Institute of Oceanology, Chinese Academy of Sciences
Article Information
- HAN Xuekai, ZHENG Yuyu, DAI Chaoling, DUAN Hu, GAO Meirong, Rayhan ALI Md, SUI Liying
- Effect of polystyrene microplastics and temperature on growth, intestinal histology and immune responses of brine shrimp Artemia franciscana
- Journal of Oceanology and Limnology, 39(3): 979-988
- http://dx.doi.org/10.1007/s00343-020-0118-2
Article History
- Received Mar. 24, 2020
- accepted in principle May. 18, 2020
- accepted for publication Jun. 28, 2020
Nowadays, plastic pollution has become one of the serious environmental problems worldwide (Andrady, 2011; PlasticsEurope, 2017). It has been reported that 10% of the world's plastic waste eventually enters the ocean (Secretariat of the Convention on Biological Diversity and Scientific and Technical Advisory Panel GEF, 2012), and that up to 90% of the waste in ocean are small fragments (Eriksen et al., 2014). Microplastics (MP), usually referring particles less than 5 mm, has become an emerging threat to marine environment and biodiversity due to its toxic and bio-accumulative effects (Jambeck et al., 2015; Bakir et al., 2016). MP can easily get into aquatic food web and result negative impact on marine organisms, and thus threaten the food safety of fisheries and aquaculture products (Wright et al., 2013; Lusher, 2015; Jabeen et al., 2017; Barboza et al., 2018b; Li et al., 2018). Being as a main planktonic consumer of the marine food web, zooplankton plays an important role in energy transfer and greatly contributes to the diversity of marine ecosystem. Excess intake of MP causes a series of adverse impacts on zooplankton, including intake disorders, acute oxidative stress, reduced reproduction, and survivals (Lee et al., 2013; Cole et al., 2015; Jeong et al., 2016). In addition, the retention of MP in the body may be transferred and accumulated along marine trophic webs, further affecting the stability of the entire marine ecosystem (Wang et al., 2016; Martins and Guilhermino, 2018).
Although MP has proven many negative impacts on zooplankton, few studies have investigated the combined effects of MP and other environmental factors on marine species. The interaction of environmental factors are not only the sum of the independent responses but mostly be synergistic or antagonistic (Crain et al., 2008). Temperature is one of the key drivers of biological response, which determine the physiological fitness and distribution range of a species by influencing its growth, feeding, metabolism, reproduction, and behavior (Place et al., 2008). In recent years anthropogenic environmental changes likely cause global warming and result in the rise of ocean temperatures (Brierley and Kingsford, 2009). Therefore, marine organisms are facing threats posed by the simultaneous presence of MP and elevated temperature.
Brine shrimp Artemia is a filter-feeding zooplankton that can adapt to a wide salinity range. It has some virtues such as shorter life cycle, bigger offspring number per brood, and easy to handle in the laboratory (Lavens and Sorgeloos, 1996), and thus has been widely used as a test organism for ecotoxicology study (Manfra et al., 2015; Vannuccini et al., 2015; Ekonomou et al., 2019). Nowadays, Artemia are increasingly used as test organisms for marine ecotoxicology study, with special focus on the toxicity of micro- and nano-particles on Artemia growth, morphology and physiology properties (Rodd et al., 2014; Bergami et al., 2017; Minetto et al., 2017; Rotini et al., 2018; Sarkheil et al., 2018; Varó et al., 2019). These studies mostly focused on short-term toxicity test with Artemia nauplii. In fact, the increase of MP concentration may lead to increased mortality of some marine species after a long-term exposure. Thereby, it is necessary to provide evidence of the chronic effects by exposing Artemia to MP for long time course.
In this study, the 14-d culture was conducted to evaluate the effects of MP and temperature on Artemia in the laboratory condition. Our hypothesis is that MP and temperature have combined effects on Artemia for a long-term exposure, and cause a series of responses at physiological, biochemical, molecular and histological level. To test this hypothesis, the survival rate was determined in time course. After 14-d exposure, Artemia growth was determined (Bergami et al., 2017). The immune-related oxidative enzymes acid phosphatase (ACP) and catalase (CAT) were used as indicators of biochemical responses (Hou et al., 2000; Bhuvaneshwari et al., 2018). Two immune genes adrenergic receptor alpha-1B (ADRA1B) and cyclic AMP-responsive elementbinding protein 3 (CREB3) were used as indicators of apoptosis pathways (Zhang et al., 2018). Moreover, intestinal histology was further examined to assess possible damage of epithelial cells lining the digestive tract (Wang et al., 2019). The outcome of this study may provide insight into the combined effect of MP and temperature on marine organism.
