Institute of Oceanology, Chinese Academy of Sciences
Article Information
- ZHANG Hua, LÜ Songhui, CEN Jingyi, LI Yang, LI Qun, WU Zhen
- Morphology and molecular phylogeny of three species of Coolia (Dinophyceae) from Hainan Island, South China Sea
- Journal of Oceanology and Limnology, 39(3): 1020-1032
- http://dx.doi.org/10.1007/s00343-020-9326-z
Article History
- Received Dec. 27, 2019
- accepted in principle Feb. 4, 2020
- accepted for publication May. 21, 2020
2 Shenzhen Academy of Environmental Science, Shenzhen 518001, China;
3 Southern Marine Science and Engineering Guangdong Laboratory, Zhuhai 519000, China;
4 College of Life Science, South China Normal University, Guangzhou 510631, China
Benthic dinoflagellate species in the genus Coolia Meunier are distributed from temperate to tropical waters around the world (Meunier, 1919; Fukuyo, 1981; Faust, 1992; 1995; Ten-Hage et al., 2000; Fraga et al., 2008; Leaw et al., 2010; Karafas et al., 2015; David et al., 2020). These species live attached to various substrates, including seaweeds, rocks, corals, mangroves, sands, and also be found in water column (GEOHAB, 2012; Hoppenrath et al., 2014; Leung et al., 2017).
Currently, there are eight Coolia species, including the recently reported C. santacroce Karafas, Tomas and York, C. palmyrensis Karafas, Tomas & York and C. guanchica H. David, Laza-Martínez, F. Rodríguez & S. Fraga (Meunier, 1919; Faust, 1995; Ten-Hage et al., 2000; Fraga et al., 2008; Leaw et al., 2010; Karafas et al., 2015; David et al., 2020). C. monotis Meunier, C. tropicalis, and C. areolata L. Ten-Hage, J. Turquet, J. P. Quod and Couté were originally described on the basis of morphological data (Meunier, 1919; Faust, 1995; Ten-Hage et al., 2000), while the other species were described with both morphological and molecular data (Fraga et al., 2008; Leaw et al., 2010; Karafas et al., 2015; David et al., 2020).
Species in this genus are differentiated by their main morphological features, including shape, size, thecal plate arrangement, and ornamentation (TenHage, 2000; Fraga et al., 2008; Leaw et al., 2010). However, the range of cell sizes among Coolia conspecifics is wide and overlapping, with the exception of the recently reported small species C. palmyrensis (Karafas et al., 2015). C. tropicalis, C. areolata, C. canariensis S. Fraga, and C. guanchica share the feature that the 1′ plate is the largest in the epitheca, located centrally on the cell (Faust, 1995; Ten-Hage et al., 2000; Fraga et al., 2008; David et al., 2014, 2020), while C. monotis complex (C. monotis, C. malayensis, C. palmyensis, and C. santacroce) have a 1′ plate positioned on the left side of the epitheca and a 6″ plate as the largest (Leaw et al., 2010; David et al., 2014; Karafas et al., 2015; Lewis et al., 2018). The thecal plate ornamentation is the principal trait distinguishing C. tropicalis, C. areolata, and C. canariensis (Nascimento et al., 2019). While cell surface is smooth in C. tropicalis, it is strongly areolated in C. areolata and thecal ornamentation is restricted to the hypotheca for C. canariensis complex (Ten-Hage et al., 2000; Fraga et al., 2008; David et al., 2014; Karafas et al., 2015). Molecular genetic analyses have been widely used to recognize and delineate species of Coolia (Penna et al., 2005; Dolapsakis et al., 2006; Leaw et al., 2010). The C. monotis complex have similar morphologies but distinct phylogenies (Leaw et al., 2010, 2016; Karafas et al., 2015; Karafas and Tomas, 2015; Leung et al., 2017). Species in the C. monotis complex generally can be differentiated from C. areolata, C. tropicalis, and C. canariensis on the basis of both morphology and genetics (Karafas et al., 2015; Karafas and Tomas, 2015; Leaw et al., 2016; Nascimento et al., 2019).
Recently, we isolated several Coolia strains from macroalgae collected from seaweed in the waters around Hainan Island, South China Sea. Cultures were established for morphological and molecular analyses. Three benthic dinoflagellates, C. canariensis, C. tropicalis and C. malayensis, were analyzed using D1/D2 large ribosomal subunit (LSU) rDNA, internal transcribed spacer (ITS) region (ITS1- 5.8S-ITS2) sequences and scanning electron microscopy (SEM) micrograph acquisitions. Our report on the morphological and phylogenetic diversity of Coolia species from Hainan Island, South China Sea, provides a detailed understanding of Coolia species of this area.
