Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHONG Zhihai, LIU Zhengyi, ZHUANG Longchuan, SONG Wanlin, CHEN Weizhou
- Effects of temperature on photosynthetic performance and nitrate reductase activity in vivo assay in Gracilariopsis lemaneiformis (Rhodophyta)
- Journal of Oceanology and Limnology, 39(1): 362-371
- http://dx.doi.org/10.1007/s00343-020-9256-9
Article History
- Received Nov. 20, 2019
- accepted in principle Dec. 30, 2019
- accepted for publication Feb. 6, 2020
2 Shantou University, Shantou 515063, China
Nitrate assimilation starts when alga takes up nitrate from the external medium. Nitrate reductase (EC 1.6.6.1) reduces nitrate to nitrite by using NAD(P)H as the electron donor (Dovis et al., 2014). Nitrite is then transported into the chloroplast, and subsequently reduced to ammonium, in which nitrite reductase (NiR) uses reduced ferredoxin as an electron donor (Chow et al., 2007; González-Galisteo et al., 2019). The overall rate of nitrate assimilation is often limited by the first reduction step of nitrate reductase activity. This step serves as a key point in nitrogen metabolism (Crawford and Arst, 1993; Campbell, 1999), and can be used as an index to nitrate assimilation in the field (Collos and Slawyk, 1977).
Nitrate reductase activity and photosynthesis are regulated by various environmental factors, including temperature, light, nitrate, iron, and other regulators of algal growth (Eppley et al., 1970; Lapointe et al., 1984; Gao et al., 1992; Young et al., 2007; Chen et al., 2018). Among them, temperature is a key physiological factor on the algal growth, distribution, and reproduction by affecting the sensitivity of the main cellular components (proteins and membranes). The temperature responses of species involve mainly three types: genetic adaptation (in thousands of millions of years), phenotypic acclimation (in hours to days), and short-term physiological regulation (in seconds to minutes). Besides, temperature is also considered to function by regulating C and N assimilation strongly associated with the photosynthesis and enzyme activities (Berry and Bjorkman, 1980; Gao et al., 2017, 2018). Furthermore, previous studies have pointed out that the metabolism of photosynthetic products is closely associated with nitrate reduction in photosynthetic tissues (Gao et al., 1992). The reason is that nitrite reduction needs reducing power and energy provided by photosynthesis (Chow et al., 2013; Varela et al., 2018). However, there are few studies about the regulation of temperature on photosynthesis and nitrate reductase activity by phenotypic acclimation.
In vitro and in vivo assays of nitrate reductase activity are two methods to measure nitrate reductase activity. The in vitro assay first ruptures the cells, then extracts and stabilizes enzyme, and at last tests the enzyme (Dovis et al., 2014). The in vivo assay to increase the membrane permeability, increase the rate of substrate (NO3-), NADH (nicotinamide adenine dinucleotide (NAD) + hydrogen (H)), and reaction production (NO2-) in and out of the cells. The in vivo assay is faster and easier, and the more important is that it represents nitrate reductase activity with the current level of cellular NADH (Chow et al., 2004; Dovis et al., 2014). Previous studies have mainly concentrated on the in vitro assay (Chow et al., 2001, 2004; Vona et al., 2004), but in vivo assay is scarcely discussed (Corzo and Niell, 1991). Only few papers concerned the optimum temperature for the nitrate reductase activity assay in vivo (Corzo and Niell, 1991; Zou, 2005; Teichberg et al., 2007; Cabello Pasini et al., 2011; Chen et al., 2015), most of those studies reported that the incubation temperature for the in vivo assay should be 30 ℃ or room temperature, regardless of the species and the actual physiology of the alga in the field. When nitrate reductase is used as an index of the nitrate assimilation, the optimum temperature for the in vivo assay and the relationship between photosynthesis and nitrate reductase activity are important for evaluating C and N assimilation (Kristiansen, 1983).
Gracilariopsis lemaneiformis (Rhodophyta) is an economic macroalgae cultured in a large-scale in China for providing quality raw material for agar industry and feed for abalone aquaculture (Yu and Yang, 2008; Gu et al., 2017; Chen et al., 2018; Liu et al., 2019). By absorbing and utilizing nutrients from the seawater, G. lemaneiformis acts as ideal biofilters to control eutrophication, and improve the health and stability of the marine ecosystem (Yang et al., 2005). As a result, much attention has been paid to the aquaculture techniques and the ecophysiology of this alga (Yang et al., 2006). However, little research focused on the effects of temperature on nitrate reductase activity and photosynthesis in G. lemaneiformis. In this study, the relationship among photosynthesis, growth, content of soluble protein, and nitrate reductase activity assay in vivo were explored and the optimum temperature for in vivo assay of nitrate reductase activity was determined.
2 MATERIAL AND METHOD 2.1 Plant materialGracilariopsis lemaneiformis was sampled from Shen'ao Bay (23.46°N, 117.09°E), Nan'ao Island, Shantou, China. Samples were kept at 5 ℃, and transported to the laboratory in 4 h. The algae were then stored in a glass aquarium tank containing filtered natural seawater (salinity: 28, temperature: 20 ℃) under irradiance of 120 μmol photons/(m2·s), and in 12 h L꞉12 h D photoperiod scheme for 3 days. Healthy thalli were selected for subsequent experiments.