2 MATERIAL AND METHOD 2.1 Experimental designThe cysts of Artemia franciscana from the Great Salt Lake, USA were hatched following the general procedure. Exact 0.100-g cysts were placed in a glass cone containing 200-mL artificial seawater (30 salinity, ASW, Instant Ocean, USA), and with continuous aeration and illumination (1 000 lx) at 28 ℃. Polystyrene microplastic beads with particle size of 4–6 μm were purchased from Tianjin BaseLine ChromTech Research Centre (Tianjin, China). The MP suspension (25 mg/mL) were prepared using deionized water and were sonicated prior to use.
Experiment was conducted in combination of three temperature (22, 26, and 30 ℃) and three MP concentrations (0, 0.2, and 2.0 mg/L) with nine treatments. Each treatment was run in three replicates. The temperature degrees were selected according to surface temperature variability (20–30 ℃) of the ocean (Shen et al., 2007). For the MP levels, 0.2 mg/L was considered ecologically relevant MP concentration in marine environments (Barboza et al., 2018a), and 2.0 mg/L was selected considering a severe scenario where MP pollution occurs.
One hundred newly hatched nauplii were randomly transferred into a 250-mL glass bottle containing 200-mL artificial seawater (ASW) (salinity 30). Artemia were fed twice a day with microalgae Dunaliella salina at a final density of 100 000 cells/ mL. To evaluate the effect of MP on Artemia during long-term exposure, the survival rate was determined after 1-, 3-, 6-, 10-, and 14-d culture. Artemia were put back in the bottles after counting the survivals. The survival rate was calculated as follows:

where N is the survived Artemia number, and N0 is the initial Artemia number.
On day 14, thirty Artemia from each replicate were randomly collected, the average individual body length was measured under a stereomicroscope.
The effect of three MP concentrations (0, 0.2, and 2.0 mg/L) combined with 30 ℃ were studied to evaluate the immune-related enzyme activity, immune gene expression and intestine histology of Artemia in bigger scale setup. Newly hatched Artemia nauplii were randomly distributed into 9 plastic cones containing 5-L ASW. The initial density of Artemia was 100 ind./L. Each treatment was run in three replicates. Artemia were reared at 30 ℃ with 14 h L꞉ 10 h D photoperiod for 14 d, and were covered to prevent water evaporation. The nauplii were fed D. salina twice a day at a final density of 100 000 cells/mL.
2.2 Enzyme activity assaysOn day 14, Artemia were collected and rinsed with deionized water. The excess water was blotted using tissue paper. Exact 0.100-g Artemia were weighed and homogenized in physiological saline (1꞉9, w꞉v). The suspension was centrifuged at 2 500 r/min for 10 min at 4 ℃, and supernatant was collected. The enzyme activity of acid phosphatase (ACP) and catalase (CAT) assays were performed using analytical kits (Nanjing Jiancheng, China) and expressed as unit per milligram of soluble protein (U/mg protein). The soluble protein content was determined using the Coomassie Brilliant Blue (G-250) method.
2.3 Intestinal histology assaysOn day 14, the intestine of Artemia in the group of 0 and 2.0-mg/L MP were dissected, and fixed in 4% paraformaldehyde for 48 h. After rinsed with phosphate buffer and dehydrated subsequently with ethanol dilutions (70%, 80%, 90%, and 100%), the tissues were made transparent with xylene, embedded in paraffin and cut in a microtome at 4-μm thickness. After hematoxylin-eosin staining, pictures were taken under Inverted Phase Contrast Microscope (NikonTiE, Japan).
2.4 RNA extraction, cDNA synthesis and real timePCRGene expression analysis was performed according to Vannuccini et al. (2015). RNA concentrations were measured using a traycell spectrophotometer (Eppendorf, Germany) and RNA quality was detected on 1.2% agarose gel. Two milligram of total RNA was transcribed to cDNA using PrimeScriptTM RT reagent Kit (Code No. RR037A). Two genes of adrenergic receptor alpha-1B (ADRA1B) and cyclic AMPresponsive element-binding protein 3 (CREB3) were chosen to perform real time-PCR, and glyceraldehyde 3-phosphatase dehydrogenase (gapdh) was selected as housekeeping gene (Chen et al., 2009).
Real time-PCR was performed using a CFX Connect thermal cycler. Amplification was performed in triplicate in a total volume of 25 μL containing 2-μL cDNA, 100 nmol/L of each primer and 12.5 μL of TB Green® Premix Ex Taq (RR820A, TaKaRa). The cycling conditions were 95 ℃ for 30 s for polymerase activation, followed by 40 PCR cycles of 95 ℃ of 10 s, at 50–60 ℃ of 30. The calculated relative expression ratio of each gene was based on the PCR efficiency (E) and Ct of sample compared with control, and expressed in comparison to the reference genes. Primers of these genes are listed in Table 1.
Differences in enzymatic activity and gene expression between treatments were analyzed using one-way ANOVA. The effects of different temperatures and MP concentrations on survival and growth of Artemia were analyzed by two-way ANOVA. All data were tested for normality and homogeneity of variance before ANOVA was done. Tukey's honest significant difference test was performed to identify differences at P < 0.05.