2 MATERIAL AND METHOD 2.1 Sampling and culturingSamples of macroalgae (mainly Sargassum sp.) were collected from seaweed beds by divers at depths of 1–3 m at Sanya (109°29′47″E, 18°12′33″N), Lingshui (109°58′37″E, 18°18′38″N), and Qionghai (110°49′15″E, 19°18′59″N) on Hainan Island, South China Sea from March, 2013 to August, 2014. Samples of macroalgae and seawater were placed into wide-mouthed polyvinylchloride bottles and immediately brought to the laboratory. These samples were vigorously shaken to detach the dinoflagellates, then filtered through 120- and 20-μm mesh filters. The residue retained on the second mesh was resuspended in filtered seawater for cell isolation. Individual cells were isolated using a single-cell capillary pipette using an inverted research microscope ECLIPSE TE2000-U (Nikon, Yokohama, Japan). Isolated cultures were established in the L1 medium (Guillard and Hargraves, 1993) at a salinity of 30 and incubated at 25 ℃ in a 12-h꞉12-h light꞉dark regime under 150 μmol photons/(m2·s) provided by fluorescent lamps.
2.2 Morphological observationMorphometric features (dorsal-ventral length and width; anteroposterior length of ≥30 cells) were determined from newly preserved cultures using a QImaging Retiga 4000R digital camera (Qimaging, Surrey, BC, Canada) and IMG Pro plus 6.0 image acquisition and analysis software (Media Cybernetic Inc., Rockville, MD, USA) at ×400 magnification. For SEM, live samples of the exponential growth phase were fixed with 4% glutaraldehyde for 1 h. The samples were then filtered through nucleopore filters (diameter of 13 mm, pore size of 3 μm, Whatman, Little Chalfont, UK), washed with distilled water, and dehydrated in ethanol with a series of concentrations from 10% to 99.9%, increasing by 10% each grade, once for 15 min at each concentration and three times for 99.9%. The samples were then critical point dried with CO2 (CPD 030, Bal-Tec, AG, Balzers, Liechtenstein). The filters were stub mounted, coated with gold (SCD 005, Bal-Tec) and observed with a field emission SEM Ultra 55 (Zeiss, Jena, Germany). The Kofoid tabulation system was adopted to name the thecal plates (Kofoid, 1909).
2.3 Molecular analysisTotal genomic DNA was extracted using the Omega HP Plant DNA kit (Omega Bio-Tek, Norcross, GA) following manufacturer's instructions. The amplification reactions were conducted using a BioRad PCR Thermal Cycler (Bio-Rad, Hemel Hempstead, UK). Subsequent PCR reactions used PCR master mix (TaKaRa, Dalian, China), template DNA and primers in a final volume of 50 μL. The D1–D3 regions of the LSU were amplified with the primers D1R and D3B (Scholin et al., 1994; Nunn et al., 1996), and the ITS region was amplified using the universal primers of ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′). The PCR amplification reactions for the LSU and ITS regions were performed as previously described (Zhang et al., 2015). Sequencing was performed using the ABI 3730XL Big Dye V3.1 Mix technique (Applied Biosystems, Foster City, CA, USA). Sequences data that support the findings of this study have been deposited in GenBank with the accession number from KR229994 to KR229997 for LSU rDNA and from MF805767 to MF805770 for ITS regions.
There were 52 and 46 sequences included in the LSU rDNA and ITS region phylogenetic analyses, respectively. These were aligned with other relatives retrieved from the GenBank database using ClustalW (Thompson et al., 1994). Only D1/D2 LSU rDNA sequences were analyzed. Ostreopsis sp. were used as outgroups for both the LSU rDNA and ITS region sequence analyses. A maximum likelihood (ML) phylogenetic tree was inferred for each region using Mega X (Kumar et al., 2018). Bayesian analyses were performed with MrBayes 3.1.2 using the best-fitting model (Ronquist and Huelsenbeck, 2003). The bestfitting models for the D1/D2 LSU rDNA and ITS regions were GTR+I+G and HKY+I+G, respectively, which were selected by hLRT in MrModeltest 2.3 (Nylander, 2004). Identical tree topologies were inferred from the ML and Bayesian analyses for both regions. Genetic distance (p-distance) was assessed using MEGA X (Kumar et al., 2018). All positions containing gaps and missing data were eliminated.
3 RESULTMorphological and phylogenetic analyses indicated that the four strains (D1C2, DS5F4, CE3, and CS4C6) of Coolia species isolated from Hainan Island were Coolia canariensis, C. tropicalis, and C. malayensis.