2.2 Experimental designThalli of approximately 4 g fresh weight were placed in flasks containing 1-L sterile natural seawater (salinity: 28; NO3-: 17.22 μmol/L; NO2-: 1.62 μmol/L; NH4+: 1.92 μmol/L; PO43-: 0.29 μmol/L) enriched with 100 μmol/L NaNO3 and 10 μmol/L NaH2PO4. The irradiance was 120 μmol photons/(m2·s) and 12 h L꞉ 12 h D photoperiod scheme. Triplicate cultures were grown at four different temperatures (15, 20, 25, and 30 ℃), and culture medium was renewed every day. Algae remained in culture for 7 days prior to experimental work.
2.3 Relative growth rate (RGR)The fresh weight of the alga was measured once a day. The relative growth rate (RGR) was calculated as follows: RGR=ln(Wt/W0)/t, where W0 is the initial fresh weight and Wt is the final fresh weight after t days. Before weighting the algae, samples were softly blotted using filter paper to remove excess water.
2.4 Determination of nitrate reductase activityThe nitrate reductase activity assay method was modified according to the in vivo method described by Corzo and Niell (1991). At the end of the culturing period (7 days), when illuminated for 4 h (Lopes et al., 1997), G. lemaneiformis samples were cut into 3-cm-long segments by scissors, then incubated in culture seawater for 1 h to minimize the cutting damage (Zou, 2005). Approximately 0.2 g of healthy algae were selected at random (Chen et al., 2015), and then put into test tubes containing 5 mL of the reactive medium (pre-cooled at 15, 20, 25, and 30 ℃, individually). The reactive medium was made using 0.1 mol/L pH 7.9 phosphate buffer, 1 mmol/L EDTA, 0.1% 1-propanol, 300 μmol/L NaNO3 and 10 μmol/L glucose. Subsequently, the medium was infused with N2 gas for approximately 2 min to remove oxygen to prevent nitrite from being oxidized to nitrate, medium was then sealed and wrapped using aluminium foil before a 2-h incubation at 15, 20, 25, and 30 ℃. After the incubation, the reaction was completed by removing the thalli from the reactive medium. Approximately 1 mL of the resulting medium was then added to a mixture of 1 mL of 1%w/v sulphanilamide and 1 mL of 0.2% w/v n-(1-napthyl) ethylenediamine dihydrochloride. The absorbance of the medium was then determined at 543 nm. Nitrite concentration in the medium was calculated using a standard curve. The nitrate reductase activity was expressed in μmol/(NO2·h·g FW).
The temperature coefficient Q10 was used to analyze the relationship between temperature and nitrate reductase activity (Rasmusson et al., 2019), and values of Q10 were calculated across temperature intervals (15–30 ℃) as: Q10=(V2/V1)10/(T2–T1), where V1 and V2 are nitrate reductase activity at different temperatures, T1 and T2 (in ℃).
2.5 Soluble protein determinationSoluble protein was extracted by grinding 0.1-g (fresh weight) thalli in 0.1-mol/L phosphate buffer (pH=7.0) in a mortar on ice. Cell debris was removed by centrifuging for 15 min at 5 000 r/min in 4 ℃, and the samples were then assayed according to the modified method of the binding of Coomassie Brilliant Blue G-250 (Bradford, 1976; Read and Northcote, 1981). Approximately 0.1 mL of extract was obtained into the 5 mL of mixture (Coomassie Brilliant Blue G-250) and the absorbance was determined at 595 nm.
The mixture: 100 mg of Coomassie Brilliant Blue G-250 was dissolved in 50 mL of 95% ethanol, and then added with 100 mL of 85% (w/v) phosphoric acid. The solution was diluted to a final volume of 1 L using distilled water.
2.6 Chlorophyll a fluorescence parametersChlorophyll a fluorescence parameters were determined using a pulse amplitude modulated fluorescence monitoring system (Maxi-ImaginePAM, Heinz Walz, Effeltrich, Germany). Samples were placed in dark at different incubation temperatures (15, 20, 25, and 30 ℃) for 10 min before beginning the measurements. The effective quantum yield of PSⅡ [Y(Ⅱ)] is Y(Ⅱ)=(F'm–F0)/F'm (Genty et al., 1989), and the non-photochemical quenching (NPQ) is NPQ=(Fm–F'm)/F′m, where Fm is the maximal fluorescence induced by a saturation pulse from a dark adapted sample, F0 is the minimal fluorescence level measured at measuring light the low frequency, and F'm is the maximal fluorescence level induced by a saturation pulse from algae in active light (111 μmol photons/(m2·s)).
The rapid light curves (RLCs) can be obtained by a series of 20 s light exposures with increasing irradiance (1, 21, 56, 111, 186, 281, 336, 396, 461, 531, and 611 μmol photons/(m2·s)). The parameters of the RLCs were calculated following the photoinhibiton (Eilers and Peeters, 1988) models as follows:
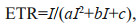 (1)
(1)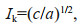 (2)
(2) (3)
(3)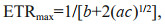 (4)
(4)where ETR is electron transport rate, ETRmax is the light-saturated electron transport rate, α is the electron transport efficiency, I is the incident irradiance, Ik is saturated irradiance, and a, b, and c are the adjustment parameters.
