Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHAO Xiaohui, YANG Xiaoqian, ZHANG Jianheng, WEN Qinlin, HE Peimin
- Karyological observations of Ulva linza chromosomes
- Journal of Oceanology and Limnology, 39(1): 259-265
- http://dx.doi.org/10.1007/s00343-020-9105-x
Article History
- Received Apr. 21, 2019
- accepted in principle Sep. 23, 2019
- accepted for publication Feb. 16, 2020
2 Water Environment & Engineering Research Center of Shanghai Institution of Higher Education, Shanghai 201306, China
Karyological studies have provided basic information about the number, size, and morphology of chromosomes. The information is then used to undertake chromosome manipulation experiments (Khan et al., 2000; Xu et al., 2016). In macroalgae, there are relatively few chromosome data, partially because their chromosome sizes are very small compared to chromosomes in higher plants (White, 1973). Macroalgae, commonly termed seaweeds, are multicellular, macroscopic benthic algae that belong to the green (phylum Chlorophyta), red (phylum Rhodophyta), and brown (phylum Phaeophyta) taxonomic groups (Kennish, 2016). However, to date, most of the researches on algal chromosomes have primarily focused on life cycles, genetic relationships, and breeding. It has also largely concentrated on species of economic importance, such as those within the genera Pyropia and Laminaria. None of the studies have focused on the karyological analysis of Ulva macroalgae.
Green macroalgae species in the genus Ulva are ubiquitous throughout the world in marine and estuarine habitats. They show a considerable ability to acclimate to adverse circumstances and are able to grow rapidly in nutrient rich waters (Tan et al., 1999; Zhang et al., 2015). Macroalgal blooms in the Yellow Sea have broken out for 12 consecutive years since 2007, resulting in great damage to the marine environment and ecosystem service functions (Liu et al., 2013; Zhang et al., 2014; Zhou et al., 2016). Ulva linza, a common species that is found around the coastal areas of China, has been identified as one of the causal species of macroalgal blooms during their early stages (Han et al., 2013).
In this study, a reliable method for preparing metaphase chromosomes from U. linza is described. The chromosomes are suitable for karyotype analysis and gene mapping by fluorescence in-situ hybridization (FISH). The objective of this study was to investigate the karyotype of this species. A karyotype analysis is a key step towards stock improvement by polyploidy manipulation, hybridization, and related genetic engineering. This paper provides detailed information about the chromosome number and karyotype of U. linza.
2 MATERIAL AND METHOD 2.1 Specimen collectionThe U. linza in this study was originally collected from Pyropia aquaculture rafts in the intertidal zone of the Jiangsu coast, China on April 8, 2018. Live samples were brought back to the laboratory and the periphyton and surface impurities were removed using sterilized seawater and a soft brush. The identity of the collected samples was confirmed using molecular methods (Wang et al., 2018). Then the identified strains were kept as unialgal cultures in an incubator at 20 ℃, under 20-30 μmol/(m2·s) light, and with a light:dark regime=12 h:12 h. Their life histories were determined from the size, phototactic response, and the zooid flagella number of at least two successive generations (Cui et al., 2018). Flagella numbers and the phototactic response of zoids were observed using the method suggested by Hiraoka et al. (2003). This method identified the sporophytes and gametophytes of U. linza, which were then used in the chromosome analysis.
