Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHANG Zhongshan, WANG Xiaomei, PAN Yongliang, WANG Zhanqi, WEN Zhengshun, LIU Feng, MAO Genxiang
- Pyropia haitanensis polysaccharide extends lifespan by inhibiting protein aggregation in Caenorhabditis elegans
- Journal of Oceanology and Limnology, 39(2): 705-713
- http://dx.doi.org/10.1007/s00343-020-0088-4
Article History
- Received Feb. 14, 2020
- accepted in principle Apr. 13, 2020
- accepted for publication May. 3, 2020
2 Zhejiang Provincial Engineering Technology Research Center of Marine Biomedical Products, School of Food Science and Pharmaceutics, Zhejiang Ocean University, Zhoushan 316022, China;
3 CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao 266071, China;
4 Zhejiang Provincial Key Lab of Geriatrics, Department of Geriatrics, Zhejiang Hospital, Hangzhou 310013, China
Anti-aging describes a method or product used to prevent aging by inhibiting changes that take place in the body simply due to aging, allowing the body to remain healthy (Jin et al., 2019). Recent advances in medical and scientific technologies against the degenerative processes of aging have resulted in methods for inhibiting the development of such events (Anandan et al., 2013; Huangfu et al., 2013). Decreased production or effectiveness of regulatory substances or pathways such as genes, reactive oxygen species, glycation, immune response, and hormones attracts increasing attention (Liang and Yin, 2010). In aging, protein or peptide assembly into amyloid fibrils (e.g., polyglutamine or polyQ), is involved in multiple events (Mazzeo et al., 2012). In some age-related neurodegenerative ailments, extended CAG-repeat mRNAs destabilize polyQ-repeat-rich proteins, which auto-assemble and form aggregates with proteotoxicity (Fan et al., 2014). Proteins with Q repeat amounts exceeding a pathological threshold of 30–40 impose a huge burden on protein homeostatic pathways in the cell, causing age-associated protein aggregation and cell toxicity (Gusella and MacDonald, 2006). In addition, the absence of toxicity of BlaP-polyQ chimeras might be explained by Caenorhabditis elegans' limitations, e.g., reduced lifespan (Burkewitz et al., 2011). Aging pathways affect the age-related onset assembly and toxicity of polyQ as well as other protein-folding sensors (Morley et al., 2002; Hsu et al., 2003; Brignull et al., 2006). Production of pathological polyQ-proteins in nematodes and cells functionally inhibits chaperone-associated, metastable proteins. Studies have been done thoroughly to identify the best products, and provide insights and knowledge that would promote a well-balanced, healthy approach to slowing and preventing the signs of aging by inhibiting protein aggregation. For example, astragalan, a polysaccharide isolated from the Chinese medicinal plant Astragalus membranaceus, was found to relieve polyQ aggregation-related neurotoxicity in C. elegans (Zhang et al., 2012).
Pyropia is a common macroalgal species resurrected from the previous genus Porphyra that comprises approximately 75 species (Sutherland et al., 2011). Particularly, Pyropia haitanensis represents an essential seaweed species whose total yield amount to about 75% of all Chinese Pyropia production (Zhao et al., 2019). Porphyran, a major P. haitanensis constituent, represents a linear sulfated polysaccharide containing D-galactose, 6-O-methyl-D-galactose, L-galactose-6-sulfate and 3, 6-anhydro-L-galactose (Bhatia et al., 2015). Previous studies have found that porphyran displays various physiological features such as antioxidant, anti-hyperlipidemic, immunomodulatory, and antitumor properties (Liu et al., 2017; Gong et al., 2018). Remarkably, few reports have assessed the neuroprotective effects of porphyran. For example, an in vivo study showed that porphyran could suppress excessive dopamine (DA) metabolism and protect dopaminergic neurons in Parkinson's disease (PD) mice induced by 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) (Liu et al., 2018). In addition, porphyran treatment results in increased total antioxidant capacity (TAOC) and superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) activities in all organs of aged mice (Sun et al., 2018). Since oxidative stress heavily affects aging, antioxidant molecules might possibly help delay aging and prolong life (Pandey et al., 2018). The present work assessed the impacts of P. haitanensis polysaccharide on C. elegans' lifespan, food intake, and reproduction. Then, the potential anti-aging mechanism of the polysaccharide though polyQ aggregation and the insulin signaling pathway was elucidated.
