Institute of Oceanology, Chinese Academy of Sciences
Article Information
- da COSTA Flávia Maria Pereira, PIRES-VANIN Ana Maria Setubal
- An illustrated checklist of macrofaunal molluscs from soft-sediments of the Abrolhos Parcel (northeastern, Brazil) with 15 new records
- Journal of Oceanology and Limnology, 39(2): 671-682
- http://dx.doi.org/10.1007/s00343-019-9161-2
Article History
- Received Jun. 29, 2019
- accepted in principle Sep. 11, 2019
- accepted for publication Dec. 3, 2019
Mollusca is one of the most specious animal groups on Earth, being surpassed only by the arthropods. In marine environments, the diversity of mollusc species (c. 52 000) is comparable to arthropods (c. 55 000 species), making up 25% of all species reported for the marine benthos (Bouchet, 2006; Appeltans et al., 2012). Mollusc diversity is also notable high in tropical waters, particularly in reef areas (Bouchet et al., 2002).
Mollusc inventories have often been conducted in different marine ecosystems (Kantor and Sysoev, 2005). In Brazil, with the exception of some pioneering work (e.g., Morretes, 1949; Rios 1970, 1985), mollusc diversity remains poorly known in many regions, and marine micromolluscs (i.e., specimens smaller than 10 mm) are especially neglected (Bouchet et al., 2002; Absalão, 2005). These small organisms require specific procedures to collect and classify, and the taxonomy of many species is very problematic (Bouchet et al., 2002). Most of studies are focused on coastal areas due to easier access and lower costs compared to the open sea. Consequently, many of these areas need to be researched to better understand their faunas. This knowledge has proved fundamental for ecological studies in view of the growing interest in biodiversity datasets for conservation and management purposes. Accordingly, preliminary species list are fundamental for environmental studies at large (Bouchet, 1997; Gofas et al., 2017).
The invertebrate fauna of the Abrolhos Reef Bank is largely unknown, despite the biological importance of the area, which houses the massive coral reef formation in the South Atlantic. Due to the ecological significance of molluscs and the area itself the present study grows in relevance.
2 MATERIAL AND METHODSpecimens were collected during the Benthos Sub-project within the PROABROLHOS Project (Productivity and Sustainability of the Abrolhos Bank) with the objective of studying the distribution and structure of benthic fauna in coral reefs of the Abrolhos Bank, Eastern Brazilian Continental Shelf.
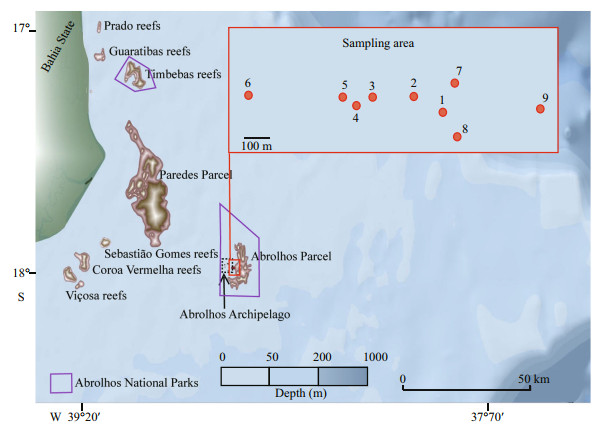
Abrolhos Bank (16°40'S-19°40'S and 37°20'W-39°10'W) is the largest extension of the Eastern Brazilian Continental Shelf, reaching up to 200 km in length. It encompasses the largest known reef complex in the South Atlantic and covers about 46 000 km2, of which about 18% is occupied by coral reefs (Melo et al., 1975; Muehe, 1987). The site is recognized unique because it has a large number of sediments〞increasing the water turbidity in some areas, mushroom-shaped reef structures, and a high degree of endemism of coral species (Leão, 1999; Leão et al., 2003; Dutra et al., 2005). The study site is located in the Abrolhos Parcel at the Abrolhos Bank, a large system of submerged reefs within the Abrolhos Marine National Park, created in 1983. It extends from north to southeast of the Abrolhos Archipelago and marks the transition between siliciclastic and carbonatic sediments.