2.2 Chromosome preparation of Ulva linzaKnown U. linza sporophyte and gametophyte generations were selected and used in the chromosome analysis. Fresh sporophyte and gametophyte tissues were separately treated with 0.1% colchicine. Different processing times were used (4, 8, 12, 16, or 20 h) so that the best processing time could be determined. Then the samples were washed three or four times in deionized water. The samples were fixed in freshly prepared Cannoy's fixative solution (100% ethanol:acetic acid, 3:1, v/v) for 24 h (Schweizer, 1976; Liu et al., 2012a, b ), which fixed the algal cells at the metaphase stage of the cell cycle. They were then washed in distilled water three times. The fixed gametophytic or sporophytic tissues were digested by gently stirring them in an enzyme mixture (cellulose, pectinase, and macerozyme R-10, 2:1:1, v/v) for 4-24 h at 37 ℃. Different enzymatic hydrolysis times were used (4, 8, 12, 16, 18, 20, or 24 h) so that the optimum time for enzymatic hydrolysis could be determined. Finally, enzymatic hydrolysis was terminated by adding 70% ethanol. Then the samples were washed three times in distilled water and centrifuged at 12 000 r/min for 3 min to remove the supernatant. The pellets were re-suspended in 60 μL acetic acid and the cell suspensions were dropped onto slides (6-10 μL per slides). Then they were stored in a humid chamber. The chromosomes were counterstained with 15-μL DAPI (Lin et al., 1977; Yabu, 1991; Liu et al., 2012a), and the slides were stored in the dark for 10 min before microscopic analysis. The slides were screened under a phase contrast microscope for chromosomes that had reached the mitotic metaphase stage. The images were captured with a Leica DM4000B digital camera using the LAS V4 controller program and the chromosomes were measured using Image-Pro Plus software. The karyotypic patterns of the gametophytes and sporophytes, which were based on the size of chromosomes, were edited using Adobe Photoshop software. Color contrast and brightness uniformity were also analyzed using this software (Liu et al., 2012a).
3 RESULT AND DISCUSSION 3.1 Species verification of Ulva linzaThe transverse section of the U. linza type had a special structure that contained two layers of cells that were separated at the margin, but had adhered together in the middle (Fig. 1). The thalli of most Ulva macroalgae change their morphology in response to different environmental conditions (Tan et al., 1999; Zhang et al., 2015). Six algal samples were identified as most likely belonging to U. linza, based on morphology, ITS, and the 5S sequence. The yellow-green or dark green thallus has long linear to lanceolate or obovate bands with mostly wavy edges. It grows into long, narrow, ruffled blades that are two cell layers thick and up to 10-50-cm tall. The cells are 10-15 μm in diameter. Additionally, each cell contained one pyrenoid, but could occasionally contain 2-3 pyrenoids. The six samples had the highest sequence similarity with U. linza (AB298685.1, HM031140.1), and 100% 5S sequence identity. This information, when combined with its morphological characteristics, confirmed that the samples were U. linza. The six U. linza thalli were also confirmed as having a typical sexual life history, which was isomorphic with biflagellate anisogametes and quadriflagellate meiospores. Four thalli were sporophytes and two were gametophytes.

|
| Fig.1 Morphological characteristics of U. linza a. U. linza thalli; b. surface view; c. transverse section. |
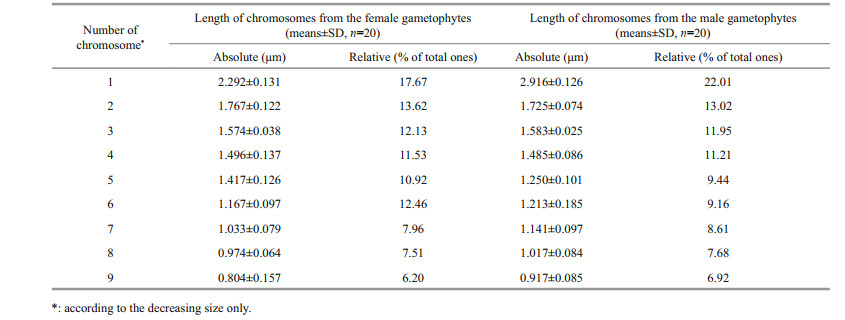
Numerous early botanists/cytogeneticists, who attempted to refine karyological techniques, demonstrated that colchicine was a mitotic inhibitor that could be applied as a pretreatment to shorten plant chromosomes and thus allow superior visualization of the somatic chromosomes prior to staining (Burrell, 1939; Nichols, 1941; Kindiger, 1994). However, the colchicine treatment time has a considerable impact on chromosome preparation.
The results indicated that if the colchicine treatment time was insufficient (6 h) (Fig. 2a), then chromosomes were still in the chromatin state and it was difficult to clearly distinguish individual chromosomes. If the treatment time was too long (18 h) (Fig. 2c), then the chromosomes could become too short or tiny round dots, which meant that it was also difficult to see any chromosome differences. However, there were more metaphase cells if the colchicine treatment time was about 12 h (Fig. 2b). Furthermore, the chromosomes were well distributed, and the morphology was clear.