2 MATERIAL AND METHOD 2.1 Strains and maintenanceCaenorhabditis elegans strains were purchased from Caenorhabditis Genetics Center (CGC, USA) and included Bristol N2 (wild type), AM141 (Genotype: rmIs133 [unc-54p: : Q40:: yellow fluorescent protein (YFP)]), and TJ356 (Genotype: zIs356 [daf-16p: : daf-16a/b: : green fluorescent protein (GFP)+rol-6(su1006)]). Nematode synchronization followed the routine alkaline hypochlorite technique. Nematode assays were carried out at 20 ℃ on the S medium with E. coli NA22 as food unless specified.
2.2 Polysaccharide preparationPyropia haitanensis was sampled in Ningbo City, China. The alga samples were quickly washed and dried at room temperature before use. The dried algae were immersed in distilled water for 4 times at 105 ℃ for 2 h. The extract was dialyzed and precipitated with four times ethanol. The precipitate was lyophilized to yield porphyran. Ascorbate (10 mmol/L) and hydrogen peroxide (10 mmol/L) were added simultaneously into the 0.5% distilled water solution of porphyran (Zhao et al., 2006). After reaction for 2 h, the solution was precipitated with 80% ethanol, and the precipitate was dried to obtain degraded porphyran low molecular weight porphyran (LP).
2.3 Lifespan assessmentNematode lifespan was evaluated in 50 age-synchronized N2 and AM141 individuals (young adults). The nematodes underwent transfer into a 96-well plate pre-treated with LP and kept in an incubator at 20 ℃. Hermaphrodites were moved to freshly prepared nematode growth medium (NGM) (plus polysaccharides) within 48 h, and various solutions (15 μL) were added after 20 h. Worms were evaluated as live or dead based on body movement, and the rate of live nematodes was assessed as previously described (Petrascheck et al., 2007).
2.4 Reproduction assayIn this assay, one synchronized L4 larva treated with LP was placed on an NGM plate, and moved to another plate every 24 h. The offspring obtained in 5 days was considered the F0 generation, and the worms were counted daily until they all died (Xu et al., 2017).
2.5 Pharyngeal pumping assayAge-synchronized young adult C. elegans were underwent transfer onto freshly prepared NGM plates pre-treated with LP. After 24 h, a subsequent transfer onto fresh NGM plates was carried out. The pharyngeal pumping rate was assessed by monitoring the movement of the terminal bulb on Days 5, 8, and 10, respectively, for 60 s (Jeon and Cha, 2016). There were 10 worms or more per group.
2.6 Stress resistance assayAge-synchronized young adult N2 C. elegans underwent transfer onto freshly prepared NGM plates pre-treated with LP at 20 ℃. For the thermotolerance assay, the worms were incubated at 35 ℃ on Day 6. Worm survival was recorded by the touch-provoked method. The animals not responding to any stimulus or touch using a platinum loop were recorded as dead (Kandasamy et al., 2014). In the oxidative stress assay, the animals underwent transfer onto plates pre-treated with 250 μmol/L juglone and LP on Day 6. The nematodes alive were scored every 24 h (Waterston and Brenner, 1978).
2.7 Quantitative fluorescence assay for polyglutamic acid detectionAge-synchronized AM141 worms in the L1 stage were treated with LP for 48 h, and underwent transfer onto glass slides and anaesthesia with 10 mmol/L sodium azide. They were then assessed under a fluorescence microscope for polyQ40:: YFP expression to evaluate LP's effect on protein balance (Morley et al., 2002).
2.8 Nuclear localization of DAF-16Transgenic C. elegans TJ356 expressing DAF-16:: GFP were analyzed for nuclear DAF-16 expression. After incubation with LP for 24 h at 20 ℃, the worms in the L1 stage underwent transfer on glass slides and anaesthesia with 10 mmol/L sodium azide. The rate of worms expressing DAF-16:: GFP in the nucleus were assessed by fluorescence microscopy based on the discrete fluorescent aggregate phenotype (Wu et al., 2017).
2.9 Statistical analysisData are mean±SD of triplicate assays. One-way analysis of variance (ANOVA) and Student's t-test were performed for comparisons. P < 0.05 indicated statistical significance.