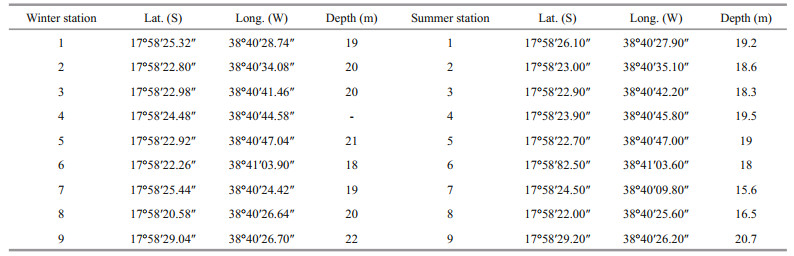
Nine sites were sampled in uncompacted sediments in two campaigns (July 2007 and January 2008) in the Abrolhos Parcel (Fig. 1; Table 1) with a 0.042-m2 van Veen grab in triplicate. The retrieved sediments were sieved through a 500-μm mesh and preserved in 70% ethanol. In the laboratory, molluscs were identified based on Rios(1985, 2009); Warén(1980, 1983) for Eulimidae; Lima and Christoffersen (2016) and Lima et al. (2013) for Caecidae; and Silva-Filho et al. (2012) for Scaphopoda. Only samples with soft parts were analyzed in order to avoid mixing species from distant locations carried by the surrounding water currents. Scientific names were updated after the World Register of Marine Species taxonomic database (WoRMS Editorial Board, 2018), and the main shell measurements were taken from digital photographs using the ImageJ software (Rasband and Image, 1997-2018). Specimens are deposited in the Museu de Zoologia (MZSP) and Coleção Biológica Prof. Edmundo F. Nonato, both from the University of São Paulo, and part in the Museu Nacional do Rio de Janeiro (MNRJ).
|
| Fig.1 Abrolhos Bank in eastern Brazilian Continental Shelf and sampling sites in the Abrolhos Parcel |
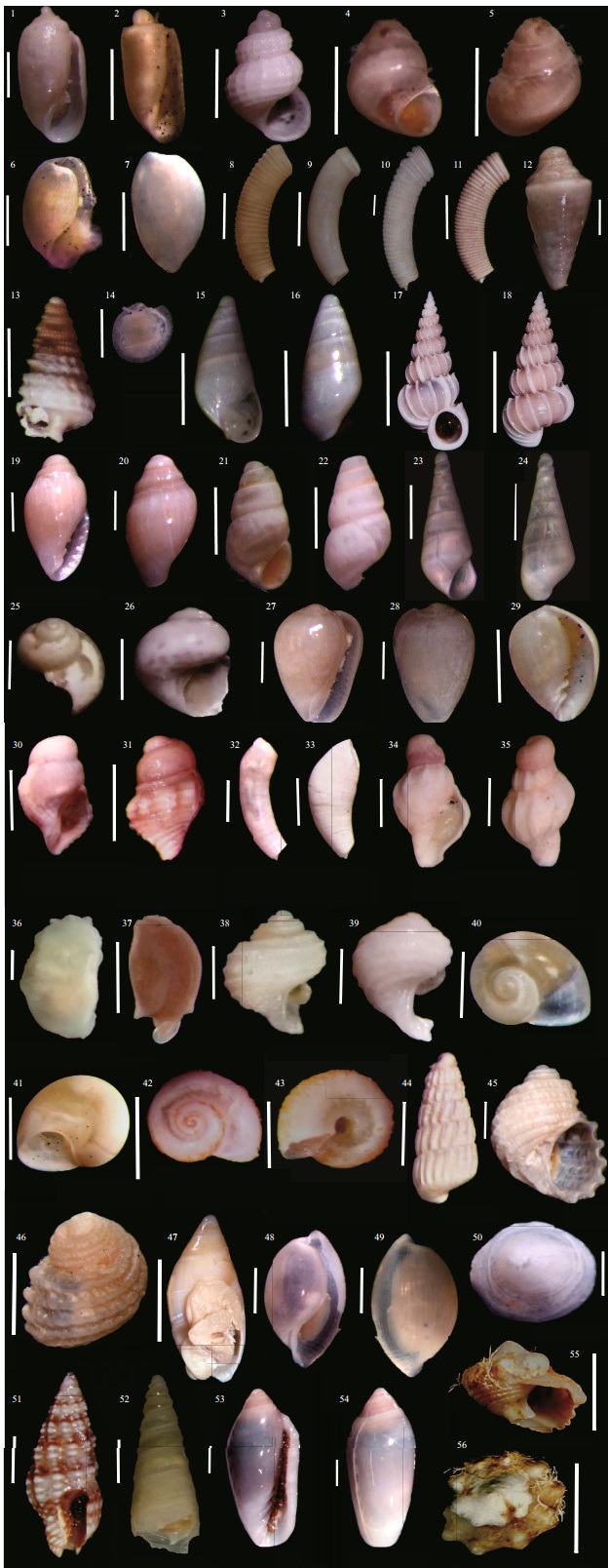
We identified 100 species belonging to 85 genera and 59 families (Table 2). Although most of the diversity of marine molluscs comprises gastropods (Appeltans et al., 2012), we found a similar number of bivalve species in the present samples. Gastropoda comprised 31 families and 42 genera, while Bivalvia was represented by 26 families and 40 genera (Figs. 2 & 3). Of the 100 species recorded, 50 were gastropods, 46 bivalves, 3 scaphopods and 1 solenogastres. In addition, many recorded taxa are probably part of complexes of species that still need revision, such as Gibberula lavalleeana, Lapinura divae and Atys sp. In some cases, juvenile specimens were classified above the species level, mainly at the genus level, and listed as "sp." like the Thyasira, Okenia, and Galeommatoidea, and await further studies.

|
|