|
| Fig.2 Effect of different colchicine pretreatment times on chromosome preparation a-c. immersion for 6, 12, and 18 h. |
Pretreatment with colchicine can destroy and inhibit the formation of microtubules in the spindle, which increases the number of cells at the metaphase stage. It also induces the chromatin superhelix to become chromosomes, which improves chromosome distribution (Oliveira et al., 1980; Peterfi and Manton, 1986). The extent of the colchicine pretreatment influence on cells was evaluated by determining the number of metaphase cells using microscopy. This can then be used to detect the effects of the pretreatment after various treatment times. Many researchers have pretreated cells with colchicine to improve chromosome distribution and morphology (Namboodiri et al., 1973; Liu et al., 2012a). However, numerous studies have shown that it is necessary to determine the optimum colchicine concentration and pretreatment time for chromosome preparation because it varies depending on the plant (Oliveira et al., 1980; Peterfi and Manton 1986). In this study, the experiments were repeated more than 85 times, and the results showed that 0.1% colchicine and a 12-h treatment time led to relatively clear chromosomes in Ulva metaphase cells.
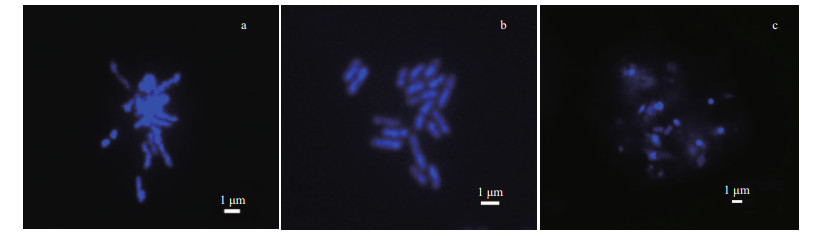
3.3 Effects of different enzymatic hydrolysis times on chromosome preparationThe dissociation fluid from the different plant materials was pretreated for various lengths of time so that the chromosomes could be isolated from single cells (Zhao et al., 2008; Chamani et al., 2012). In this study, a mixture of enzymes was used to obtain the Ulva chromosomes. The enzyme mixture digests the cell walls, and the protoplasts of the meristem cells are separated by enzymatic hydrolysis and tablet pressing. This means that there is little or no cytoplasm around the chromosome, the background is clean, and the chromosomes are well dispersed (Oliveira et al., 1980; Berends et al., 2015). It is important to select the correct enzyme for cell hydrolysis. Cellulase, pectinase, and segregation enzymes are widely used in chromosome preparation. The cellulase acts as a biocatalyst in the decomposition of cellulose and can digest cellulose into oligosaccharides or monosaccharides. Pectinase is extracted from Rhizopus. It can break down pectin and separate the cells from the tissue. The main function of macerozyme R-10 is to separate cells from the tissues into single cells (Teasdale and Rugini 1983; Dipakkore et al., 2005; Raikar et al., 2008).
The degree of enzymatic hydrolysis directly affects the quality of the chromosomes. In this study, cellulase, pectinase, and segregation enzymes were used to obtain high-quality chromosomes. The enzymolysis results were clearly influenced by treatment time (Fig. 3). The results show that if the enzyme digestion time was 4 h (Fig. 3a), the cell wall was not completely removed, the intracellular substances were not totally digested, and cell dispersion was uneven, which meant that the morphology of the chromosome was hard to discern. When the enzymatic hydrolysis time was 24 h (Fig. 3c), the chromosomes were too degraded and it was difficult to determine the morphology of the chromosomes. The results show that enzymolysis for 20 h (Fig. 3b) effectively removed the cell wall, cell membrane, and polysaccharides; the cells were evenly dispersed; and the chromosomes were clearly punctured or rod-like.