3 RESULT 3.1 Polysaccharide LPThe extract polysaccharide LP had excellent dissolubility and flow ability in water. High-performance gel permeation chromatography (HPGPC) result showed the molecular weight of LP was 8 381 Da. UV spectrum in the range of 200–800 nm indicated that LP were purified well due to no absorptions at 260 nm and 280 nm. Monosaccharide compose of LP was Gal, Glc, GalA, and Ara. The detailed structural characteristics were showed in previous report (Zhang et al., 2020).
3.2 Effects of LP on the lifespan of C. elegansAs shown in Fig. 1, the average life span of wild type nematodes was 9.30 days under normal conditions, while that of AM141 nematodes was decreased to 7.54 days under the effect of aggregation toxicity, demonstrating that aggregation toxicity could affect the longevity of nematodes. Next, the effect of LP on lifespan in C. elegans was evaluated at 250, 500, and 1 000 μg/mL, respectively. In this study, worms after exposure to LP showed prolonged lifespans at all concentrations (Fig. 1). For AM141 nematodes, a marked elevation (P < 0.05) was found in lifespan at 1 000 μg/mL levels in comparison with control values (by 12.24%). These results suggest that the anti-aging effects of LP might involve its proteotoxicity-alleviation function.
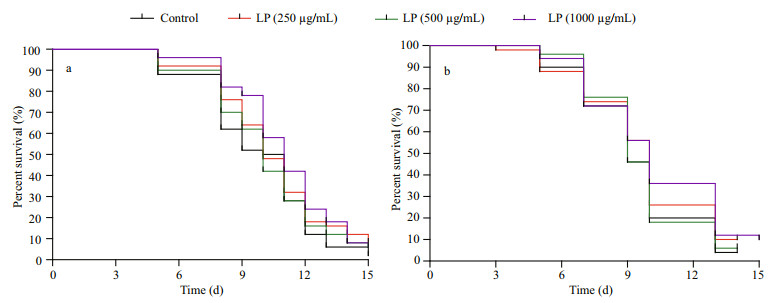
|
| Fig.1 Impact of LP on lifespan extension in N2 (a) and AM141 (b) C. elegans at 20 ℃ |
Figure 2a shows reproduction data in the F1 generation. At day 1, the total number of progenies was significantly reduced by LP (13.06% at 250 μg/mL, P < 0.05). Surprisingly, the total number of progenies was reduced by 47.50% (P < 0.001) at 1 000 μg/mL on Day 2. At days 3 to 5, the total numbers of progenies of LP-treated worms were slightly decreased at 250 μg/mL compared with control. Nematodes generally have reproductive capacity in the first four days of the L4 stage. With the increase of life span, fecundity decreases gradually until it is lost.
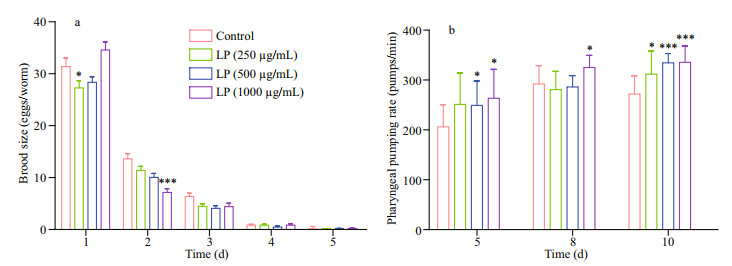
|
| Fig.2 The effect of LP on the reproduction (a) and pharyngeal pumping (b) in C. elegans |
To assess food intake in worms, pharyngeal pumping occurrences were numbered. As shown in Fig. 2b, on Days 5, 8, and 10, the basal pumping rates of the cut pharyngeal preparation in the absence of LP were increased compared with control values. There was a significant increase in pumping rate of around 23.6% in comparison with control values (P < 0.001) at 1 000 μg/mL on Day 10.
3.5 LP's impacts on thermotolerance and oxidative stressThermotolerance and the ability to cope with oxidative stress are important indexes for evaluating the aging process in organisms (Cypser et al., 2006; Zhao et al., 2018). Mean survival times of worms pretreated with LP are depicted in Fig. 3. At all three concentrations, LP showed limited effects under two stress conditions.