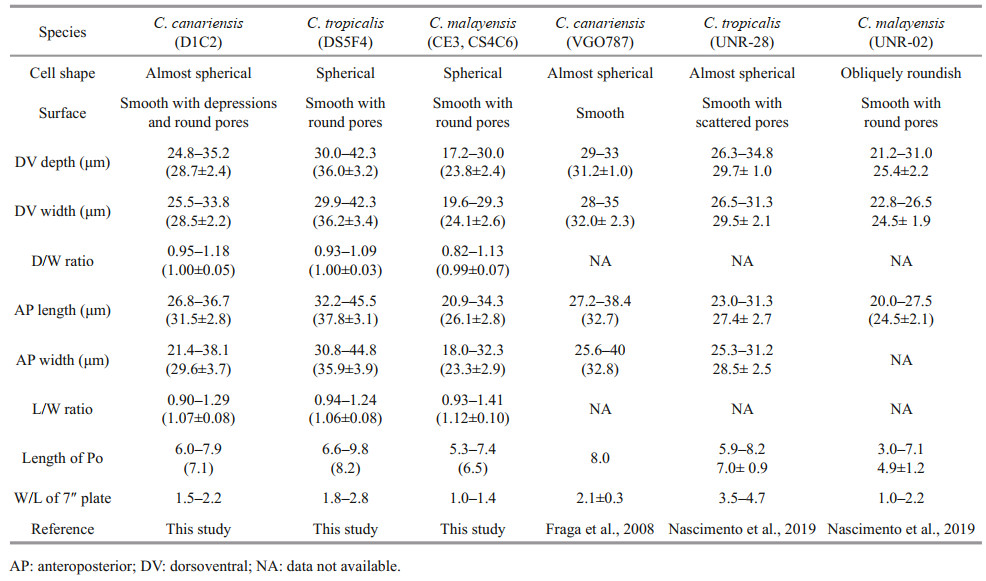
3.1 Morphology of the Hainan strain of C. canariensisThe cells of the C. canariensis Hainan strain (D1C2) were almost spherical (Fig. 1a–b) with a dorsoventral (DV) depth ranging from 24.8 to 35.2 μm (28.7±2.4 μm, n>30), width from 25.5 to 33.8 μm (28.5±2.2 μm, n>30), and anteroposterior (AP) length from 26.8 to 36.7 μm (31.5±2.8 μm, n>30) (Table 1). The plate formula was Po, 3′, 7′′, 6c, 6s, 5′′′, 2′′′′ (Fig. 1c–f). The width/length (W/L) ratio of the 7′′ plate ranged from 1.5 to 2.2 (Table 1). The cingulum was deep and slightly displaced (Fig. 1g–h). The sulcus was short and there were six sulcal plates observed: the posterior sulcal plate (S.p.), the left posterior sulcal plate (S.s.p.), the right posterior sulcal plate (S.d.p.), the right anterior sulcal plate (S.d.a.), the left anterior sulcal plate (S.s.a.), and the anterior sulcal plate (S.a.) (Fig. 1e–h). The Po plate was 6.0– 7.9 μm (average 7.1 μm) in length (Table 1). The apical pore complex (APC) was elongated and slightly curved, and was surrounded by three apical plates: 1′, 2′ and 3′ (Fig. 1c–d & i–j). The 1′ plate was hexagonal and centrally located in the epitheca (Fig. 1c–d). The edge of plate 2′ where embraced the APC was a protuberant, lip-like structure (Fig. 1i–j). A row of hard depressions appeared along the lip-like structure (Fig. 1j). The cell surface was smooth with round pores and depressions (Fig. 1k). Round depressions were found especially in the hypotheca and precingular plates (Fig. 1c–f & h). The margin in contact to cingular plates was ridged (Fig. 1l).
|