3 RESULT 3.1 Survival and growthOne-way ANOVA analysis showed higher MP concentration and temperature led to a decrease in survival rate of Artemia (Fig. 1). Throughout the experiment, Artemia exposed to 30 ℃ and 2.0 mg/L showed the lowest survival rate compared to other groups. By using two-way ANOVA analysis, temperature showed significantly influence on the survival rate of Artemia throughout the experiment (P < 0.05), with the exception after 1-d exposure (Table 2). In addition, survival rate was significantly affected by MP after 1-d exposure and day 14 (P < 0.05). There was no interaction between MP and temperature with the exception after 1-day exposure.
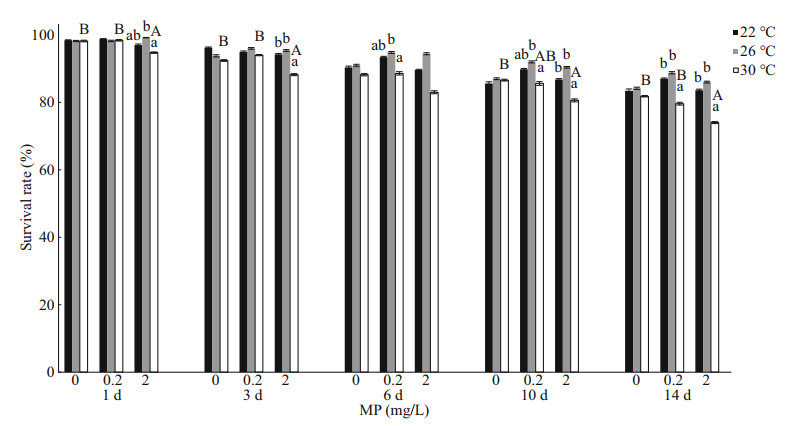
|
| Fig.1 One-way ANOVA analysis on survival rate of Artemia exposed to different temperatures and MP concentrations for 1, 3, 6, 10, and 14 d Lower-case letters indicate significant differences between temperatures for each MP concentration (P < 0.05). Capital letters indicate significant differences between MP concentrations for each temperature (P < 0.05). |
|
After 14-d exposure, Artemia length increased from 0.35–0.40 mm to 3–4 mm. High temperature and MP concentration significantly decreased Artemia growth in a long-term exposure, which is consistent with the survival rate (Fig. 2). Two-way ANOVA analysis showed that Artemia length was significantly affected by temperature (P < 0.05), and there was a significant interaction between MP and temperature (P < 0.05) (Table 3).
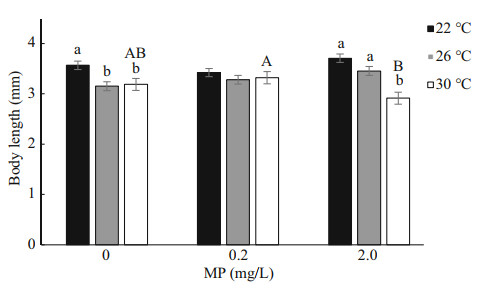
|
| Fig.2 One-way ANOVA analysis on body length of Artemia exposed to different temperatures and MP concentrations for 14 d Lower-case letters indicate significant difference between temperatures for each MP concentration (P < 0.05). Capital letters indicate significant differences between MP concentrations at each temperature (P < 0.05). |
|
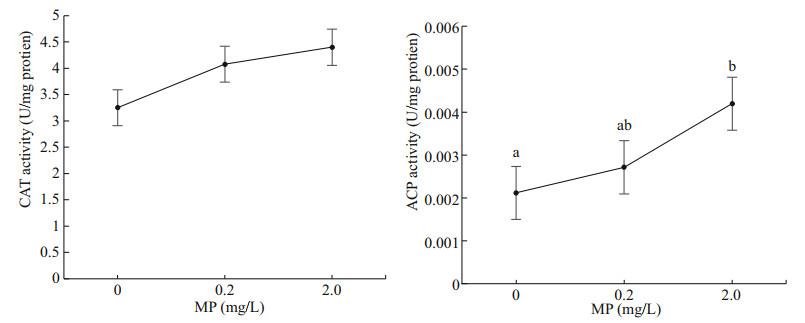
Both CAT and ACP activity increased with the increasing MP concentrations (Fig. 3). There was no significant difference in CAT activity in different groups (P>0.05), while ACP activity showed significant difference between 0 and 2.0-mg/L MP (P < 0.05).