| Fig.2 Gastropod specimens sampled in soft sediment at Abrolhos Parcel (NE Brazil) Correspondence between the name of the species and its respective code number: (1) Acteocina candei; (2) Acteocina lepta; (3) Alvania auberiana; (4-5) Amphithalamus glabrus; (6-7) Atys riiseanus; (8) Caecum brasilicum; (9) Caecum circumvolutum; (10) Caecum floridanum; (11) Caecum metamorphosicum; (12) Conus sp.; (13) Cosmotriphora melanura; (14) Crepidula sp.; (15-16) Curveulima sp.; (17-18) Epitonium novangliae; (19-20) Eratoidea janeiroensis; (21-22) Eulimastoma sp.; (23-24) Eulimostraca sp.; (25-26) Gabrielona sulcifera; (27-28) Gibberula lavalleeana; (29) Granulina ovuliformis; (30-31) Leucozonia sp.; (32) Meioceras cubitatum; (33) Meioceras nitidum; (34-35) Muricopsis necocheana; (36) Notarchus sp.; (37) Lapinura divae; (38-39) Parviturbo rehderi; (40-41) Solariorbis shimeri; (42-43) Teinostoma aff. megastoma; (44) Turbonilla sp.; (45-46) Vanikoro sulcatus; (47) Amalda josecarlosi; (48-49) Atys sp.; (50) Calyptraea centralis; (51) Steironepion minus; (52) Turritella exoleta; (53-54) Volvarina serrei; (55-56) Modulus modulus. Scale bars: 0.5 mm for 1-16, 19-35, and 37-46; 1 mm for 36 and 47-54; 5 mm for 17-18 and 55-56. |

|
| Fig.3 Bivalvia, Scaphopoda, and Solenogastres sampled in soft sediment at Abrolhos Parcel (NE Brazil) Correspondence between the name of the species and its respective code number: (57) Sheldonella bisulcata; (58) Carditopsis smithii; (59-60) Chama sp.; (61) Crassinella lunulata; (62) Crenella decussata; (63) Cyclopecten sp.; (64) Diplodonta punctata; (65) Guyanella clenchi; (66) Lasaea sp.; (67) Lioberus castanea; (68) Musculus lateralis; (69) Nucinella serrei; (70) Parvilucina pectinella; (71) Solemya notialis; (72) Semele proficua; (73) Temnoconcha galathaea; (74) Yoldia sp.; (75) Clathrolucina costata; (76-77) Corbula operculata; (78) Crassinella lunulata; (79) Crassinella martinicensis; (80) Caryocorbula dietziana; (81) Ervilia nitens; (82) Galeommatoidea sp.; (83) Tucetona pectinata; (84) Gouldia cerina; (85) Limaria pellucida; (86) Musculus lateralis; (87) Nucula crenulata; (88) Nuculana concentrica; (89) Papyridea semisulcata; (90) Pinctada imbricata; (91) Pleurolucina sp.; (92) Similipecten sp.; (93) Episiphon sowerbyi; (94) Thyasira trisinuata; (95) Transennella cubaniana; (96) anterior aperture of Dentalium laqueatum; (97) Dentalium laqueatum; (98) anterior aperture of Paradentalium gouldii; (99) Paradentalium gouldii; (100) Chione cancellata; (101) Euvola ziczac; (102) Ameritella sybaritica; (103) Solenogastres sp. Scale bars: 0.5 mm for 57-74; 1 mm for 75-94; 5 mm for 95, 100 and 102; 2 mm for 96-99; 10 mm for 101; 0.2 mm for 103. |