|
| Fig.3 Effects of different enzymatic hydrolysis times on chromosome preparation a-c. immersion in enzyme solution for 4, 18, and 24 h. |
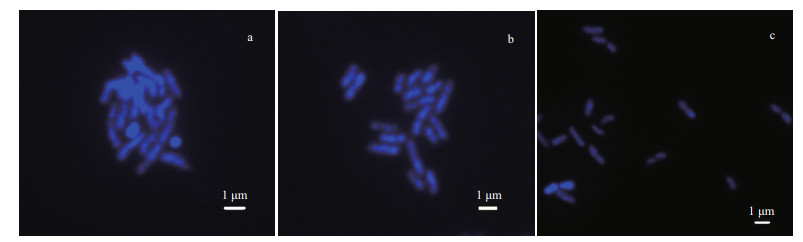
In this study, high quality chromosomes were obtained by dropping prepared suspended chromosomes onto clean glass slides. Dropping suspended chromosomes onto a slide broke down the enzyme lysed extracellular envelope and the nuclear membrane, which led to well dispersed chromosomes (Liu et al., 2012a, b ). The results showed that at a height of about 10 cm (Fig. 4a), the chromosome background was blurred and the cells had not dispersed well, which meant it was difficult to distinguish individual chromosomes. When the drop height was about 30 cm (Fig. 4b), the cells were well dispersed and the chromosomes could be easily observed. However, when the height was greater than 50 cm (Fig. 4c), the cells and chromosomes were excessively dispersed, which made it difficult to distinguish the cell chromosomes.
|
| Fig.4 Effects of different drop heights on chromosome preparation a-c. samples dropped from heights of 10, 30, and 50 cm. |
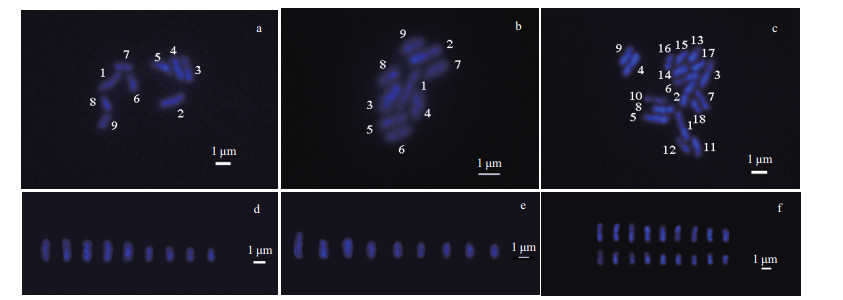
Chromosomes are the carriers of genetic material in biological organisms (Wang and Yu, 2016). Chromosome preparation is a basic technique in cytogenetic studies. In this study, we describe a basic protocol for U. linza metaphase chromosome preparation. A clear division phase for the U. linza chromosome was obtained by continuous optimization of the U. linza chromosome preparation method. The chromosomes were dispersed evenly and their morphology was clear (Fig. 5). A total of 100 female and male gametophytes, and sporophyte cells were selected and used to determine the number of chromosomes in U. linza. The chromosome number of 89% of the female and male gametophyte cells was n=9 (Fig. 5a & b), and the sporophyte was 2n=18 in more than 85% of the cells (Fig. 5c). The karyotype index of the chromosome was based on the absolute length of the chromosomes from large to small (Fig. 5d, e & f).
|
| Fig.5 Chromosome images of the gametophytes, and analysis of the sporophytes karyotypes of U. linza a & d. female gametophyte; b & e. male gametophyte; c & f. the sporophytes. |
The average absolute length (Table 1) of the chromosomes shown in the 20 images taken from the female slides was 0.804 μm to 2.292 μm, whereas the male length ranged from 0.917 μm to 2.916 μm. The size of the male chromosomes was larger than the corresponding females, but there was no significant difference between their relative lengths, both of which approximately represented 6% to 22% of the total chromosome length (Table 1). The gametophytes and sporophytes in a karyogram can be ranked according to their sizes (Fig. 5a, b & c).
Chromosome number and size is an important basis for species identification and genetic relationships. U. linza is the dominant species in green tides, but there have been few studies on its chromosomes. In this study, a reliable method for preparing metaphase chromosomes from U. linza is described. The karyotypes and chromosomes in U. linza were observed after a series of treatments, including pretreatment with 0.1% colchicine for about 12 h, Carnoy's fixative, and a mixture of enzymes. The samples were then dropped from 30 cm height onto glass slides to spread the surface coat. The prepared chromosomes were stained with 4', 6-diamidino-2-phenylindole (DAPI), and the results showed that there were n=9 chromosomes in the haploid male and female gametophytes, and 2n=18 chromosomes in the diploid sporophytes. The results from this study provide a good technical foundation for karyotype analysis and gene mapping using molecular markers.
