlin, Heidelberg. 499p, https://doi.org/10.1007/978-3-540-77598-0_22.
|
Santos A, Mayoral E. 2008. Bioerosion versus colonisation on Bivalvia: a case study from the Upper Miocene of Cacela (southeast Portugal). Geobios, 41(1): 43-59.
DOI:10.1016/j.geobios.2007.01.009 |
Schmarda L K. 1861. Neue Wirbellose Thiere Beobachtet und Gesammelt auf Einer Reise um Die Erde 1853 bis 1857. Turbellarien, Rotatorien und Anneliden. 164p.
|
Taylor P D, Wilson M A, Bromley R G. 1999. A new ichnogenus for etchings made by cheilostome bryozoans into calcareous substrates. Palaeontology, 42(4): 595-604.
DOI:10.1111/1475-4983.00087 |
Taylor P D, Wilson M A, Bromley R G. 2013. Finichnus, a new name for the ichnogenus Leptichnus Taylor, Wilson and Bromley, 1999, preoccupied by Leptichnus Simroth, 1896 (Mollusca, Gastropoda). Palaeontology, 56(2): 456.
DOI:10.1111/pala.12000 |
Taylor P D, Wilson M A. 2002. A new terminology for marine organisms inhabiting hard substrates. Palaios, 17(5): 522-525.
DOI:10.1669/0883-1351(2002)017<0522:ANTFMO>2.0.CO;2 |
Taylor P D, Wilson M A. 2003. Palaeoecology and evolution of marine hard substrate communities. Earth-Science Reviews, 62(1-2): 1-103.
DOI:10.1016/S0012-8252(02)00131-9 |
Taylor P D. 2016. Competition between encrusters on marine hard substrates and its fossil record. Palaeontology, 59(4): 481-497.
DOI:10.1111/pala.12239 |
Vinn O, Ten Hove H A, Mutvei H, Kirsimäe K. 2008. Ultrastructure and mineral composition of serpulid tubes (Polychaeta, Annelida). Zoological Journal of the Linnean Society, 154(4): 633-650.
DOI:10.1111/j.1096-3642.2008.00421.x |
Voigt E. 1965. Über parasitische Polychaeten in Kreide-Austern sowie einige andere in Muschelschalen bohrende Würmer. Paläontologische Zeitschrift, 39(3-4): 193-211.
DOI:10.1007/BF02990164 |
Wilson B. 2013. Intertidal rocky shores. In: Wilson B ed. The Biogeography of the Australian North West Shelf: Environmental Change and Life's Response. Elsevier, Burlington, MA. p.59-106.
|
 2021, Vol. 39
2021, Vol. 39