|
| Fig.3 CAT and ACP activity of Artemia exposed to different MP concentrations at 30 ℃ for 14 d Lower-case letters indicate significant differences at P < 0.05. |
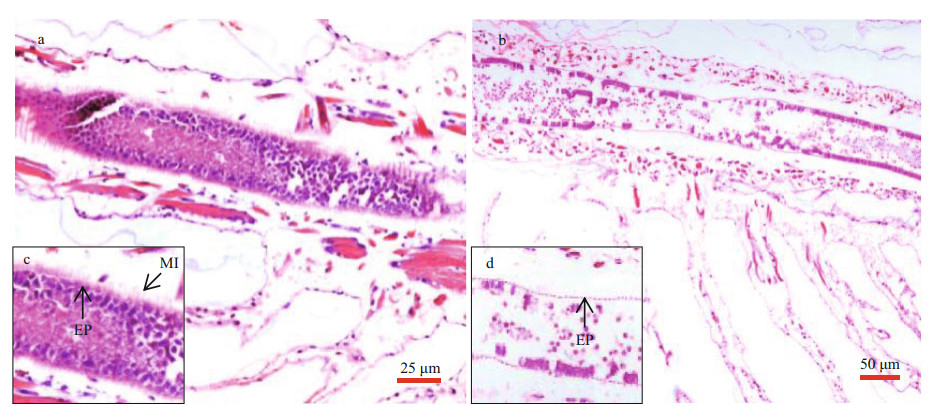
Longitudinal sections of Artemia intestines in the group of 0 and 2.0-mg/L MP were shown in Fig. 4. After 14-d exposure, the intestinal epithelial cells were closely connected and microvilli were regularly-ordered in control group. By contrast, the number of intestinal microvilli decreased and epithelia cells exfoliated in 2.0-mg/L MP treatment, which implied that 2.0-mg/L MP could damage the intestinal tissue of Artemia.
|
| Fig.4 Intestinal tissue of Artemia exposure to 0 (a) and 2.0-mg/L (b) MP at 30 ℃ for 14 d c and d are enlarged images for intestinal tissue in a and b, respectively. Scale bar was 25 and 50 μm for a and b, respectively. The abbreviation EP stands for epithelial cell layers and MI stands for microvilli. |
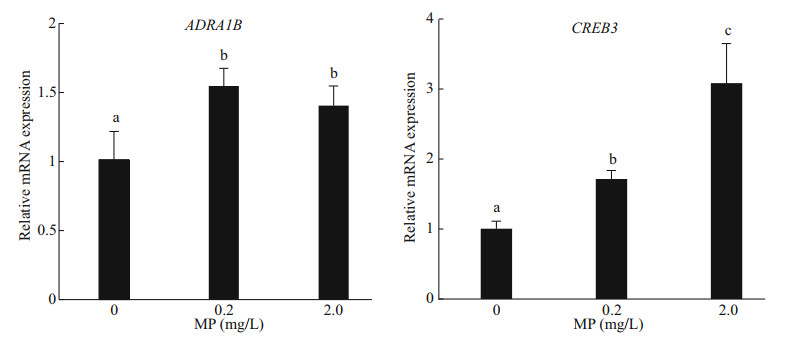
A further confirmation of the defense mechanism is given by the gene expression of both ADRA1B and CREB3, which was significantly upregulated in Artemia exposed to 0.2- and 2.0-mg/L MP for 14 d. After long-term exposure, a dose-dependent modulation of CREB3 was observed, and the gene expression increased with the increase of MP concentration (P < 0.05). Compared to the control, 0.2- and 2.0-mg/L MP exposure resulted in significant up-regulation of ADRA1B (P < 0.05) (Fig. 5).
|
| Fig.5 Genes expression of ADRA1B and CREB3 in Artemia after 14-d exposure to different MP concentrations at 30 ℃ Lower-case letters indicate significant differences (P < 0.05). |
To the best of our knowledge, the present study is the first to investigate the long-term effects of both MP and temperature on juvenile and adult A. franciscana, using multi-effect criteria, such as survival rate, growth, intestinal histology, and immune responses. Researchers believed that increases in temperature beyond the marine organism's suitable temperature range directly affect the metabolism and cause growth and physiological stress (Barber and Blake, 2006). Our results showed that temperature had significant effects on survival and growth of Artemia after a long-term exposure, this is consistent with the funding of Browne and Wanigasekera (2000). Given that the temperature was the main factor affecting oxygen consumption (Irwin et al., 2007), it is a reasonable inference that survival rate and growth relate to the fact that rising temperatures impose additional developmental stress by speeding metabolism.
Artemia nauplii is capable of ingesting MP beads ranging from 1 to 20 μm, and even bigger (Batel et al., 2016; Cole et al., 2016). A recent report suggested that there were no significant detrimental effects on growth and survival after a 14-d exposure to MP with 0.4, 0.8, and 1.6 mg/L at 25 ℃, and Artemia can ingest and egest MPs when continuously exposed to these concentrations (Peixoto et al., 2019). However, our study found that MP had significant effects on the survival rate of Artemia after 14-d exposure, and survival rate and body length were significantly decreased at 30 ℃ and 2.0-mg/L MP concentration. Bergami et al. (2017) reported that naonoplastics PSNH2 were able to disrupt the physiology and the energy flow in developing A. franciscana, and we can certainly deduce that the ingestion and accumulation of polystyrene MP particles (4–6 μm) in the digestive tract might limit food intake and significantly affect growth and development of Artemia (Besseling et al., 2014; Cole et al., 2016; Wang et al., 2019). Moreover, although temperature had significant effects on survival and growth of Artemia after a long-term exposure, MP and temperature showed a combined stress on Artemia, especially at relatively higher level. Prior research has suggested that temperature was the dominant factor affecting oxygen consumption rates in Artemia, which showed a significant increase with increasing temperature from 0 to 30 ℃ (Irwin et al., 2007). Therefore, it can be hypothesized that the higher temperature could increase MP ingestion, metabolism and energy consumption of Artemia, and thus aggravating the toxic effect of MP.