| Fig.1 SEM micrographs of C. canariensis a–b. lateral view showing the nearly spherical cells; c–d. apical view showing the epithecal plates; e–f. antapical view showing the hypothecal plates and sulcus; g–h. ventral view showing the deep cingulum and short sulcus; i–j. the apical pore complex (APC) and the slightly curved apical pore inside. The edge of plate 2′ is a protuberant, lip-like structure (arrow). A row of hard depressions appeared along the lip-like structure (arrowheads); k. detailed thecal surface; l: the ridged margin in contact to cingular plates (arrows). Scale bars in a–h=5 μm; i–l=2 μm. |
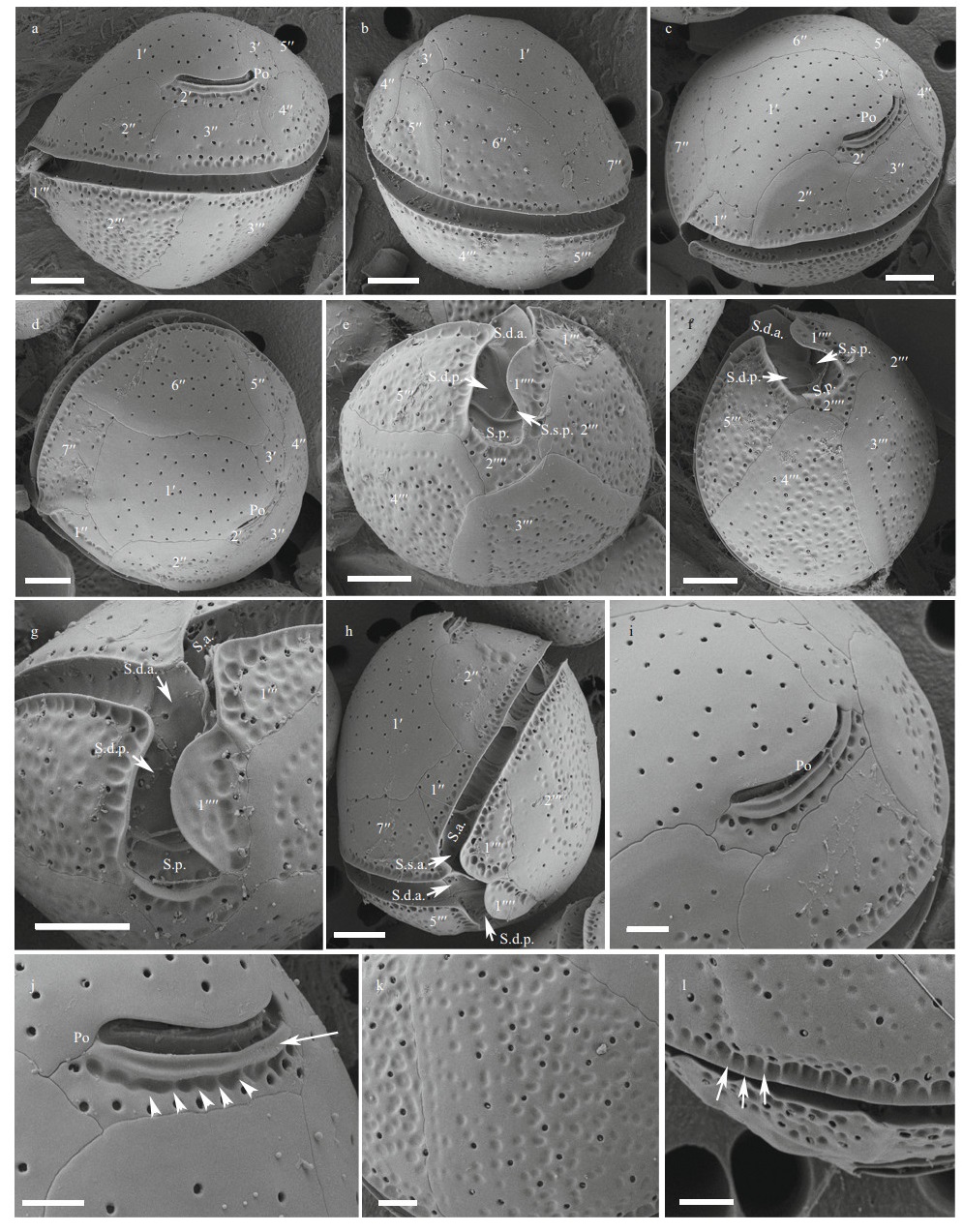
The cells of the C. tropicalis Hainan strain (DS5F4) were spherical (Fig. 2a–b) and the epitheca was smaller than the hypotheca (Fig. 2a–b). Cell sizes ranged from 30.0 to 42.3 μm (36.0±3.2 μm, n>30) in DV depth, 29.9 to 42.3 μm (36.2±3.4 μm, n>30) in width and 32.2 to 45.5 μm (37.8±3.1 μm, n>30) in AP length (Table 1). The plate formula was Po, 3′, 7′′, 6c, 6s, 5′′′, 2′′′′ (Fig. 2c–f). The 1′ plate was pentagonal and centrally located in the epitheca (Fig. 2c–d). The W/L ratio of the 7′′ plate ranged from 1.8 to 2.8 (Table 1). The cingulum was deep and narrow (Fig. 2g–h). The sulcus was short and only five sulcal plates could be observed: S.p., S.d.p. S.d.a., S.s.a., and S.a. (Fig. 2g–h). The Po plate was 6.6–9.8 μm (average 8.2 μm) in length (Table 1). The APC was slightly curved with the apical pore and round pores inside (Fig. 2i–j). The edge of plate 2′ where contacted the APC was ridged like a collar (Fig. 2j). The cell surface was smooth with round pores (Fig. 2k–l).