2.7 Data analysisETR was turned into a C-fixation rate according to Silva and Santos (2004), and the nitrate reductase activity was changed to N-incorporation rate according to Collos and Slawyk (1977).
Significance among treatments was tested using the one-way analysis of variance (one-way ANOVA) in SPSS (Version 19). The significant level was set at 0.05. All data were expressed as the mean ± standard deviation (SD, n=3).
3 RESULT 3.1 GrowthGracilariopsis lemaneiformis was cultured for 7 days at 15, 20, 25, and 30 ℃, separately. The maximum RGR (4.36%/d) (P < 0.05) occurred at 20 ℃. The RGR decreased when the temperature was higher than 20 ℃ (P < 0.05) (25 ℃: 3.43%/d; 30 ℃: 3.05%/d; Fig. 1). The appropriate temperature for algal growth was between 15 and 25 ℃, with the optimum temperature being 20 ℃.
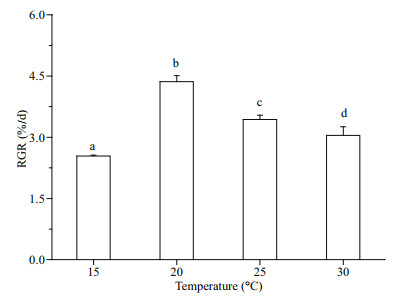
|
| Fig.1 The relative growth rate (RGR) of G. lemaneiformis grown at temperatures of 15, 20, 25, and 30 ℃ The date represents mean±SD (n=3). Different letters represent significant difference between values. |
The nitrate reductase activity was determined at different incubation temperatures (15, 20, 25, and 30 ℃) and the instantaneous responses of nitrate reductase activity to temperature were detected. Figure 2 illustrates the variation of nitrate reductase activity as a function of temperature. Irrespective of growth temperature, the maximum nitrate reductase activity of algae occurred at 30 ℃. Maximum nitrate reductase activity occurred at 15 ℃, and decreased with increasing of temperature (Fig. 2). After comparing nitrate reductase activity determined at the growth temperature, we found that maximum nitrate reductase activity occurred at 20 ℃ (Table 1), which is in accordance with the results of algal growth (the maximum RGR occurred at 20 ℃).
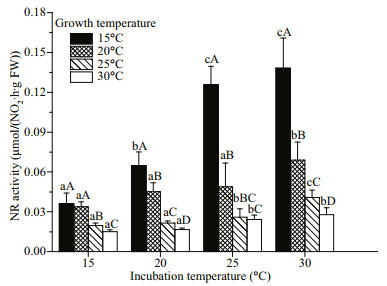
|
| Fig.2 The nitrate reductase activity of G. lemaneiformis determined under different incubation temperature The data are shown in mean±SD (n=3). Different lowercase letters represent significant differences between incubation temperature treatments at the same growth temperature, while different uppercase letters indicate significant differences between growth temperature treatments at the same incubation temperature. |

|
For the nitrate reductase activity measurements, the Q10 values differed substantially with the growth temperature. The Q10 value at 15 ℃ was highest among all the growth temperatures. No significant (P>0.05) was found over the range from 20 to 30 ℃ (Table 1).
Increasing temperatures led to lower soluble protein contents, indicating that lower temperature promoted the accumulation of soluble protein (Table 1).
3.3 Changes in PSⅡ photosynthetic capabilitiesAfter G. lemaneiformis had acclimated to different growth temperatures for 7 days, chlorophyll fluorescence parameters were determined by Maxiimagine PAM. Figure 3 shows the changes of the two photosynthetic properties of PSⅡ, effective quantum yield of PSII [Y(Ⅱ)] and NPQ. The highest Y(Ⅱ) (about 0.36) was found at 20 ℃, which was significantly greater than that at other temperatures (P < 0.05) (15 ℃: 0.28; 25 ℃: 0.28; 30 ℃: 0.24) (Fig. 3a). Contrary to the tendency of changes about Y(Ⅱ) to temperature, the lowest NPQ occurred at 20 ℃. Higher NPQ was found at 25 and 30 ℃, which was almost twice the value at 20 ℃ (Fig. 3b).
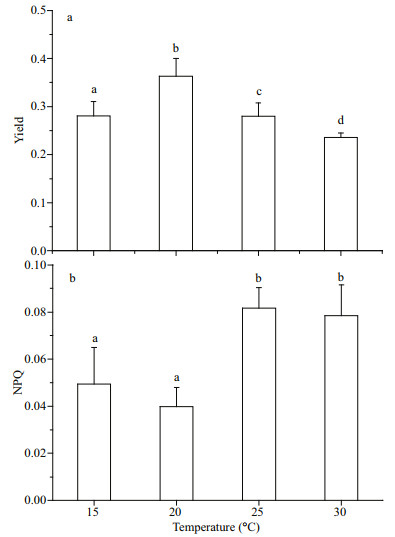
|
| Fig.3 Changes of yield (a) and NPQ (b) of G. lemaneiformis grown at 15, 20, 25, and 30 ℃, individually, under 111 μmol photons/(m2·s) The data represents mean±SD (n=3). Different letters represent significant difference between values |
The rapid light curve shows significantly different responses of photosynthetic performance to temperature (P < 0.05) (Fig. 4). At 20 ℃, the lightsaturated electron transport rate (ETRmax) reached its maximum value, which was significantly greater (P < 0.05) than other temperatures'. The increasing temperature led to a gradual decrease in ETRmax to a lower extent. When the growth temperature increased to 30 ℃, ETRmax decreased to about two fifths of the value at 20 ℃ (P < 0.05) (Table 2). However, irrespective of the growth temperature, the electron transport efficiency (α) showed no significant difference (P>0.05). The saturation light (Ik) of G. lemaneiformis cultured at 15 and 20 ℃ was almost equal, but was significantly greater than that at 25 ℃ (almost 1.7 times), and was even twice as much as the value at 30 ℃ (P < 0.05).