Oxidative stress occurs when the antioxidant defenses are overwhelmed by the production of reactive oxygen species (ROS). ACP is often used as a marker for intracellular lysosomal detection and as a reliable tool for environmental pollution assessment (Rajalakshmi and Mohandas, 2005). CAT is well known as an antioxidant enzyme that catalyzes hydrogen peroxide to water and oxygen, and plays an important role against hydroxyl radical toxicity (Bagnyukova et al., 2005; Ighodaro and Akinloye, 2018). Our data showed that the enzyme activity of CAT and ACP increased with the increasing of MP concentration after 14 d. In particular, a significant increase in ACP activity was observed in Artemia when exposed to 30 ℃ and 2-mg/L MP concentration, revealing that high temperature and MP concentration could induced oxidative stress on Artemia more easily. This may be due to environmental stimuli that lead to lysosomal membrane instability and therefore to increased ACP activity (Pampanin et al., 2002).
For most of marine organisms, especially zooplankton, their alimentary canal (gut) is the most important target organ where MP accumulates (Browne et al., 2008; Jemec et al., 2016). Wang et al. (2019) reported that after 24 h of exposure to 10-μm polystyrene at 10 and 100 particles/mL, the decreased and disordered microvilli, increased mitochondria and the presence of autophagosomes were observed in Artemia intestine tissue. Similarly, we found that the intestinal microvilli and epithelia cells of Artemia decreased significantly and arranged disorderly when exposed to 2.0-mg/L MP at 30 ℃, suggesting the relatively high MP concentration and temperature can cause an adverse impact on intestinal tissue. Since intestinal microvilli and epithelia are involved in a series of physiological processes, we can speculate that the damage of intestinal tissue could affect the nutrient absorption and energy metabolism, and ultimately pose a threat to Artemia growth and health (Gunasekara et al., 2011).
Apoptosis is a programmed cell death that is regulated by genes, which is essential for normal development and homeostasis in plants and throughout the animal kingdom (Kiss, 2010). CREB3 and ADRA1B have been identified as apoptosis-related genes in Artemia (Zhang et al., 2018). Our study showed that ADRA1B and CREB3 genes had high expressions in response to high MP concentration and temperature, indicating that the two genes may play an important role in regulating apoptosis, development and metabolism (Ping et al., 2011; Tressel et al., 2011). The strong induction of CREB3 and ADRA1B genes indicates that high MP concentration and temperature may cause immunological stress, and affect the energy flow as well as the normal physiological processes in Artemia over prolonged exposure.
5 CONCLUSIONMP and temperature have a combined adverse effect on survival and growth of Artemia, especially at relatively high temperature and MP concentration. Temperature had significant effect on Artemia growth, and had significant interaction with MP concentrations on growth at 14 d. Exposure of Artemia at 30 ℃ and 2.0-mg/L MP significantly decreased the growth and survival rate of Artemia, reduced the number of intestinal microvilli and epithelia cell, as well as induced oxidative stress and immunological stress. Our research provides a scientific basis for the risk assessment of MP pollution and elevated temperature on zooplankton in the marine environment. Further research is required to evaluate the combined effects of MP and temperature on intestinal absorptive function and intestinal microflora, and their possible impacts on the immune function of Artemia.
6 CONFLICT OF INTERESTThe authors confirm that this article content has no conflict of interest.
7 DATA AVAILABILITY STATEMENTThe research data used in this study can be shared upon request.
8 ETHICS STATEMENTThe study protocol was approved by the Committee on the Ethics of Animal Experiments of Tianjin University of Science and Technology.
Andrady A L. 2011. Microplastics in the marine environment. Marine Pollution Bulletin, 62(8): 1596-1605.
DOI:10.1016/j.marpolbul.2011.05.030 |
Bagnyukova T V, Vasylkiv O Y, Storey K B, Lushchak V I. 2005. Catalase inhibition by amino triazole induces oxidative stress in goldfish brain. Brain Research, 1052(2): 180-186.
DOI:10.1016/j.brainres.2005.06.002 |
Bakir A, O'Connor I A, Rowland S J, Hendriks A J, Thompson R C. 2016. Relative importance of microplastics as a pathway for the transfer of hydrophobic organic chemicals to marine life. Environmental Pollution, 219: 56-65.