Although the sampling method employed here is most suitable for collecting endofaunistic organisms, we found a significant number of epifaunal species, such as Crenella decussata, Lioberus castanea, Musculus lateralis, Pinctada imbricata, Cyclopecten sp., Similipecten sp., Chama sp., Euvola ziczac, Limaria pellucida, Calyptraea centralis, Conus sp., Aplysia parvula and Solenogastres, as well as parasitic groups such as Curveulima sp., Eulimastoma sp., Eulimostraca sp., Melanella sp., and Turbonilla sp.
The species reported here represent almost 6% of all marine mollusc diversity recorded for Brazil according to Rios (2009). The present paper adds 48 species to the previous list by Absalão (2005) of the Abrolhos Bank, which highlights the relevance of the present samples.
Lamellaria branca Simone, 2004 is currently recorded in Uruguay and São Paulo State, Brazil, from 58-134 m depth, and the Brazilian endemic species Amphithalamus glabrus Simone, 1996 has been found in shallow waters (5 m) off São Paulo State (23°S). The geographic distribution of both species is extended herein north to Abrolhos, Bahia State (17°58'S), and their bathymetric range is expanded to 18 and 16.5 m, respectively. Similarly, Amalda josecarlosi, Diplodonta punctata, Solariorbis shimeri, Temnoconcha galathaea, Transennella cubaniana, Eratoidea janeiroensis, Nuculana concentrica, and Solemya notialis are firstly reported to Bahia State, but their depth ranges remain the same. Notarchus, of which two species are known from the Mediterranean and the Indo-Pacific, and Curveulima, recorded for northern Atlantic and Indo-Pacific, are herein recorded for the first time in Brazil. Moreover, two additional species have their distribution extended to Abrolhos: Guyanella clenchi, previously reported from the Caribbean Sea, and Okenia impexa from São Paulo State. Furthermore, some other species had their geographic and bathymetric ranges addressed, such as Nucinella serrei and Similipecten sp., usually reported in deeper waters (Taylor and Glover, 2010).