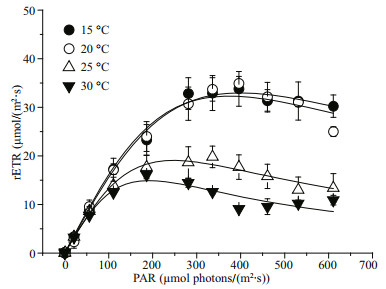
|
| Fig.4 Rapid light curve (RLC) of G. lemaneiformis grown at 15, 20, 25, and 30 ℃ The values were represented as means±SD (n=3). |
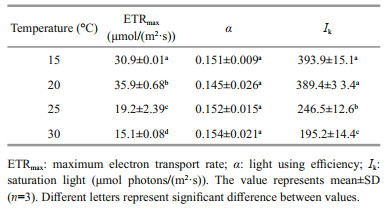
|
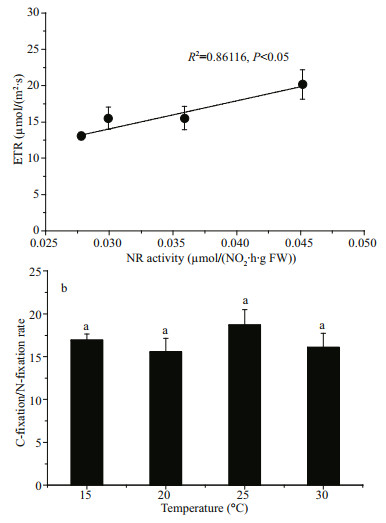
The correlation between nitrate reductase activity and electron transport rate (ETR) illustrates the relationship between nitrate assimilation, photosynthetic character and physiological status. The convincing correlation observed indicated a strong relationship between nitrate assimilation and photosynthesis (R2=0.861 16, P < 0.05; Fig. 5a). As shown in Fig. 5b, the maximal ratio of C-fixation rate and N-incorporation rate occurred at 25 ℃. At higher temperature (30 ℃) the ratio tended to decrease.
|
| Fig.5 Correlation between nitrate reductase activity (measured at its growth temperature) and ETR (a) and the C-fixation / N-fixation rate varied with temperature (b) The values were represented as means±SD (n=3). |
Although the growth of G. lemaneiformis remained positive under different temperatures, and clear effects of temperature on the growth were observed (Fig. 1). We found the maximum RGR occurred at 20 ℃ with 4.36%/d, Zou and Gao (2009) found G. lemaneiformis at 20±1 ℃ with RGR 6%/d, which was 37.6% more, but Xu and Gao (2009) found that RGR (2.4%/d) is less 80%. Different results may be due to the different cultural conditions.
Gracilariopsis lemaneiformis exhibited identical thermal responses for the nitrate reductase activity in the present study. To investigate the optimum temperature for the nitrate reductase activity of G. lemaneiformis in vivo assay, four different incubation temperatures (15, 20, 25, and 30 ℃) were determined, with the incubation temperature optima for the nitrate reductase activity assay in vivo should be the growth temperature in the present study. At 20 ℃, the maximum nitrate reductase activity was 0.045 μmol/(NO2·h·g FW), which is 31% less than that Xu and Gao (2012) found. NH+4 concentration in our natural filtrated seawater was 1.92 μmol/L, which might inhibit nitrate reductase activity partly (Chow and De Oliveira, 2008). The natural filtrated seawater we enriched with 100-μmol/L NaNO3. Nitrate is one of the most important factors regulating nitrate reductase activity (Chow and De Oliveira, 2008). Addition of nitrate can induce high nitrate reductase activity and reduce the toxic effect on the algae. On the optimum incubation temperature selected for the nitrate reductase activity assay in vivo, previous studies suggested that the nitrate reductase activity shall be determined at the optimum incubation temperature, which should be close to the maximum activity (Kristiansen, 1983; Corzo and Niell, 1991; Gao et al., 2000; Chow et al., 2004). Regardless of species and growth temperature. G. lemaneiformis cultured at different temperatures had its maximum nitrate reductase activity at 30 ℃ (incubation temperature), 15 ℃-cultured alga had 0.135 μmol/(NO2·h·g FW) similar to the nitrate reductase activity of Ulva rigida (Corzo and Niell, 1991) and Hizikia fusiforme (Zou, 2005) when assayed in vivo at 30 ℃, but lower than Gracilaria chilensis assayed in vitro, which represents a theoretical maximum for in vivo activity of the enzyme (Chow and De Oliveira, 2008). According to previous studies, 30 ℃ should be the optimum incubation temperature for the nitrate reductase activity assay, as shown in the Fig. 2. However, the actual optimum temperature for algal growth was 20 ℃ (Fig. 1), the same as the temperature for the maximum nitrate reductase activity of thalli determined at its growth temperature (Table 1). The optimum temperature for the maximum nitrate reductase activity did not accord with the optimum temperature for the algal growth. For Thalassiosira nordenskioeldii and Heterocapsa triquetra, the optimum temperature for nitrate reductase activity corresponded well with the optimum temperature for the growth (Jitts et al., 1964). Considering the importance of algal physiological status, we believe that the optimum temperature for the nitrate reductase activity in vivo assay is 20 ℃.