|
| Fig.2 SEM micrographs of C. tropicalis a–b. lateral view showing the spherical cells; c–d. apical view showing the epithecal plates; e–f. antapical view showing the hypothecal plates; g–h. ventral view showing the deep and narrow cingulum and short sulcus; i–j. the apical pore complex (APC) with apical pore and round pores inside. The edge of plate 2′ where embraces the APC is ridged like a collar; k–l. detailed cell surface. Scale bars in a–h=5 μm; i–l=2 μm. |
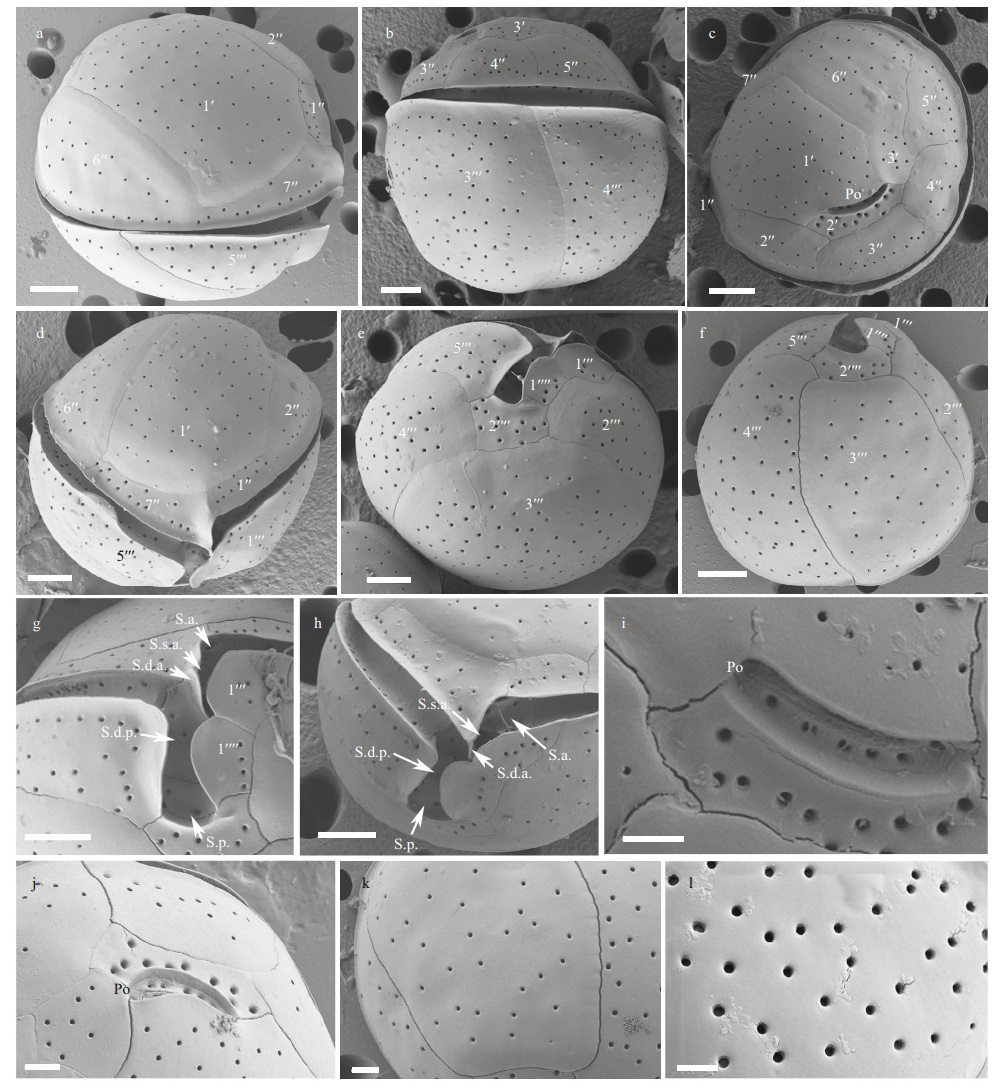
The cells of the Hainan C. malayensis strains (CE3/ CS4C6) were spherical (Fig. 3a–b) with a DV depth of 17.2–30.0 μm (23.8±2.4 μm, n>30), width of 19.6– 29.3 μm (24.1±2.6 μm, n>30), and AP length of 20.9– 34.3 μm (26.1±2.8 μm, n>30) (Table 1). The plate formula was Po, 3′, 7′′, 6c, 6s, 5′′′, 2′′′′ (Fig. 3c–f). The 1′ plate was oblong and positioned to the left of centre (Fig. 3c–d). The W/L ratio of the 7′′ plate ranged from 1.0 to 1.4 (Table 1). The cingulum was wide (Fig. 3g–h), while the sulcus was short with six sulcal plates: S.p., S.d.p., S.s.p., S.d.a., S.s.a., and S.a. (Fig. 3g–h). The Po plate was 5.3–7.4 μm (average 6.5 μm) in length (Table 1). The APC was almost straight with a narrow apical pore and a line of round pores inside (Fig. 3i–j). The cell surface was smooth with round thecal pores (Fig. 3k). There was very fine perforation in the thecal pores (Fig. 3l).