The largest number of specimens was grouped in Caecidae and Semelidae, with six and four species, respectively. Despite the overall high number of species registered in the area, only seven were found in all sites. In terms of abundance, Caecum brasilicum, Caecum floridanum, Crenella decussata, and Solenogastres sp. represented over 50% of the specimens. The genus Caecum Fleming, 1813 was widely distributed in the area and is the largest group with 634 specimens, with the minute Caecum floridanum Stimpson, 1851 as the most abundant species (20.7% of all molluscs sampled). This occurrence was probably related to the ability of this species to exploit a large number of habitats. Caecum species are typically associated with mangrove roots, rocks, limestone substrates, and sediment grains adjacent to the reefs (Abbott, 1974; Mello and Maestrati, 1986; Pizzini et al., 1998).
The presence of the bivalve families Lucinidae (5 spp.), Thyasiridae (2 spp.) and Solemyidae (1 sp.) in the Abrolhos Parcel is remarkable. Species of these families often live in organically enriched environments such as mangroves, dense algal beds (Glover et al., 2008) and sewage disposal sites (Reid and Brand, 1986) associated with chemosynthetic bacteria (Dame, 1996). In Lucinidae, the most diverse group of symbiotic molluscs, the endosymbiotic relationship with sulphide-oxidizing or even methane-oxidizing bacteria is mandatory (Taylor and Glover, 2016; Mikkelsen and Bieler, 2008). Although already addressed to oligotrophic environments (Glover and Taylor, 2007), the presence of these species in poorly-known areas reinforces the relevance of inventory studies. This is especially true when these areas are compared to other heavily sample reef environments, such as those of the Indo-West Pacific, which are known to have Lucinidae as one of the most abundant bivalve families (Bouchet et al., 2002).
The present work also contributed to the expansion of the geographical distribution of Solemya notialis Simone, 2009 and Guyanella clenchi Altena, 1968, both species previously known to have very restricted distributions. The first one is described to Southern Brazil and the second was previously reported to the Caribbean Sea (Taylor and Glover, 2016).
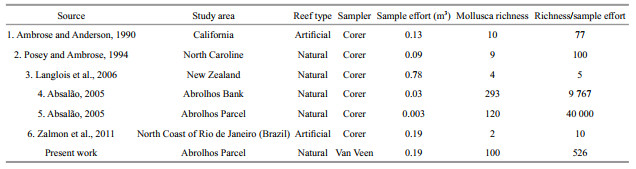
Regarding the relatively small area and narrow bathymetric range accessed, the high number of reported species suggests that much more can be found with a significant intensification of sampling. However, we assume that species richness continually increases with increasing sediment volume, but, as this may not be entirely true, this value may be overestimated. Table 3 lists the species richness data obtained from other papers dealing with the number of mollusc species in areas elsewhere. The sample effort of each data was transformed to cubic meters (m3) to allow comparisons. Our results indicate a significant number of species for the Abrolhos Parcel, about 5 times higher than that reported for the 23-mile Reef in North Carolina (Posey and Ambrose, 1994). In fact, the number of species is astonishing in the Abrolhos Bank as a whole. Despite the raw number reported by Absalão (2005) for Abrolhos Bank being almost thrice that from our results, we found a similar number of species when considering only the Abrolhos Parcel. The small discrepancy in numbers that favors the results of Absalão may be related to the origin of the material, as the author examined live specimens and empty shells in his study. Moreover, considering the similar research discussed, the list presented here shows an important and rich malacofauna living in the unconsolidated sediments around the Abrolhos reefs.
|
One hundred species were identified in the unconsolidated sediment of the Abrolhos Parcel. The high number of species in a relatively small area suggests that the Abrolhos Parcel sustains a high diversity of molluscs, superior to other previously studied reef areas. The well-preserved specimens allowed us to extend the distribution range of 15 species. Some of them were previously recognized only for their empty shells, which are not always a good element to consider in ecological studies. The high number of samples with soft parts found in this study may support future approaches in studies on Mollusca.