To determine the optimum temperature for nitrate reductase activity in vivo assay, we studied the relationship between the photosynthesis and nitrate reductase activity. The assimilation of nitrate and the synthesis of enzymes, such as nitrate reductase are linked fundamentally with photosynthesis in alga (Thomas et al., 1976). After long-term acclimation (7 days), the physiological performances of alga were changed. Thalli usually have a series of mechanisms to respond the changes caused by environmental factors variation. For example, plants can optimize photosynthesis irradiance at different temperatures (Staehr and Wernberg, 2009) and increase carbon concentration mechanism expression when light intensity increases (Raven et al., 2011). Photosynthesis often displays an optimal temperature, which corresponds to the median of the non-harmful range, and decreases when the temperature increases above the thermal optimum (Sage and Kubien, 2007). At 15 and 20 ℃, the cultured algae had better photosynthetic performance than those cultured at 25 and 30 ℃ (Fig. 4). This tendency of change about ETRmax was the same as the nitrate reductase activity measured at its growth temperature. To promote the growth of alga, sufficient carbohydrate and protein are required. As a result, nitrate assimilation and carbon metabolism are tightly correlated (Turpin and Weger, 1988; Vanlerberghe et al., 1990; Turpin, 1991). This close connection arises from the reducing power and carbon-skeleton requirements of the synthesis of amino acids from ammonium produced during nitrate assimilation. Consequently, if either of them is affected, the other will also be affected. Meanwhile, the occurrence of maximal activities of both processes should be synchronized because it is crucial in decreasing nitrite toxicity, given that nitrite assimilation reduces nitrite to ammonium and requires reduced ferredoxin. Therefore, tight regulation between nitrate assimilation and photosynthesis has been found in many macroalgae (Chow et al., 2004; Gao et al., 2016; Xu et al., 2017), as showed in Fig. 5.
Temperature is one of the most important factors controlling plant distribution and productivity (Davison, 1991; Sage and Kubien, 2007). When algae adapted to different temperatures for a long term (7 days), its photosynthetic performance showed significantly different characteristics (Figs. 3 & 4). Low temperature can impair the synthesis and function of photosynthetic pigment-protein complexes and down-regulate the activities of key enzymes in the Calvin cycle to influence the photosynthesis, while the fluidity of membranes can be enhanced in high temperature, leading to a disintegration of the lipid bilayer ultimately (Nie et al., 1995; Los and Murata, 2004). Temperature at 20 ℃ was the optimum temperature for algal photosynthesis, at which algae had the maximum ETRmax and Y(Ⅱ), but the lowest NPQ, indicating that plants cultured at 20 ℃ could provide more energy for carboxylation operation, stimulated organic material synthesis for faster growth. At the same time, the maximum nitrate reductase activity occurred also at 20 ℃ when determined at its growth temperature, so nitrate assimilation could obtain enough needed material and power to insert the nitrogen into the carbon skeleton without producing more toxic nitrite to damage alga (Chow et al., 2004). When the growth temperature decreased to 15 ℃, algal photosynthesis remained relatively unchanged (Fig. 4), but the nitrate reductase activity was much lowered when determined at its growth temperature (Table 1), showing lower nitrate assimilation. As a consequence, the growth of alga was relatively lower (Fig. 1). According to the results in Table 1, the lower temperature promoted high soluble protein contents, and at 15 ℃ cultured alga had the maximum nitrate reductase activity measured at different incubation temperatures and Q10 (15, 20, 25, and 30 ℃; Table 1). This may be attributed to the fact that the contents of nitrate reductase was higher at 15 ℃. However, when the growth temperature increased to 25 and 30 ℃, both algal photosynthesis and nitrate reductase activity were strongly inhibited. Compared with that of algae cultured at 15 and 20 ℃, high-temperature cultured algae had lower photosynthetic performance and nitrate reductase activity but higher NPQ levels, demonstrating that at 25 and 30 ℃ damage had already occurred in the thalli. At the end of the culturing period at 30 ℃, a part of the alga had begun to decompose (data not shown), indicating that G. lemaneiformis cannot tolerate high temperature beyond 25 ℃. Besides, C꞉N fixation rate ratio varied with temperature, and the optimum temperature was found at 25 ℃. It began to decrease at 30 ℃, which might be due to the inhibited photosynthesis.