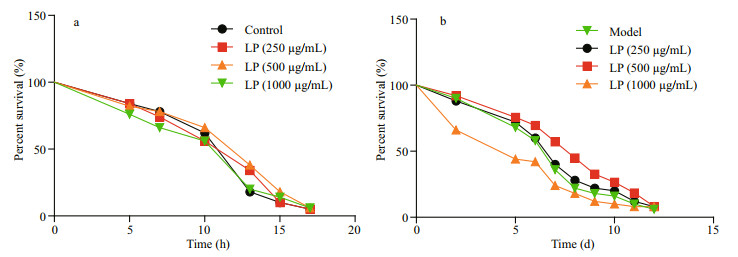
|
| Fig.3 The effect of LP on thermotolerance (a) and oxidative stress (b) in C. elegans |
Caenorhabditis elegans strain AM141 is broadly employed for assessing proteostasis and polyQ toxicity-associated pathways (Garcia et al., 2007). Therefore, in the current study this transgenic model was used to assess whether LP could prevent polyQ aggregation. As shown in Fig. 4, the nematodes showed overt differences in overall health and development following LP administration. The amounts of polyQ40:: YFP aggregates were decreased significantly (24.39%, P < 0.05) after treatment with 1 000 μg/mL LP, which demonstrated LP's anti-aggregation effects in the C. elegans polyQ model.

|
| Fig.4 The effects of LP on polyglutamic acid fluorescence in C. elegans *P < 0.05 vs. untreated control; a. control; b. LP at 250 μg/mL; c. LP at 500 μg/mL; d. LP at 1000 μg/mL. |
In this assay, we assessed whether LP affects the lifespan of C. elegans mutated for the IIS pathway, a critical lifespan, stress-resistance and metabolic modulator also associated with the aggregation of toxic proteins as well as neurodegeneration (Gan, 2007; Cohen and Dillin, 2008). Since DAF-16 and FOXO represent the major IIS pathway effectors, we evaluated whether DAF-16 translocation into the nucleus and its transcriptional activity were altered by LP. It was found that about 46.7% of control nematodes expressed DAF-16:: GFP in the cytosol while 16.7% of worms showed nuclear expression (Fig. 5). Significantly increased nuclear DAF-16:: GFP expression in nuclear exposed to paraquat was observed after treatment with LP. The high dose (1 000 μg/mL) sharply increased DAF-16 translocation into the nucleus over intermediate and cytoplasmic expressions in comparison with control treatment (P < 0.001) suggesting LP-associated DAF-16 translocation from cytosol (20.0%) to nucleus (60.1%).

|
| Fig.5 The effect of LP on nuclear translocation of DAF-16 in C. elegans **P < 0.01; ***P < 0.001 vs. untreated control; a. control; b. LP at 250 μg/mL; c. LP at 500 μg/mL; d. LP at 1000 μg/mL. |
The time preceding polyQ pathology occurrence is slightly associated with lifespan in organisms in the process of late-onset neurodegeneration (Kim et al., 2017). In this study, we analyzed LP for its capability to increase lifespan in N2 (wild type) and AM141 (polyQ40 in the muscle) strains of C. elegans, by culturing them with or without LP pre-treatment of plates at 20 ℃. The C. elegans strain AM141 expresses polyQ40:: YFP fusion proteins in body wall muscle cells and this aggregation toxicity could affect the life span of nematodes. To a certain extent, it can be elucidated the relationship between the effect of LP on the life span of C. elegans and the aggregation toxicity.
Reproductive capability is a critical index for assessing aging. A "Trade-off" mechanism has been proposed by which molecules reduce the reproductive ability while increasing lifespan duration although such mechanism does not always play a role (Kim et al., 2014; Large et al., 2017). Our results showed that LP impeded senescence in nematodes with reduced or lost reproductive capacity. Jointly, the above findings suggest LP's regulatory effects on reproduction may be associated with prolonged lifespan.