5 DATA AVAILABILITY STATEMENTThe datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Berends P, Merz M, Kranz B, Thaler T, Appel D, Rabe S, Blank I, Stressler T, Fischer L. 2015. Optimization of an enzymatic wheat gluten hydrolysis process in an enzyme membrane reactor using a design of experiment approach. European Food Research and Technology, 242(10): 1 735-1 746.
|
Burrell P C. 1939. Root-tip smear method for difficult material. Stain Technology, 14(4): 147-149.
DOI:10.3109/10520293909110318 |
Chamani E, Tahami S K, Zare N, Asghari-Zakaria R, Mohebodini M, Joyce D. 2012. Effect of different cellulase and pectinase enzyme treatments on protoplast isolation and viability in Lilium ledebeourii bioss. Notulae Botanicae Horti Agrobotanici Cluj-Napoca, 40(2): 123-128.
DOI:10.15835/nbha4028055 |
Cui J J, Shi J T, Zhang J H, Wang L T, Fan S Y, Xu Z Y, Huo Y Z, Zhou Q Y, Lu Y W, He P M. 2018. Rapid expansion of Ulva blooms in the Yellow Sea, China through sexual reproduction and vegetative growth. Marine Pollution Bulletin, 130: 223-228.
DOI:10.1016/j.marpolbul.2018.03.036 |
Dipakkore S, Reddy C R K, Jha B. 2005. Production and seeding of protoplasts of Porphyra okhaensis (Bangiales, Rhodophyta) in laboratory culture. Journal of Applied Phycology, 17(4): 331-337.
DOI:10.1007/s10811-005-7291-8 |
Han W, Chen L P, Zhang J H, Tian X L, Hua L, He Q, Huo Y Z, Yu K F, Shi D J, Ma J H, He P M. 2013. Seasonal variation of dominant free-floating and attached Ulva species in Rudong coastal area, China. Harmful Algae, 28: 46-54.
DOI:10.1016/j.hal.2013.05.018 |
Hiraoka M, Dan A, Shimada S, Hagihira M, Migita M, Ohno M. 2003. Different life histories of Enteromorpha prolifera (Ulvales, Chlorophyta) from four rivers on Shikoku Island, Japan. Phycologia, 42(3): 275-284.
DOI:10.2216/i0031-8884-42-3-275.1 |
Kennish M J. 2016. Macroalgae. Netherlands: Springer.
|
Khan T A, Bhise M P, Lakra W S. 2000. Chromosome manipulation in fish-a review. The Indian Journal of Animal Sciences, 70(2): 213-221.
|
Kindiger B. 1994. A technique for the preparation of somatic chromosomes of Maize. In: Freeling M, Walbot V eds. The Maize Handbook. Springer, New York.
|
Lin M S, Comings D E, Alfi O S. 1977. Optical studies of the interaction of 4'-6 diamidino-2-phenylindole with DNA and metaphase chromosomes. Chromosoma, 60(1): 15-25.
DOI:10.1007/BF00330407 |
Liu D Y, Keesing J K, He P M, Wang Z L, Shi Y J, Wang Y J. 2013. The world's largest macroalgal bloom in the Yellow Sea, China: formation and implications. Estuarine, Coastal and Shelf Science, 129: 2-10.
DOI:10.1016/j.ecss.2013.05.021 |
Liu Y, Bi Y H, Gu J G, Li L H, Zhou Z G. 2012b. Localization of a female-specific marker on the chromosomes of the brown seaweed Saccharina japonica using fluorescence in situ hybridization. PLoS One, 7(11): e48784.
DOI:10.1371/journal.pone.0048784 |
Liu Y, Bi Y H, Zhou Z G. 2012a. Karyological observation on Saccharina japonica chromosomes stained with DAPI. Journal of Fisheries of China, 36(1): 50-54.
(in Chinese with English abstract) DOI:10.3724/SP.J.1231.2012.27590 |
Namboodiri A N. 1973. Colchicine pretreatment and maceration for improved squashes of dipterocarpaceae. Stain Technology, 48(2): 98-100.
DOI:10.3109/10520297309116593 |
Nichols C. 1941. Spontaneous chromosome aberrations in allium. Genetics, 26(1): 89-100.
DOI:10.1093/genetics/26.1.89 |
Oliveira L, Bisalputra T, Antia N J. 1980. Ultrastructural observation of the surface coat of Dunaliella tertiolecta from staining with cationic dyes and enzyme treatments. New Phytologist, 85(3): 385-392.