|
| Fig.3 SEM micrographs of C. malayensis a–b. lateral view showing the spherical cells; c–d. apical view showing the epithecal plates; e–f. antapical view showing the hypothecal plates; g–h. ventral view showing the wide cingulum and short sulcus; i–j. The apical pore complex (APC) with a narrow apical pore and a line of round pores inside; k–l. detailed cell surface. very fine perforation in the thecal pores (arrows). Scale bars in a–h=5 μm; i–k=2 μm; l=0.5 μm. |
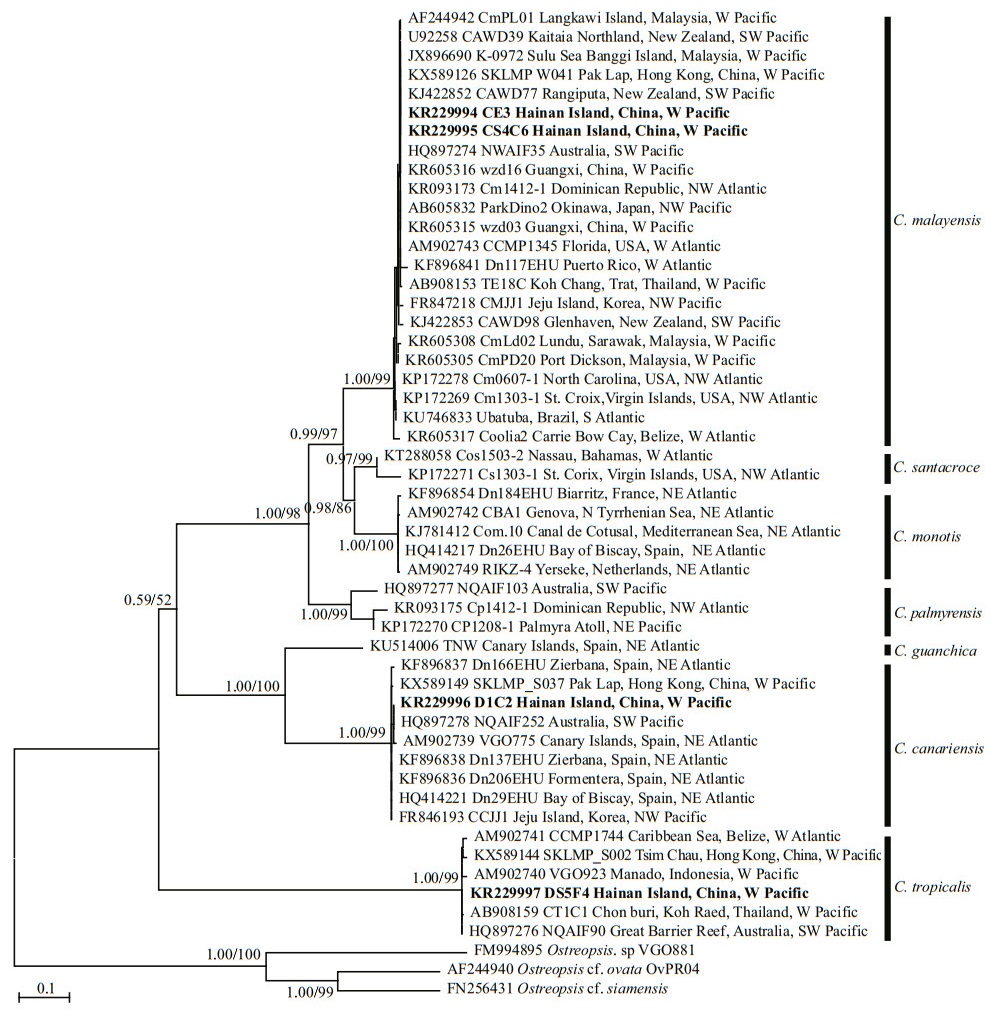
Based on the phylogenetic analysis, the LSU rDNA sequences were clustered into seven major clades: C.monotis, C.santacroce, C.malayensis, C.palmyrensis, C. guanchica, C. tropicalis, and C. canariensis (Fig. 4). Two Hainan strains (CS4C6 and CE3) belonged to C. malayensis, as did other strains from the Pacific (Japan, China, Australia, New Zealand, Korea, and Malaysia) and Atlantic (Virgin Island, USA, Brazil, Puerto Rico, Dominican Republic and Belize) with good support (1.00/99). The Hainan strain DS5F4, one Belizean strain from the West Atlantic and several strains from the Pacific, including Hong Kong of China, Thailand, Australia, and Indonesia formed clade C. tropicalis with well support (1.00/99). Hainan strain D1C2 and strains from Hong Kong of China, Korea, Australia, and Spain formed clade C. canariensis with high support (1.00/99).