5 CONCLUSIONIn conclusion, the photosynthetic performance and nitrate reductase activity of G. lemaneiformis were significantly affected by temperature, and the optimum temperature for nitrate reductase activity in vivo assay was the same as growth temperature. Additionally, low temperature (15 ℃) was in favour of accumulation of nitrate reductase, but higher temperature (30 ℃) could enhance the activity of nitrate reductase. Low temperature (15 ℃) cultured G. lemaneiformis meets sudden high temperature (25 and 30 ℃) is beneficial to N assimilation.
6 DATA AVAILABILITY STATEMENTThe authors declare that all data in the present study are available upon request.
Berry J, Bjorkman O. 1980. Photosynthetic response and adaptation to temperature in higher plants. Annual Review of Plant Biology, 31(1): 491-543.
DOI:10.1146/annurev.pp.31.060180.002423 |
Bradford M M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72(1): 248-254.
DOI:10.1016/0003-2697(76)90527-3 |
Cabello-Pasini A, Macías-Carranza V, Abdala R, Korbee N, Figueroa F L. 2011. Effect of nitrate concentration and UVR on photosynthesis, respiration, nitrate reductase activity, and phenolic compounds in Ulva rigida(Chlorophyta). Journal of Applied Phycology, 23(3): 363-369.
DOI:10.1007/s10811-010-9548-0 |
Campbell W H. 1999. Nitrate reductase structure, function and regulation:bridging the gap between biochemistry and physiology. Annual Review of Plant Biology, 50(1): 277-303.
DOI:10.1146/annurev.arplant.50.1.277 |
Chen B B, Zou D H, Du H, Ji Z W. 2018. Carbon and nitrogen accumulation in the economic seaweed Gracilaria lemaneiformis affected by ocean acidification and increasing temperature. Aquaculture, 482: 176-182.
DOI:10.1016/j.aquaculture.2017.09.042 |
Chen B B, Zou D H, Jiang H. 2015. Elevated CO2 exacerbates competition for growth and photosynthesis between Gracilaria lemaneiformis and Ulva lactuca. Aquaculture, 443: 49-55.
DOI:10.1016/j.aquaculture.2015.03.009 |
Chow F, Oliveira M C. 2008. Rapid and slow modulation of nitrate reductase activity in the red macroalga Gracilaria chilensis (Gracilariales, Rhodophyta):influence of different nitrogen sources. Journal of Applied Phycology, 20: 775-782.
DOI:10.1007/s10811-008-9310-z |
Chow F, Capociama F V, Faria R, Oliveira M C. 2007. Characterization of nitrate reductase activity In vitro in Gracilaria caudata J. Agardh (Rhodophyta, Gracilariales).Brazilian Journal of Botany, 30(1): 123-129.
DOI:10.1590/S0100-84042007000100012 |
Chow F, Macchiavello J, Cruz S S, Fonck E, Olivares J. 2001. Utilization of Gracilaria chilensis (Rhodophyta:Gracilariaceae) as a biofilter in the depuration of effluents from tank cultures of fish, oysters, and sea urchins. Journal of the World Aquaculture Society, 32(2): 215-220.
DOI:10.1111/j.1749-7345.2001.tb01098.x |
Chow F, Oliveira M C, Pedersén M. 2004. In vitro assay and light regulation of nitrate reductase in red alga Gracilaria chilensis. Journal of Plant Physiology, 161(7): 769.
DOI:10.1016/j.jplph.2004.01.002 |
Chow F, Pedersén M, Oliveira M C. 2013. Modulation of nitrate reductase activity by photosynthetic electron transport chain and nitric oxide balance in the red macroalga Gracilaria chilensis (Gracilariales, Rhodophyta). Journal of Applied Phycology, 25(6): 1847-1853.
DOI:10.1007/s10811-013-0005-8 |
Collos Y, Slawyk G. 1977. Nitrate reductase activity as a function of in situ nitrate uptake and environmental factors of euphotic zone profiles. Journal of Experimental Marine Biology and Ecology, 29(2): 119-130.
DOI:10.1016/0022-0981(77)90043-0 |
Corzo A, Niell F X. 1991. Determination of nitrate reductase activity in Ulva rigida C. Agardh by the in situ method.Journal of Experimental Marine Biology and Ecology, 146(2): 181-191.
DOI:10.1016/0022-0981(91)90024-Q |
Crawford N M, Arst Jr H N. 1993. The molecular genetics of nitrate assimilation in fungi and plants. Annual Review of Genetics, 27(1): 115-146.
DOI:10.1146/annurev.ge.27.120193.000555 |
Davison I R. 1991. Environmental effects on algal photosynthesis:temperature. Journal of Phycology, 27(1): 2-8.
DOI:10.1111/j.0022-3646.1991.00002.x |
Dovis V L, Hippler F W R, Silva K I, Ribeiro R V, Machado E C, Mattos Jr D. 2014. Optimization of the nitrate reductase activity assay for citrus trees. Brazilian Journal of Botany, 37(4): 383-390.
DOI:10.1007/s40415-014-0083-0 |
Eilers P, Peeters J. 1988. A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecological Modelling, 42(3): 199-215.
DOI:10.1016/0304-3800(88)90057-9 |
Eppley R W, Packard T T, MacIsaac J J. 1970. Nitrate reductase in Peru Current phytoplankton. Marine Biology, 6(3): 195-199.