Dietary restriction (DR), which is defined as reduction of food intake, increases lifespan and delays the onset of multiple age-associated ailments in many species, including mammals (Masoro, 2005). In C. elegans, pharyngeal pumping and food intake decrease with increasing age (Jeon and Cha, 2016). Therefore, the pharyngeal pumping rate in nematodes is considered the most efficient index for identifying novel diet restriction mimetics (Powolny et al., 2011). However, how DR extends the lifespan is largely unknown. In this study, we observed that LP induced a significant dose-dependent elevation of pharyngeal pumping rate at 8 and 10 days of adulthood. However, LP-treated worms showed no marked reduction in food intake compared with control animals, which suggested that LP-associated regulation of lifespan does not depend on calorie restriction. LP treatment resulted in marked improvement of the swallowing muscle in C. elegans.
Multiple age-associated pathologies show protein misfolding and aggregation. It was found that environmental stressors, including temperature, oxidative stress, and osmotic stress, cause protein degradation. Actually, some chemical compounds have been demonstrated to contribute to increased survival in C. elegans under stress (Monickaraj et al., 2013; Zhou et al., 2018). Meanwhile, a previous report also showed that heat shock (37 ℃) and oxidative stress (200 μmol/L juglone) do not induce polyQ aggregation in C. elegans' intestine (Liang and Yin, 2010). In other words, a protein could show different aggregation states according to the implicated stressor, and stress and aging affect the proteome in linked but distinct mechanisms. Taken together, the above findings indicate that LP regulates bio-physiological processes in worms via other signaling networks.
Protein homeostasis maintenance is performed by the proteostasis network, which includes pathways controlling protein metabolism (Powers et al., 2009). It was found that several small molecules and bio-macromolecules could induce proteostasis via binding to and stabilization of particular proteins or by inducing the proteostasis network (Levy et al., 2008; Wang et al., 2008; Sutherland et al., 2011). This study showed a distinct fluorescent aggregate phenotype in adult worms due to polyQ40:: YFP fusion proteins expressed in body wall muscle cells. Combined with the above results that LP could increase the lifespan of AM141 nematodes, we concluded that LP protects worms from polyQ-associated neurotoxicity.
In C. elegans, it was found that DAF-16 could control aging likely by affecting biochemical events concerning protein homeostasis as assessed by protein aggregation. Active DAF-16 from decreased IIS causes dauer formation, induced stress response and prolonged lifespan (Lin et al., 2001). Research on polyQ-associated diseases showed that knockdown of DAF-16 accelerates the aggregation of the polyQ protein (Hsu et al., 2003). In this study, LP significantly increased the lifespan of N2 and AM141 C. elegans, preventing polyQ aggregation. We considered that LP could extend the lifespan and reduce protein aggregation in C. elegans in a DAF-16-dependent manner. However, the FOXO transcription factor DAF-16 could be also activated by some factors in other pathways, such as autophagy, oxidative stress, and so on. These pathways may play an important role in the prevention of age-associated protein misfolding diseases. In the further study, the mutant strain of upstream gene will be used to explore the target point of LP.
5 CONCLUSIONIn this study, the anti-aging activity of LP was evaluated by observing the lifespan, reproduction, pharyngeal pumping, stress response, quantitative fluorescence of polyglutamic acid, and nuclear localization of DAF-16 of worms with C. elegans models. The results reveal that LP could extend the adult lifespan of wild-type and polyQ nematodes, indicating a connection of its anti-aging benefit with the toxicity-suppressing effect. The number of polyglutamic acid aggregates in high groups significantly decreased compared to the control. The high-dose group strongly induced DAF-16 nuclear translocation over intermediate and cytosolic localizations. These findings provide insights into possible lifespan modulators for preventing proteotoxic ailments as well as a solid basis for developing neurodegenerative glycol-therapeutics using other carbohydrates.
6 DATA AVAILABILITY STATEMENTAll data used for this project are publicly available and accessible online. We have annotated the entire data building process and empirical techniques presented in the paper. The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Anandan R, Ganesan B, Obulesu T, Mathew S, Asha K K, Lakshmanan P T, Zynudheen A A. 2013. Antiaging effect of dietary chitosan supplementation on glutathione-dependent antioxidant system in young and aged rats. Cell Stress and Chaperones, 18(1): 121-125.
DOI:10.1007/s12192-012-0354-2 |
Bhatia S, Sharma K, Bera T. 2015. Structural characterization and pharmaceutical properties of porphyran. Asian Journal of Pharmaceutics, 9(2): 93-101.