|
| Fig.4 Phylogenetic analysis of Coolia species inferred from D1/D2 LSU rDNA sequences using Bayesian inference (BI) and Maximum-likelihood (ML) Both analyses constructed identical topology. Support values for the nodes are shown as posterior probabilities from BI and bootstrap values from ML (BI/ML). Posterior probabilities ≥0.50 and bootstrap values ≥50 are shown. |
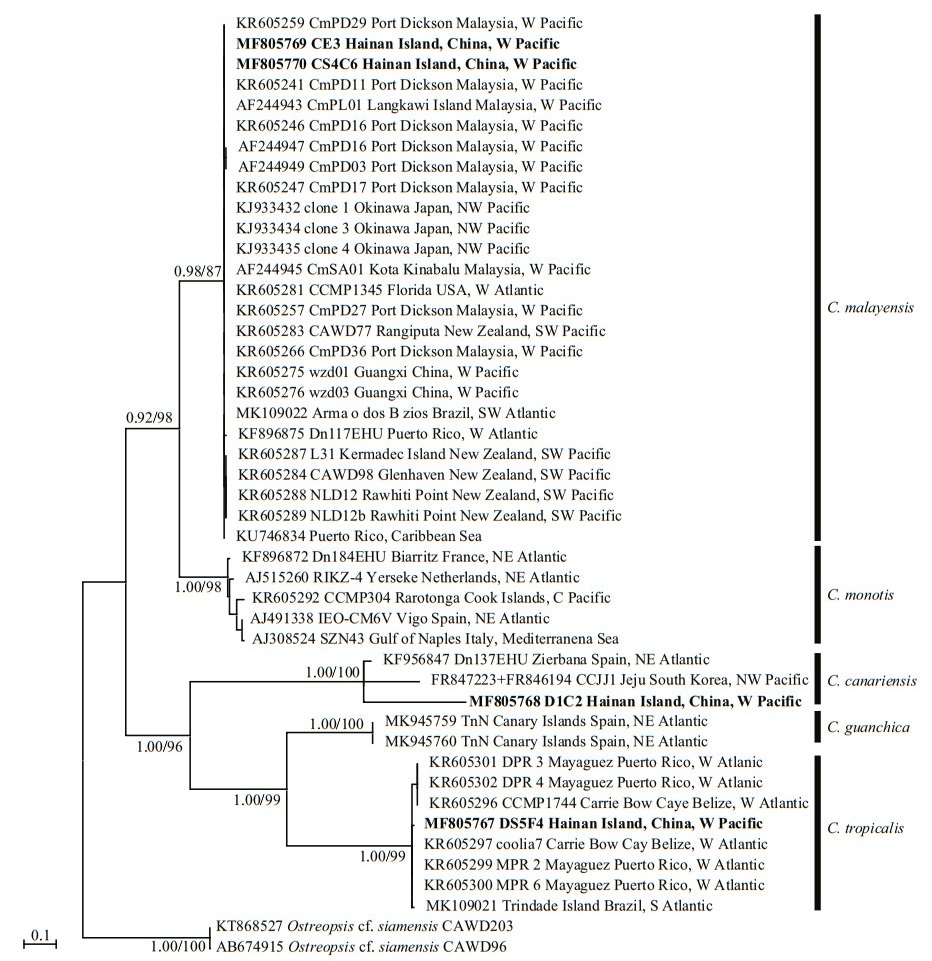
The phylogenetic analysis of ITS regions (Fig. 5) led to the categorisation of five clades: C. monotis, C. malayensis, C. tropicalis, C. guanchica and C. canariensis. The strain DS5F4 of C. tropicalis from Hainan Island was included in the same clade as the strains from Belize, Puerto Rico, and Brazil with good support (1.00/99). The C. malayensis clade was formed with strains from Malaysia, Florida, Puerto Rico, New Zealand, Japan, Brazil, and China, including the strains CE3 and CS4C6 from Hainan Island (0.98/87). The C. canariensis clade comprised strains from Hainan Island, Spain, and Korea with full support.
|
| Fig.5 Phylogenetic analysis inferred from ITS region (ITS1-5.8S-ITS2) sequences using Bayesian inference (BI) and Maximum-likelihood (ML) Both analyses constructed identical topology. Support values for the nodes are shown as posterior probabilities from BI and bootstrap values from ML (BI/ML). Posterior probabilities ≥0.50 and bootstrap values ≥50 are shown. |
The highest estimated genetic distance (p-distances) values of 0.420 4–0.436 5 between the LSU rDNA sequences for selected species of Coolia were observed between C. canariensis and C. tropicalis, followed by values between C. tropicalis and C. monotis at 0.412 6–0.418 5, and between C. tropicalis and C. palmyrensis at 0.392 6–0.412 0 (Supplementary Table S1). The lowest value of 0.103 0–0.132 9 was observed between C. monotis and C. santacroce. An estimated value of evolutionary divergence of approximately 0.4 was found between C. tropicalis and C. malayensis. The values observed between C. canariensis and C. tropicalis, C. monotis, C. santacroce and C. palmyrensis varied from 0.35 to 0.43. Values between C. guanchica and C. canariensis varied from 0.234 8 to 0.243 0. The values among different strains within species were 0–0.025 5, 0–0.013 4, 0–0.015 9, 0–0.005 8, 0.041 6, 0.024 8– 0.104 7 and 0.006 5 for C. malayensis, C. canariensis, C. tropicalis, C. monotis, C. santacroce, C. palmyrensis and C. guanchica, respectively. No differences in the LSU rDNA sequences were found between the Hainan C. malayensis strains and strains from New Zealand, Florida of USA, Malaysia, Japan, Dominican Republic and Guangxi and Hong Kong of China. For C. canariensis and C. tropicalis, no differences in the LSU rDNA sequences were found between the Hainan strains (D1C2 and DS5F4, respectively) and Australian strains (NQAIF252 and NQAIF90, respectively).