DOI:10.1016/j.envpol.2016.09.046 |
Barber B J, Blake N J. 2006. Reproductive physiology. In: Shumway S E, Parsons G J eds. Scallops: Biology, Ecology and Aquaculture. Elsevier, Amsterdam. 59pp, https://doi.org/10.1016/S0167-9309(06)80033-5.
|
Barboza L G A, Vieira L R, Branco V, Figueiredo N, Carvalho F, Carvalho C, Guilhermino L. 2018a. Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquatic Toxicology, 195: 49-57.
DOI:10.1016/j.aquatox.2017.12.008 |
Barboza L G A, Vieira L R, Guilhermino L. 2018b. Single and combined effects of microplastics and mercury on juveniles of the European seabass (Dicentrarchus labrax): changes in behavioural responses and reduction of swimming velocity and resistance time. Environmental Pollution, 236: 1014-1019.
DOI:10.1016/j.envpol.2017.12.082 |
Batel A, Linti F, Scherer M, Erdinger L, Braunbeck T. 2016. [a]pyrene from microplastics to Artemia nauplii and further to zebrafish via a trophic food web experiment: CYP1A induction and visual tracking of persistent organic pollutants. Environmental Toxicology and Chemistry, 35(7): 1656-1666.
DOI:10.1002/etc.3361 |
Bergami E, Pugnalini S, Vannuccini M L, Manfra L, Faleri C, Savorelli F, Dawson K A, Corsi I. 2017. Long-term toxicity of surface-charged polystyrene nanoplastics to marine planktonic species Dunaliella tertiolecta and Artemia franciscana. Aquatic Toxicology, 189: 159-169.
DOI:10.1016/j.aquatox.2017.06.008 |
Besseling E, Wang B, Lürling M, Koelmans A A. 2014. Nanoplastic affects growth of S. obliquus and reproduction of D. magna. Environmental Science & Technology, 48(20): 12336-12343.
DOI:10.1021/es503001d |
Bhuvaneshwari M, Thiagarajan V, Nemade P, Chandrasekaran N, Mukherjee A. 2018. Toxicity and trophic transfer of P25 TiO2 NPs from Dunaliella salina to Artemia salina effect of dietary and waterborne exposure. Environmental Research, 160: 39-46.
DOI:10.1016/j.envres.2017.09.022 |
Brierley A S, Kingsford M J. 2009. Impacts of climate change on marine organisms and ecosystems. Current Biology, 19(14): R602-R614.
DOI:10.1016/j.cub.2009.05.046 |
Browne M A, Dissanayake A, Galloway T S, Lowe D M, Thompson R C. 2008. Ingested microscopic plastic translocates to the circulatory system of the mussel, Mytilus edulis (L. ). Environmental Science & Technology, 42(13): 5026-5031.
DOI:10.1021/es800249a |
Browne R A, Wanigasekera G. 2000. Combined effects of salinity and temperature on survival and reproduction of five species of Artemia. Journal of Experimental Marine Biology and Ecology, 244(1): 29-44.
DOI:10.1016/S0022-0981(99)00125-2 |
Chen W H, Ge X M, Wang W W, Yu J, Hu S N. 2009. A gene catalogue for post-diapause development of an anhydrobiotic arthropod Artemia franciscana. BMC Genomics, 10: 52.
DOI:10.1186/1471-2164-10-52 |
Cole M, Lindeque P K, Fileman E, Clark J, Lewis C, Halsband C, Galloway T S. 2016. Microplastics alter the properties and sinking rates of zooplankton faecal pellets. Environmental Science & Technology, 50(6): 3239-3246.
DOI:10.1021/acs.est.5b05905 |
Cole M, Lindeque P, Fileman E, Halsband C, Galloway T S. 2015. The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus. Environmental Science & Technology, 49(2): 1130-1137.
DOI:10.1021/es504525u |
Crain C M, Kroeker K, Halpern B S. 2008. Interactive and cumulative effects of multiple human stressors in marine systems. Ecology Letters, 11(12): 1304-1315.
DOI:10.1111/j.1461-0248.2008.01253.x |
Ekonomou G, Lolas A, Castritsi-Catharios J, Neofitou C, Zouganelis G D, Tsiropoulos N, Exadactylos A. 2019. Mortality and effect on growth of Artemia franciscana exposed to two common organic pollutants. Water, 11(8): 1614.
DOI:10.3390/w11081614 |
Eriksen M, Lebreton L C M, Carson H S, Thiel M, Moore C J, Borerro J C, Galgani F, Ryan P G, Reisser J. 2014. Plastic pollution in the world's oceans: more than 5 trillion plastic pieces weighing over 250, 000 tons afloat at Sea. PLoS One, 9(12): e111913.
DOI:10.1371/journal.pone.0111913 |
Gunasekara R A Y S A, Rekecki A, Cornillie P, Cornelissen M, Sorgeloos P, Simoens P, Bossier P, Van den Broeck W. 2011. Morphological characteristics of the digestive tract of gnotobiotic Artemia franciscana nauplii. Aquaculture, 321(1-2): 1-7.