DOI:10.4103/0973-8398.154698 |
Brignull H R, Moore F E, Tang S J, Morimoto R I. 2006. Polyglutamine proteins at the pathogenic threshold display neuron-specific aggregation in a pan-neuronal Caenorhabditis elegans model. Journal of Neuroscience, 26(29): 7 597-7 606.
DOI:10.1523/JNEUROSCI.0990-06.2006 |
Burkewitz K, Choe K, Strange K. 2011. Hypertonic stress induces rapid and widespread protein damage in C. elegans. American Journal of Physiology-Cell Physiology, 301(3): C566-C576.
DOI:10.1152/ajpcell.00030.2011 |
Cohen E, Dillin A. 2008. The insulin paradox: aging, proteotoxicity and neurodegeneration. Nature Reviews Neuroscience, 9(10): 759-767.
DOI:10.1038/nrn2474 |
Cypser J R, Tedesco P, Johnson T E. 2006. Hormesis and aging in Caenorhabditis elegans. Experimental Gerontology, 41(10): 935-939.
DOI:10.1016/j.exger.2006.09.004 |
Fan H C, Ho L I, Chi C S, Chen S J, Peng G S, Chan T M, Lin S Z, Harn H J. 2014. Polyglutamine (PolyQ) diseases: genetics to treatments. Cell Transplantation, 23(4-5): 441-458.
DOI:10.3727/096368914X678454 |
Gan L. 2007. Therapeutic potential of sirtuin-activating compounds in Alzheimer's disease. Drug News & Perspectives, 20(4): 233.
|
Garcia S M, Casanueva M O, Silva M V, Amaral M D, Morimoto R I. 2007. Neuronal signaling modulates protein homeostasis in Caenorhabditis elegans post-synaptic muscle cells. Genes & Development, 21(22): 3 006-3 016.
|
Gong G P, Zhao J X, Wang C J, Wei M, Dang T T, Deng Y N, Sun J, Song S, Huang L J, Wang Z F. 2018. Structural characterization and antioxidant activities of the degradation products from Porphyra haitanensis polysaccharides. Process Biochemistry, 74: 185-193.
DOI:10.1016/j.procbio.2018.05.022 |
Gusella J F, MacDonald M E. 2006. Huntington's disease: seeing the pathogenic process through a genetic lens. Trends in Biochemical Sciences, 31(9): 533-540.
DOI:10.1016/j.tibs.2006.06.009 |
Hsu A L, Murphy C T, Kenyon C. 2003. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science, 300(5622): 1 142-1 145.
DOI:10.1126/science.1083701 |
Huangfu J F, Liu J, Sun Z, Wang M F, Jiang Y, Chen Z Y, Chen F. 2013. Antiaging effects of astaxanthin-rich alga Haematococcus pluvialis on fruit flies under oxidative stress. Journal of Agricultural and Food Chemistry, 61(32): 7 800-7 804.
DOI:10.1021/jf402224w |
Jeon H, Cha D S. 2016. Anti-aging properties of Ribes fasciculatum in Caenorhabditis elegans. Chinese Journal of Natural Medicines, 14(5): 335-342.
|
Jin L, Song Q R, Zhang W L, Geng B, Cai J. 2019. Roles of long noncoding RNAs in aging and aging complications. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease, 1865(7): 1 763-1 771.
DOI:10.1016/j.bbadis.2018.09.021 |
Kandasamy S, Khan W, Evans F D, Critchley A T, Zhang J Z, Fitton J H, Stringer D N, Gardiner V A, Prithiviraj B. 2014. A fucose containing polymer-rich fraction from the brown alga Ascophyllum nodosum mediates lifespan increase and thermal-tolerance in Caenorhabditis elegans, by differential effects on gene and protein expression. Food & Function, 5(2): 275-284.
|
Kim D K, Cho K W, Ahn W J, Perez-Acuña D, Jeong H, Lee H J, Lee S J. 2017. Cell-to-cell transmission of polyglutamine aggregates in C. elegans. Experimental Neurobiology, 26(6): 321-328.
DOI:10.5607/en.2017.26.6.321 |
Kim D K, Jeon H, Cha D S. 2014. 4-Hydroxybenzoic acid-mediated lifespan extension in Caenorhabditis elegans. Journal of Functional Foods, 7: 630-640.