DOI:10.1007/BF00347227 |
Gao G, Clare A S, Rose C, Caldwell G S. 2017. Intrinsic and extrinsic control of reproduction in the green tide-forming alga, Ulva rigida. Environmental and Experimental Botany, 139: 14-22.
DOI:10.1016/j.envexpbot.2017.03.016 |
Gao G, Clare A S, Rose C, Caldwell G S. 2018. Ulva rigida in the future ocean:potential for carbon capture, bioremediation and biomethane production. Global Change Biology Bioenergy, 10: 39-51.
DOI:10.1111/gcbb.12465 |
Gao G, Zhong Z H, Zhou X H, Xu J T. 2016. Changes in morphological plasticity of Ulva prolifera under different environmental conditions:a laboratory experiment. Harmful Algae, 59: 51-58.
DOI:10.1016/j.hal.2016.09.004 |
Gao Y, Smith G J, Alberte R S. 1992. Light regulation of nitrate reductase in Ulya fenestrata (Chlorophyceae). Marine Biology, 112(4): 691-696.
DOI:10.1007/BF00346188 |
Gao Y, Smith G J, Alberte R S. 2000. Temperature dependence of nitrate reductase activity in marine phytoplankton:biochemical analysis and ecological implications. Journal of Phycology, 36(2): 304-313.
DOI:10.1046/j.1529-8817.2000.99195.x |
Genty B, Briantais J M, Baker N R. 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta (BBA)-General Subjects, 990(1): 87-92.
DOI:10.1016/S0304-4165(89)80016-9 |
González-Galisteo S, Packard T T, Gómez M, Herrera A, Dugdale R C, Wilkerson F P, Barber R T, Blasco D, Christensen J P, Codispoti L A. 2019. Calculating new production from nitrate reductase activity and light in the Peru current upwelling. Progress in Oceanography, 173: 78-85.
DOI:10.1016/j.pocean.2019.02.009 |
Gu Y Y, Cheong K L, Du H. 2017. Modification and comparison of three Gracilaria spp. Agarose with methylation for promotion of its gelling properties. Chemistry Central Journal, 11: 104.
DOI:10.1186/s13065-017-0334-9 |
Jitts H R, McAllister C D, Stephens K, Strickland J D H. 1964. The cell division rates of some marine phytoplankters as a function of light and temperature. Journal of the Fisheries Research Board of Canada, 21(1): 139-157.
DOI:10.1139/f64-012 |
Kristiansen S. 1983. The temperature optimum of the nitrate reductase assay for marine phytoplankton. Limnology and Oceanography, 28(4): 776-780.
DOI:10.4319/lo.1983.28.4.0776 |
Lapointe B E, Duke C S. 1984. Biochemical strategies for growth of Gracilaria tikvahiae (Rhodophyta) in relation to light intensity and nitrogen availability. Journal of Phycology, 20(4): 488-495.
DOI:10.1111/j.0022-3646.1984.00488.x |
Liu X J, Wen J Y, Chen W Z, Du H. 2019. Physiological effects of nitrogen deficiency and recovery on the macroalga Gracilariopsis lemaneiformis (Rhodophyta). Journal of Phycology, 55: 830-839.
DOI:10.1111/jpy.12862 |
Lopes P F, Oliveira M C, Colepicolo P C. 1997. Diurnal fluctuation of nitrate reductase activity in the marine red alga Gracilaria tenuistipitata (Rhodophyta). Journal of Phycology, 33(2): 225-231.
DOI:10.1111/j.0022-3646.1997.00225.x |
Los D A, Murata N. 2004. Membrane fluidity and its roles in the perception of environmental signals. Biochimica et Biophysica Acta, 1666: 142-157.
DOI:10.1016/j.bbamem.2004.08.002 |
Nie G Y, Robertson E J, Fryer M J, Leech R M, Baker N R. 1995. Response of the photosynthetic apparatus in maize leaves grown at low temperature on transfer to normal growth temperature. Plant, Cell & Environment, 18: 1-12.
DOI:10.1111/j.1365-3040.1995.tb00538.x |
Rasmusson L M, Gullström M, Gunnarsson P C B, George R, Björk M. 2019. Estimation of a whole plant Q10 to assess seagrass productivity during temperature shifts. Scientific Reports, 9: 12667.
DOI:10.1038/s41598-019-49184-z |
Raven J A, Giordano M, Beardall J, Maberly S C. 2011. Algal and aquatic plant carbon concentrating mechanisms in relation to environmental change. Photosynthesis Research, 109(1-3): 281-296.
DOI:10.1007/s11120-011-9632-6 |
Read S M, Northcote D. 1981. Minimization of variation in the response to different proteins of the Coomassie blue G dyebinding assay for protein. Analytical Biochemistry, 116(1): 53-64.
DOI:10.1016/0003-2697(81)90321-3 |
Sage R F, Kubien D S. 2007. The temperature response of C3 and C4 photosynthesis. Plant, Cell and Environment, 30: 1086-1106.
DOI:10.1111/j.1365-3040.2007.01682.x |
Silva J, Santos R. 2004. Can chlorophyll fluorescence be used to estimate photosynthetic production in the seagrass Zostera noltii?. Journal of Experimental Marine Biology and Ecology, 307(2): 207-216.