DOI:10.1016/j.aquaculture.2011.07.037 |
Hou L, Wang Y, Zou X Y. 2000. Expression characterizations of alkaline phosphatase (ALP) and acid phosphatase (ACP) isozymic genes of bisexual Artemia populations from China. Donghai Marine Science, 18(4): 22-28.
(in Chinese) |
Ighodaro O M, Akinloye O A. 2018. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alexandria Journal of Medicine, 54(4): 287-293.
DOI:10.1016/j.ajme.2017.09.001 |
Irwin S, Wall V, Davenport J. 2007. Measurement of temperature and salinity effects on oxygen consumption of Artemia franciscana K. , measured using fibre-optic oxygen microsensors. Hydrobiologia, 575: 109-115.
DOI:10.1007/s10750-006-0358-y |
Jabeen K, Su L, Li J N, Yang D Q, Tong C F, Mu J L, Shi H H. 2017. Microplastics and mesoplastics in fish from coastal and fresh waters of China. Environmental Pollution, 221: 141-149.
DOI:10.1016/j.envpol.2016.11.055 |
Jambeck J R, Geyer R, Wilcox C, Siegler T R, Perryman M, Andrady A, Narayan R, Law K L. 2015. Plastic waste inputs from land into the ocean. Science, 347(6223): 768-771.
DOI:10.1126/science.1260352 |
Jemec A, Horvat P, Kunej U, Bele M, Kržan A. 2016. Uptake and effects of microplastic textile fibers on freshwater crustacean Daphnia magna. Environmental Pollution, 219: 201-209.
DOI:10.1016/j.envpol.2016.10.037 |
Jeong C B, Won E J, Kang H M, Lee M C, Hwang D S, Hwang U K, Zhou B S, Souissi S, Lee S J, Lee J S. 2016. Microplastic size-dependent toxicity, oxidative stress induction, and p-JNK and p-p38 activation in the Monogonont rotifer (Brachionus koreanus). Environmental Science & Technology, 50(16): 8849-8857.
DOI:10.1021/acs.est.6b01441 |
Kiss T. 2010. Apoptosis and its functional significance in molluscs. Apoptosis, 15(3): 313-321.
DOI:10.1007/s10495-009-0446-3 |
Lavens P, Sorgeloos P. 1996. Manual on the Production and Use of Live Food for Aquaculture. FAO, Rome, 172p.
|
Lee K W, Shim W J, Kwon O Y, Kang J H. 2013. Size-dependent effects of micro polystyrene particles in the marine copepod Tigriopus japonicus. Environmental Science & Technology, 47(19): 11278-11283.
DOI:10.1021/es401932b |
Li J N, Green C, Reynolds A, Shi H H, Rotchell J M. 2018. Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom. Environmental Pollution, 241: 35-44.
DOI:10.1016/j.envpol.2018.05.038 |
Lusher A. 2015. Microplastics in the marine environment: distribution, interactions and effects. In: Bergmann M, Gutow L, Klages M eds. Marine Anthropogenic Litter. Springer, Cham. p. 245-307, https://doi.org/10.1007/978-3-319-16510-3_10.
|
Manfra L, Savorelli F, Di Lorenzo B, Libralato G, Comin S, Conti D, Floris B, Francese M, Gallo M L, Gartner I, Guida M, Leoni T, Marino G, Martelli F, Palazzi D, Prato E, Righini P, Rossi E, Volpi G A, Migliore L. 2015. Intercalibration of ecotoxicity testing protocols with Artemia franciscana. Ecological Indicators, 57: 41-47.
DOI:10.1016/j.ecolind.2015.04.021 |
Martins A, Guilhermino L. 2018. Transgenerational effects and recovery of microplastics exposure in model populations of the freshwater cladoceran Daphnia magna Straus. Science of the Total Environment, 631-632: 421-428.
DOI:10.1016/j.scitotenv.2018.03.054 |
Minetto D, Libralato G, Marcomini A, Volpi Ghirardini A. 2017. Potential effects of TiO2 nanoparticles and TiCl4 in saltwater to Phaeodactylum tricornutum and Artemia franciscana. Science of the Total Environment, 579: 1379-1386.
DOI:10.1016/j.scitotenv.2016.11.135 |
Pampanin D M, Loriano B, Carotenuto L, Marin M G. 2002. Air exposure and functionality of Chamelea gallina haemocytes: effects on haematocrit, adhesion, phagocytosis and enzyme contents. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 131(3): 605-614.
DOI:10.1016/S1095-6433(01)00512-8 |
Peixoto D, Amorim J, Pinheiro C, Oliva-Teles L, Varó I, Rocha R D M, Vieira M N. 2019. Uptake and effects of different concentrations of spherical polymer microparticles on Artemia franciscana. Ecotoxicology and Environmental Safety, 176: 211-218.