DOI:10.1016/j.jff.2013.12.022 |
Large E E, Padmanabhan R, Watkins K L, Campbell R F, Xu W, McGrath P T. 2017. Modeling of a negative feedback mechanism explains antagonistic pleiotropy in reproduction in domesticated Caenorhabditis elegans strains. PLoS Genetics, 13(5): e1006769.
DOI:10.1371/journal.pgen.1006769 |
Levy M, Porat Y, Bacharach E, Shalev D E, Gazit E. 2008. Phenolsulfonphthalein, but not phenolphthalein, inhibits amyloid fibril formation: implications for the modulation of amyloid self-assembly. Biochemistry, 47(22): 5 896-5 904.
DOI:10.1021/bi800043d |
Liang Z H, Yin D Z. 2010. Preventive treatment of traditional Chinese medicine as antistress and antiaging strategy. Rejuvenation Research, 13(2-3): 248-252.
DOI:10.1089/rej.2009.0867 |
Lin K, Hsin H, Libina N, Kenyon C. 2001. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nature Genetics, 28(2): 139-145.
DOI:10.1038/88850 |
Liu Q M, Xu S S, Li L, Pan T M, Shi C L, Liu H, Cao M J, Su W J, Liu G M. 2017. In vitro and in vivo immunomodulatory activity of sulfated polysaccharide from Porphyra haitanensis. Carbohydrate Polymers, 165: 189-196.
DOI:10.1016/j.carbpol.2017.02.032 |
Liu Y J, Geng L H, Zhang J J, Wang J, Zhang Q, Duan D L, Zhang Q B. 2018. Oligo-porphyran ameliorates neurobehavioral deficits in Parkinsonian mice by regulating the PI3K/Akt/Bcl-2 pathway. Marine Drugs, 16(3): 82.
DOI:10.3390/md16030082 |
Masoro E J. 2005. Overview of caloric restriction and ageing. Mechanisms of Ageing and Development, 126(9): 913-922.
DOI:10.1016/j.mad.2005.03.012 |
Mazzeo L E M, Dersh D, Boccitto M, Kalb R G, Lamitina T. 2012. Stress and aging induce distinct polyQ protein aggregation states. Proceedings of the National Academy of Sciences of the United States of America, 109(26): 10 587-10 592.
DOI:10.1073/pnas.1108766109 |
Monickaraj F, Aravind S, Nandhini P, Prabu P, Sathishkumar C, Mohan V, Balasubramanyam M. 2013. Accelerated fat cell aging links oxidative stress and insulin resistance in adipocytes. Journal of Biosciences, 38(1): 113-122.
DOI:10.1007/s12038-012-9289-0 |
Morley J F, Brignull H R, Weyers J J, Morimoto R I. 2002. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America, 99(16): 10 417-10 422.
DOI:10.1073/pnas.152161099 |
Pandey S, Tiwari S, Kumar A, Niranjan A, Chand J, Lehri A, Chauhan P S. 2018. Antioxidant and anti-aging potential of Juniper berry (Juniperus communis L.) essential oil in Caenorhabditis elegans model system. Industrial Crops and Products, 120: 113-122.
DOI:10.1016/j.indcrop.2018.04.066 |
Petrascheck M, Ye X L, Buck L B. 2007. An antidepressant that extends lifespan in adult Caenorhabditis elegans. Nature, 450(7169): 553-556.
DOI:10.1038/nature05991 |
Powers E T, Morimoto R I, Dillin A, Kelly J W, Balch W E. 2009. Biological and chemical approaches to diseases of proteostasis deficiency. Annual Review of Biochemistry, 78: 959-991.
DOI:10.1146/annurev.biochem.052308.114844 |
Powolny A A, Singh S V, Melov S, Hubbard A, Fisher A L. 2011. The garlic constituent diallyl trisulfide increases the lifespan of C. elegans via skn-1 activation. Experimental Gerontology, 46(6): 441-452.
DOI:10.1016/j.exger.2011.01.005 |
Sun C J, Wu F, Chen D D, Ge J B. 2018. Therapeutic effects of polysaccharides extracted from Porphyra yezoensis in rats with cerebral ischemia/reperfusion injury. Archives of Biological Sciences, 70(2): 233-239.