5 DATA AVAILABILITY STATEMENTThe data that support the findings are all presented herein.
6 ACKNOWLEDGMENTThe authors express their gratitude to the following taxonomists that helped in identifying several species: Sergio A. Vanin (Instituto de Biociências da Universidade de São Paulo, IBUSP), Luiz Simone (Museu de Zoologia da Universidade de São Paulo, MZSP), Carlo Magenta (Universidade Metropolitana de Santos, UNIMES), Silvio Lima (Universidade Federal de Campina Grande, UFCG), Alexandre Pimenta (Museu Nacional do Rio de Janeiro, MNRJ), Leonardo Souza (MNRJ), and Bárbara Valentas-Romera (MZSP). This work was conducted as part of the multidisciplinary project "Produtividade, Sustentabilidade e Utilização do Banco de Abrolhos", Subproject Benthos, supported by "Conselho Nacional de Desenvolvimento Científico e Tecnológico"- CNPq - given to AMSP-V (proc. #420219/2005-6) and in part by the MSc fellowship from CNPq given to FMPC (proc. #130159/2016-6). The materials were collected under the permit number 722773 from IBAMA, Brazilian Ministry of Environment, to Eurico C. Oliveira Filho of the University of São Paulo.
Abbott R T. 1974. American Seashells: The Marine Mollusks of the Atlantic and Pacific Coasts of North America. 2nd edn. Van Nostrand Reinhold, New York. 663p.
|
Absalão R S. 2005. Mollusca recorded during the Abrolhos RAP survey. In: Dutra G F, Allen G R, Werner T, McKenna S A eds. A Rapid Marine Biodiversity Assessment of the Abrolhos Bank, Bahia, Brazil. Conservation International, Washington. p. 82-86.
|
Ambrose R F, Anderson T W. 1990. Influence of an artificial reef on the surrounding infaunal community. Marine Biology, 107(1): 41-52.
DOI:10.1007/BF01313240 |
Appeltans W, Ahyong S T, Anderson G, Angel M V, Artois T, Bailly N, Bamber R, Barber A, Bartsch I, Berta A, Błażewicz-Paszkowycz M, Bock P, Boxshall G, Boyko C B, Brandão S N, Bray R A, Bruce N L, Cairns S D, Chan T Y, Cheng L N, Collins A G, Cribb T, Curini-Galletti M, Dahdouh-Guebas F, Davie P J F, Dawson M N, de Clerck O, Decock W, de Grave S, de Voogd N J, Domning D P, Emig C C, Erséus C, Eschmeyer W, Fauchald K, Fautin DG, Feist S W, Fransen C H, Furuya H, Garcia-Alvarez O, Gerken S, Gibson D, Gittenberger A, Gofas S, Gómez-Daglio L, Gordon D P, Guiry M D, Hernandez F, Hoeksema B W, Hopcroft R R, Jaume D, Kirk P, Koedam N, Koenemann S, Kolb J B, Kristensen R M, Kroh A, Lambert G, Lazarus D B, Lemaitre R, Longshaw M, Lowry J, Macpherson E, Madin L P, Mah C, Mapstone G, McLaughlin P A, Mees J, Meland K, Messing C G, Mills C E, Molodtsova T N, Mooi R, Neuhaus B, Ng P K L, Nielsen C, Norenburg J, Opresko D M, Osawa M, Paulay G, Perrin W, Pilger J F, Poore G C B, Pugh P, Read G B, Reimer J D, Rius M, Rocha R M, Saiz-Salinas J I, Scarabino V, Schierwater B, Schmidt-Rhaesa A, Schnabel K E, Schotte M, Schuchert P, Schwabe E, Segers H, Self-Sullivan C, Shenkar N, Siegel V, Sterrer W, Stöhr S, Swalla B, Tasker M L, Thuesen E V, Timm T, Todaro M A, Turon X, Tyler S, Uetz P, van der Land J, Vanhoorne B, van Ofwegen L P, van Soest R W M, Vanaverbeke J, Walker-Smith G, Walter T C, Warren A, Williams G C, Wilson S P, Costello M J. 2012. The magnitude of global marine species diversity. Current Biology, 22(23): 2189-2202.