DOI:10.1111/j.1469-8137.1980.tb03177.x |
Peterfi L S, Manton I. 1986. Observations with the electron microscope on Asteromonas gracilis Artari emend. (Stephanoptera gracilis (Artari) Wisl.), with some comparative observations on Dunaliella sp.. British Phycological Bulletin, 3(3): 423-440.
|
Raikar S V, Braun R H, Bryant C, Conner A J, Christey M C. 2008. Efficient isolation, culture and regeneration of Lotus corniculatus protoplasts. Plant Biotechnology Reports, 2(3): 171.
DOI:10.1007/s11816-008-0058-3 |
Schweizer D. 1976. Reverse fluorescent chromosome banding with chromomycin and DAPI. Chromosoma, 58(4): 307-324.
DOI:10.1007/BF00292840 |
Tan I H, Blomster J, Hansen G, Leskinen E, Maggs C A, Mann D G, Sluiman H J, Stanhope M J. 1999. Molecular phylogenetic evidence for a reversible morphogenetic switch controlling the gross morphology of two common genera of green seaweeds, Ulva and Enteromorpha. Molecular Biology and Evolution, 16(8): 1 011-1 018.
DOI:10.1093/oxfordjournals.molbev.a026190 |
Teasdale R D, Rugini E. 1983. Preparation of viable protoplasts from suspension-cultured loblolly pine (Pinus taeda) cells and subsequent regeneration to callus. Plant Cell, Tissue and Organ Culture, 2(3): 253-261.
DOI:10.1007/BF00033563 |
Wang K, Yu W C. 2016. Chromosome preparation in Rice (Oryza sativa). Current Protocols in Plant Biology, 1(1): 67-77.
DOI:10.1002/cppb.20006 |
Wang S Y, Huo Y Z, Zhang J H, Cui J J, Wang Y, Yang L L, Zhou Q Y, Lu Y W, Yu K F, He P M. 2018. Variations of dominant free-floating Ulva species in the source area for the world's largest macroalgal blooms, China: differences of ecological tolerance. Harmful Algae, 74: 58-66.
DOI:10.1016/j.hal.2018.03.007 |
White G B. 1973. Comparative studies on sibling species of the Anopheles gambiae Giles complex (Dipt., Culicidae). III. The distribution, ecology, behaviour and vectorial importance of species D in Bwamba County, Uganda, with an analysis of biological, ecological, morphological and cytogenetical relationships of Ugandan species D. Bulletin of Entomological Research, 63(1): 65-97.
DOI:10.1017/S0007485300050896 |
Xu C H, Yu W C. 2016. Artificial chromosomes in Rice (Oryza sativa). Current Protocols in Plant Biology, 1(1): 107-120.
DOI:10.1002/cppb.20008 |
Yabu H, Yasui H. 1991. Chromosome number in four species of Laminaria (Phaeophyta). Japanese Journal of Phycology, 39: 185-187.
|
Zhang J H, Huo Y Z, Wu H L, Yu K F, Kim J K, Yarish C, Qin Y T, Liu C C, Xu R, He P M. 2014. The origin of the Ulva macroalgal blooms in the Yellow Sea in 2013. Marine Pollution Bulletin, 89(1-2): 276-283.
DOI:10.1016/j.marpolbul.2014.09.049 |
Zhang J H, Liu C C, Yang L L, Gao S, Ji X, Huo Y Z, Yu K F, Xu R, He P M. 2015. The source of the Ulva blooms in the East China Sea by the combination of morphological, molecular and numerical analysis. Estuarine, Coastal and Shelf Science, 164: 418-424.
DOI:10.1016/j.ecss.2015.08.007 |
Zhao H J, Zhang B, Chen A P, Li P Y, Xiao Y. 2008. Effects of different enzymolysis conditions on the cotyledons protoplast isolation of Alfalfa. Acta Agrestia Sinica, 16(1): 50-53.
(in Chinese with English abstract) |
Zhou L J, Wang L K, Zhang J H, Cai C E, He P M. 2016. Complete mitochondrial genome of Ulva linza, one of the causal species of green macroalgal blooms in Yellow Sea, China. Mitochondrial DNA Part B, 1(1): 31-33.
DOI:10.1080/23802359.2015.1137806 |
 2021, Vol. 39
2021, Vol. 39