DOI:10.2298/ABS170621039S |
Sutherland J E, Lindstrom S C, Nelson W A, Brodie J, Lynch M D J, Hwang M S, Choi H G, Miyata M, Kikuchi N, Oliveira M C, Farr T, Neefus C, Mols-Mortensen A, Milstein D, Müller K M. 2011. A new look at an ancient order: generic revision of the bangiales (Rhodophyta). Journal of Phycology, 47(5): 1 131-1 151.
DOI:10.1111/j.1529-8817.2011.01052.x |
Wang H, Duennwald M L, Roberts B E, Rozeboom L M, Zhang Y L, Steele A D, Krishnan R, Su L J, Griffin D, Mukhopadhyay S, Hennessy E J, Weigele P, Blanchard B J, King J, Deniz A A, Buchwald S L, Ingram V M, Lindquist S, Shorter J. 2008. Direct and selective elimination of specific prions and amyloids by 4, 5-dianilinophthalimide and analogs. Proceedings of the National Academy of Sciences of the United States of America, 105(20): 7 159-7 164.
DOI:10.1073/pnas.0801934105 |
Waterston R H, Brenner S. 1978. A suppressor mutation in the nematode acting on specific alleles of many genes. Nature, 275(5682): 715-719.
DOI:10.1038/275715a0 |
Wu M Y, Kang X, Wang Q, Zhou C Y, Mohan C, Peng A. 2017. Regulator of G protein signaling-1 modulates paraquat-induced oxidative stress and longevity via the insulin like signaling pathway in Caenorhabditis elegans. Toxicology Letters, 273: 97-105.
DOI:10.1016/j.toxlet.2017.03.027 |
Xu J, Jiang Y J, Wan L, Wang Q, Huang Z B, Liu Y M, Wu Y L, Chen Z Y, Liu X. 2017. Feeding recombinant E. coli with GST-mBmKTX fusion protein increases the fecundity and lifespan of Caenorhabditis elegans. Peptides, 89: 1-8.
DOI:10.1016/j.peptides.2017.01.003 |
Zhang H R, Pan N, Xiong S Q, Zou S L, Li H F, Xiao L Y, Cao Z J, Tunnacliffe A, Huang Z B. 2012. Inhibition of polyglutamine-mediated proteotoxicity by Astragalus membranaceus polysaccharide through the DAF-16/FOXO transcription factor in Caenorhabditis elegans. Biochemical Journal, 441(1): 417-424.
DOI:10.1042/BJ20110621 |
Zhang Z S, Wang X M, Lv F, Xie X C, Zhang S Y, Cai C E, Jia R, Pan Y L, Liu F. 2020. Anti-complementary activity of a degraded sulfated heterogalactan from red alga Pyropia haitanensis. International Journal of Biological Macromolecules, 147: 527-533.
DOI:10.1016/j.ijbiomac.2020.01.045 |
Zhao P, Niu J F, Huan L, Gu W H, Wu M J, Wang G C. 2019. Agar extraction from Pyropia haitanensis residue after the removal and purification of phycobiliproteins. Journal of Applied Phycology, 31(4): 2 497-2 505.
DOI:10.1007/s10811-019-1735-z |
Zhao S X, Cheng Q, Peng Q, Yu X S, Yin X Q, Liang M, Ma C W, Huang Z B, Jia W Z. 2018. Antioxidant peptides derived from the hydrolyzate of purple sea urchin (Strongylocentrotus nudus) gonad alleviate oxidative stress in Caenorhabditis elegans. Journal of Functional Foods, 48: 594-604.
DOI:10.1016/j.jff.2018.07.060 |
Zhao T T, Zhang Q B, Qi H M, Zhang H, Niu X Z, Xu Z H, Li Z E. 2006. Degradation of porphyran from Porphyra haitanensis and the antioxidant activities of the degraded porphyrans with different molecular weight. International Journal of Biological Macromolecules, 38(1): 45-50.
DOI:10.1016/j.ijbiomac.2005.12.018 |
Zhou L, Wang L, Bai S J, Xing S, Li W N, Ma J F, Fu X Q. 2018. Knockdown of LMW-PTP enhances stress resistance in Caenorhabditis elegans. International Journal of Biological Macromolecules, 113: 1 015-1 023.
DOI:10.1016/j.ijbiomac.2018.03.014 |
 2021, Vol. 39
2021, Vol. 39