DOI:10.1016/j.cub.2012.09.036 |
Bouchet P. 1997. Inventorying the molluscan diversity of the world: what is our rate of progress?. The Veliger, 40(1): 1-11.
|
Bouchet P. 2006. The magnitude of marine biodiversity. In: Duarte C M ed. The Exploration of Marine Biodiveristy: Scientific and Technological Challenges. Fundación BBVA, France. p. 31-62.
|
Bouchet P, Lozouet P, Maestrati P, Heros V. 2002. Assessing the magnitude of species richness in tropical marine environments: exceptionally high numbers of molluscs at a New Caledonia site. Biological Journal of the Linnean Society, 75(4): 421-436.
DOI:10.1046/j.1095-8312.2002.00052.x |
Dame R F. 1996. Ecology of Marine Bivalves: An Ecosystem Approach. CRC Press, Boca Raton, FL. 256.
|
Dutra G F, Allen G R, Werner T, McKenna SA. 2005. A rapid Marine Biodiversity Assessment of the Abrolhos Bank, Bahia, Brazil. Conservation International, Washington, DC. 160p.
|
Glover E A, Taylor J D. 2007. Diversity of chemosymbiotic bivalves on coral reefs: Lucinidae (Mollusca, Bivalvia) of New Caledonia and Lifou. Zoosystema, 29(1): 109-181.
|
Glover E A, Taylor J D, Williams S T. 2008. Mangrove, associated lucinid bivalves of the central Indo-West Pacific: review of the "Austriella" group with a new genus and species (Mollusca: Bivalvia: Lucinidae). The Raffles Bulletin of Zoology, 18(S): 25-40.
|
Gofas S, Luque Á A, Templado J, Salas C. 2017. A national checklist of marine Mollusca in Spanish waters. Scientia Marina, 81(2): 241-254.
DOI:10.3989/scimar.04543.21A |
Kantor Y I, Sysoev A V. 2005. A preliminary analysis of biodiversity of molluscs of Russia and adjacent territories. Ruthenica, 14(2): 107-118.
|
Langlois T J, Anderson M J, Babcock R C. 2006. Inconsistent effects of reefs on different size classes of macrofauna in adjacent sand habitats. Journal of Experimental Marine Biology and Ecology, 334(2): 269-282.
DOI:10.1016/j.jembe.2006.02.001 |
Leão Z M A N. 1999. Abrolhos - O complexo recifal mais extenso do Oceano Atlántico Sul. In: Schobbenhaus C, Campos D A, Queiroz E T, Winge M, Berbert-Born M eds. Sítios Geológicos e Paleontológicos do Brasil. p. 345-359.
|
Leão Z M A N, Kikuchi R K P, Testa V. 2003. Corals and coral reefs of Brazil. In: Cortés J ed. Latin American Coral Reefs. Elsevier, Amsterdam, Netherlands. p. 9-52.
|
Lima S F B, Christoffersen M L. 2016. Redescription and designation of a neotype for Caecum floridanum (Littorinimorpha, Truncatelloidea, Caecidae) with a characterization of the protoconch and growth stages. ZooKeys, 585: 17-31.
DOI:10.3897/zookeys.585.7646 |
Lima S F B, Santos F N, Absalão R S. 2013. New species of Caecum (Caenogastropoda: Rissooidea: Caecidae) from the Atlantic Coast of South America (Brazil) with a description of the protoconch and growth stages. Zoological Science, 30(9): 767-778.