DOI:10.1016/j.jembe.2004.02.009 |
Staehr P A, Wernberg T. 2009. Physiological responses of Ecklonia radiata (Laminariales) to a latitudinal gradient in ocean temperature. Journal of Phycology, 45(1): 91-99.
DOI:10.1111/j.1529-8817.2008.00635.x |
Teichberg M, Heffner L R, Fox S, Valiela I. 2007. Nitrate reductase and glutamine synthetase activity, internal N pools, and growth of Ulva lactuca:responses to long and short-term N supply. Marine Biology, 151(4): 1249-1259.
DOI:10.1007/s00227-006-0561-4 |
Thomas R J, Hipkin C R, Syrett P J. 1976. The interaction of nitrogen assimilation with photosynthesis in nitrogen deficient cells of Chlorella. Planta, 133(1): 9-13.
DOI:10.1007/BF00385999 |
Turpin D H, Weger H G. 1988. Steady-state chlorophyll a fluorescence transients during ammonium assimilation by the N-limited green alga Selenastrum minutum. Plant Physiology, 88(1): 97-101.
DOI:10.1104/pp.88.1.97 |
Turpin D H. 1991. Effects of inorganic N availability on algal photosynthesis and carbon metabolism. Journal of Phycology, 27(1): 14-20.
DOI:10.1111/j.0022-3646.1991.00014.x |
Vanlerberghe G C, Schuller K A, Smith R G, Feil R, Plaxton W C, Turpin D H. 1990. Relationship between NH4+ assimilation rate and in vivo phosphoenolpyruvate carboxylase activity:regulation of anaplerotic carbon flow in the Green Alga Selenastrum minutum. Plant Physiology, 94(1): 284-290.
DOI:10.1104/pp.94.1.284 |
Varela D A, Hernríquez L A, Fernández P A, Leal P, HernándezGonzález M C, Figueroa F L, Buschmann A H. 2018. Photosynthesis and nitrogen uptake of the giant kelp Macrocystis pyrifera (Ochrophyta) grown close to salmon farms. Marine Environmental Research, 135: 93-102.
DOI:10.1016/j.marenvres.2018.02.002 |
Vona V, Martino Rigano Di V, Lobosco O, Carfagna S, Esposito S, Rigano C. 2004. Temperature responses of growth, photosynthesis, respiration and NADH:nitrate reductase in cryophilic and mesophilic algae. New Phytologist, 163(2): 325-331.
DOI:10.1111/j.1469-8137.2004.01098.x |
Xu Z G, Gao G, Xu J T, Wu H Y. 2017. Physiological response of a golden tide alga (Sargassum muticum) to the interaction of ocean acidification and phosphorus enrichment. Biogeosciences, 14: 671-681.
DOI:10.5194/bg-14-671-2017 |
Xu Z G, Gao K S. 2009. Impacts of UV radiation on growth and photosynthetic carbon acquisition in Gracilaria lemaneiformis (Rhodophyta) under phosphorus-limited and replete conditions. Functional Plant Biology, 36: 1057-1064.
DOI:10.1071/FP09092 |
Xu Z G, Gao K S. 2012. NH+4 enrichment and UV radiation interact to affect the photosynthesis and nitrogen uptake of Gracilaria lemaneiformis (Rhodophyta). Marine Pollution Bulletin, 64: 99-105.
DOI:10.1016/j.marpolbul.2011.10.016 |
Yang H, Zhou Y, Mao Y Z, Li X X, Liu Y, Zhang F. 2005. Growth characters and photosynthetic capacity of Gracilaria lemaneiformis as a biofilter in a shellfish farming area in Sanggou Bay, China. Journal of Applied Phycology, 17(3): 199-206.
DOI:10.1007/s10811-005-6419-1 |
Yang Y F, Fei X G, Song J M, Hu H Y, Wang G C, Chung I K. 2006. Growth of Gracilaria lemaneiformis under different cultivation conditions and its effects on nutrient removal in Chinese coastal waters. Aquaculture, 254(1): 248-255.
DOI:10.1016/j.aquaculture.2005.08.029 |
Young E B, Dring M J, Savidge G, Birkett D A, Berges J A. 2007. Seasonal variations in nitrate reductase activity and internal N pools in intertidal brown algae are correlated with ambient nitrate concentrations. Plant, Cell and Environment, 30(6): 764-774.
DOI:10.1111/j.1365-3040.2007.01666.x |
Yu J, Yang Y F. 2008. Physiological and biochemical response of seaweed Gracilaria lemaneiformis to concentration changes of N and P. Journal of Experimental Marine Biology and Ecology, 367(2): 142-148.
DOI:10.1016/j.jembe.2008.09.009 |
Zou D H, Gao K S. 2009. Effects of elevated CO2 on the red seaweed Gracilaria lemaneiformis (Gigartinales, Rhodophyta) grown at different irradiance levels. Phycologia, 48(6): 510-517.
DOI:10.2216/08-99.1 |
Zou D H. 2005. Effects of elevated atmospheric CO2 on growth, photosynthesis and nitrogen metabolism in the economic brown seaweed, Hizikia fusiforme(Sargassaceae, Phaeophyta). Aquaculture, 250(3): 726-735.
DOI:10.1016/j.aquaculture.2005.05.014 |
 2021, Vol. 39
2021, Vol. 39