DOI:10.1016/j.ecoenv.2019.03.100 |
Ping C C, Hang K K, Yan J D. 2011. CREB3 subfamily transcription factors are not created equal: recent insights from global analyses and animal models. Cell & Bioscience, 1(1): 6.
DOI:10.1186/2045-3701-1-6 |
Place S P, O'Donnell M J, Hofmann G E. 2008. Gene expression in the intertidal mussel Mytilus californianus: physiological response to environmental factors on a biogeographic scale. Marine Ecology Progress Series, 356: 1-14.
DOI:10.3354/meps07354 |
PlasticsEurope. 2017. An analysis of European plastics production, demand and waste data. Plastics Europe Association of Plastics Manufacturers, Brussels, Belgium. 44p, https://www.plasticseurope.org/application/files/1715/2111/1527/Plastics_the_facts_2017_FINAL_for_website.pdf.
|
Rajalakshmi S, Mohandas A. 2017. Copper-induced changes in tissue enzyme activity in a freshwater mussel. Ecotoxicology and Environmental Safety, 62(1): 140-143.
DOI:10.1016/j.ecoenv.2005.01.003 |
Rodd A L, Creighton M A, Vaslet C A, Rangel-Mendez J R, Hurt R H, Kane A B. 2014. Effects of surface-engineered nanoparticle-based dispersants for marine oil spills on the model organism Artemia franciscana. Environmental Science & Technology, 48(11): 6419-6427.
DOI:10.1021/es500892m |
Rotini A, Gallo A, Parlapiano I, Berducci M T, Boni R, Tosti E, Prato E, Maggi C, Cicero A M, Migliore L, Manfra L. 2018. Insights into the CuO nanoparticle ecotoxicity with suitable marine model species. Ecotoxicology and Environmental Safety, 147: 852-860.
DOI:10.1016/j.ecoenv.2017.09.053 |
Sarkheil M, Johari S A, An H J, Asghari S, Park H S, Sohn E K, Yu I J. 2018. Acute toxicity, uptake, and elimination of zinc oxide nanoparticles (ZnO NPs) using saltwater microcrustacean, Artemia franciscana. Environmental Toxicology and Pharmacology, 57: 181-188.
DOI:10.1016/j.etap.2017.12.018 |
Secretariat of the Convention on Biological Diversity and Scientific and Technical Advisory Panel GEF. 2012. Impacts of Marine Debris on Biodiversity: current status and potential solutions. Convention on Biological Diversity, Montreal. 61p.
|
Shen J H, Zhou S F, Dong Y L, Cui Y L. 2012. Analysis on the status of surface temperature structure of the East China Sea and partial Yellow Sea in 2006. Marine Fisheries, 29(2): 179-185.
(in Chinese) |
Tressel S L, Koukos G, Tchernychev B, Jacques S L, Covic L, Kuliopulos A. 2011. Pharmacology, biodistribution, and efficacy of GPCR-based pepducins in disease models. In: Langel Ü ed. Cell-Penetrating Peptides: Methods and Protocols. Humana Press, New York. p. 259-275, https://doi.org/10.1007/978-1-60761-919-2_19.
|
Vannuccini M L, Grassi G, Leaver M J, Corsi I. 2015. Combination effects of nano-TiO2 and 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) on biotransformation gene expression in the liver of European sea bass Dicentrarchus labrax. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 176-177: 71-78.
DOI:10.1016/j.cbpc.2015.07.009 |
Varó I, Perini A, Torreblanca A, Garcia Y, Bergami E, Vannuccini M L, Corsi I. 2019. Time-dependent effects of polystyrene nanoparticles in brine shrimp Artemia franciscana at physiological, biochemical and molecular levels. Science of the Total Environment, 675: 570-580.
DOI:10.1016/j.scitotenv.2019.04.157 |
Wang J D, Tan Z, Peng J P, Qiu Q X, Li M M. 2016. The behaviors of microplastics in the marine environment. Marine Environmental Research, 113: 7-17.
DOI:10.1016/j.marenvres.2015.10.014 |
Wang Y, Zhang D, Zhang M X, Mu J L, Ding G H, Mao Z, Cao Y F, Jin F, Cong Y, Wang L J, Zhang W W, Wang J Y. 2019. Effects of ingested polystyrene microplastics on brine shrimp, Artemia parthenogenetica. Environmental Pollution, 244: 715-722.
DOI:10.1016/j.envpol.2018.10.024 |
Wright S L, Thompson R C, Galloway T S. 2013. The physical impacts of microplastics on marine organisms: a review. Environmental Pollution, 178: 483-492.
DOI:10.1016/j.envpol.2013.02.031 |
Zhang Y L, Wang D, Zhang Z, Wang Z P, Zhang D C, Yin H. 2018. Transcriptome analysis of Artemia sinica in response to Micrococcus lysodeikticus infection. Fish & Shellfish Immunology, 81: 92-98.
DOI:10.1016/j.fsi.2018.06.033 |
 2021, Vol. 39
2021, Vol. 39