DOI:10.2108/zsj.30.767 |
Mello R L S, Maestrati P. 1986. A família Caecidae Gray, 1850 no nordeste do Brasil. Caderno Ômega, 2: 145-166.
|
Melo U, Summerhayes C P, Ellis J P. 1975. Salvador to Vitória, Southeastern Brazil. In: Milliman J D, Summerhayes C P. Upper continental margin sedimentation off Brazil. Contrib Sedimentol. p. 151-175.
|
Mikkelsen P M, Bieler R. 2008. Seashells of Southern Florida: Living Marine Mollusks of the Florida Keys and Adjacent Regions. Princeton University Press, Princeton. 496p.
|
Morretes F L. 1949. Ensaio de catálogo dos moluscos do Brasil. Arquivos do Museu Paranaense, 7: 5-226.
|
Muehe D. 1987. O arquipélago dos Abrolhos: geomorfologia e aspectos gerais. Anuário do Instituto de Geociências, 11: 90-100.
|
Pizzini M, Oliverio M, Nofroni I. 1998. Contribution to the knowledge of the family Caecidae. 4. The temporary septum formation of some caecid species (Caenogastropoda: Rissooidea). Iberus, 16: 133-140.
|
Posey M H, Ambrose Jr W G. 1994. Effects of proximity to an offshore hard-bottom reef on infaunal abundances. Marine Biology, 118(4): 745-753.
DOI:10.1007/BF00347524 |
Rasband W S, Image U S. 1997-2018. National Institutes of Health, Bethesda, Maryland, USA. https://imagej.nih.gov/ij/. Last access on December 23, 2019.
|
Reid R G B, Brand D G. 1986. Sulfide-oxidizing symbiosis in lucinaceans: implications for bivalve evolution. The Veliger, 29(1): 3-24.
|
Rios E C. 1970. Coastal Brazilian Seashells. Fundação Cidade do Rio Grande, Rio Grande, Rio Grande. 255p.
|
Rios E C. 1985. Seashells of Brazil. Fundação Universidade do Rio Grande, Rio Grande. 328p.
|
Rios E C. 2009. Compendium of Brazilian Sea Shells. Editora Evangraf, Rio Grande. 668p.
|
Silva-Filho G F, Tenório D O, Pinto S L, Alves M S. 2012. Mollusca Scaphopoda Bronn, 1862 da Costa Nordeste do Brasil. Trop. Oceanogr, 40(1): 29-103.
|
Simone L R L. 1995. A new Amphitalamus carpenter, 1864 species (Gastropoda, Rissoidea, Barleeidae) from the Brazilian coast. Journal of Conchology, 35: 329-333.
|
Simone L R L. 2004. Morphology and Phylogeny of the Cypraeoidea (Mollusca, Caenogastropoda). Papel Virtual, Rio de Janeiro. 185p.
|
Taylor J D, Glover E A. 2010. Chemosymbiotic bivalves. In: Kiel S ed. The Vent and Seep Biota. Springer, Dordrecht. p. 107-135.
|
Taylor O D, Glover E A. 2016. Lucinid bivalves of Guadeloupe: diversity and systematics in the context of the tropical Western Atlantic (Mollusca: Bivalvia: Lucinidae). Zootaxa, 4196(3): 301-380.
DOI:10.11646/zootaxa.4196.3.1 |
Warén A. 1980. Descriptions of new taxa of Eulimidae (Mollusca, Prosobranchia), with notes on some previously described genera. Zoologica Scripta, 9(1-4): 283-306.
DOI:10.1111/j.1463-6409.1980.tb00668.x |
Warén A. 1983. A generic revision of the family Eulimidae (Gastropoda, Prosobranchia). Journal of Molluscan Studies, 13: 1-96.
|
WoRMS Editorial Board. 2018. World Register of Marine Species. http://www.marinespecies.org/imis.php?dasid=1447&doiid=170. Accessed on 2018-05-16.
|
Zalmon I R, Boina C D, Almeida T C M. 2012. Artificial reef influence on the surrounding infauna-north coast of Rio de Janeiro State, Brazil. Journal of the Marine Biological Association of the United Kingdom, 92(6): 1 289-1 299.
DOI:10.1017/S0025315411001147 |
 2021, Vol. 39
2021, Vol. 39