For the ITS region, genetic distance between Coolia species ranged from 0.153 8 to 0.634 1. The lowest and highest values was observed in C. monotis vs. C. malayensis and C. canariensis vs. C. tropicalis (Supplementary Table S2). A value of 0.41–0.56 was found between C. malayensis and C. tropicalis / C. canariensis / C. guanchica, as well as that of C. monotis. The values between C. guanchica and the other species was 0.4–0.6. The values among strains within species were 0.161 6–0.320 3, 0–0.018 5, and 0–0.029 8 for C. canariensis, C. malayensis, and C. tropicalis, respectively. Based on ITS region sequences, the values between the Hainan strains and others were 0.253 9–0.320 3, 0.002 6–0.013 2, and 0.008 1–0.029 8 for C. canariensis, C. malayensis, and C. tropicalis.
4 DISCUSSIONThe overall shape of the C. canariensis Hainan strain was similar to that of the original description (Fraga et al., 2008). The AP length (31.5±2.8 μm) and width (29.6±3.7 μm) of the C. canariensis Hainan strain were similar to those of the Canary Islands strains at 32.7 μm and 32.8 μm (Fraga et al., 2008). There was considerable cell variation in DV dimensions among strains. The C. canariensis Korean strain had a smaller cell size, both in DV depth and width (28.3 μm and 27.1 μm, respectively) (Jeong et al., 2012), than those of the Biscayan strains (35.2 μm and 35.4 μm) (Laza-Martinez et al., 2011), and the Hainan strain had a moderate cell size in terms of the DV length and width among the three strains.
The cell shape of the C. tropicalis Hainan strain generally fit the original description from Belize (Faust, 1995). The cell size of the C. tropicalis Hainan strain, at 32.2–45.5 μm in AP length and 30.8–44.8 μm in AP width, was slightly larger than that of the original description, in which Faust (1995) reported that the C. tropicalis cells varied from 23 to 40 μm in length and 25 to 39 μm in width. The cell size of C. tropicalis strains collected from four sites by Mohammad-Noor et al. (2013) were 35–47 μm in length and 30–45 μm in width, larger than originally described by Faust (1995). Cell size of C. tropicalis Brazil strain UNR-28 was very similar to the Hainan strain (Table 1) (Nascimento et al., 2019).
The cell shapes of the Hainan C. malayensis strains were similar to those of the Malaysian strains described by Leaw et al. (2010). The ranges of AP length and width (respectively 20.9–34.3 μm and 18.0–32.3 μm) of the Hainan C. malayensis strains were broader than those of the Malaysian strains (28– 33 μm and 27–32.3 μm; Leaw et al., 2010) and Thailand strains (22–28 μm and 23–25 μm; Tawong et al., 2015), but similar to those of the Korean strain (20–34 μm and 18–30 μm; Jeong et al., 2012). However, C. malayensis from Okinawa had a slightly greater width of 22–33 μm compared with a length of 20–32 μm (Wakeman et al., 2015).
Cells of C. monotis, C. canariensis, C. tropicalis, C. areolata, and C. malayensis were very similar in cell shape, size, and plate arrangement (Meunier, 1919; Faust, 1995; Ten-Hage et al., 2000; Fraga et al., 2008; Leaw et al., 2010). However, these species could be distinguished by several morphological features (Ho and Nguyen, 2014). All of these species had a smooth thecal surface (Faust, 1995; Leaw et al., 2010; Laza-Martinez et al., 2011; Karafas et al., 2015) except C. canariensis and C. areolata (Ten-Hage et al., 2000; Fraga et al., 2008). There were numerous round depressions in the hypotheca and precingular plates of C. canariensis (Fraga et al., 2008; Jeong et al., 2012) and areolates were distributed in all plate surfaces except the 1′ plate of C. areolata (Ten-Hage et al., 2000; Fraga et al., 2008). Although the C. canariensis Korean strains were found to have round non-perforated dents in the hypotheca, cingulum, and bottom of the precingular plates (Jeong et al., 2012), the C. canariensis strains from Hainan had round depressions in the hypotheca and precingular plates. Therefore, the Hainan strains of C. canariensis supported the difference between C. canariensis and C. areolata. Plate 1′ was the largest of the epitheca in C. tropicalis, C. canariensis, C. areolata, and C. guanchica (Fraga et al., 2008; Jeong et al., 2012; David et al., 2014, 2020), while plate 6′′ was the largest for the other Coolia species (Ho and Nguyen, 2014; Karafas et al., 2015; Leaw et al., 2016; Leung et al., 2017). In addition, plate 1′ was oblong and narrow in C. monotis and C. malayensis but pentagonal in C. tropicalis (Mohammad-Noor et al., 2013; Momigliano et al., 2013; Ho and Nguyen, 2014), and elongated in C. palmyrensis and C. santacroce (Karafas et al., 2015). C. canariensis, C. areolata and C. guanchica had a hexagonal 1′ plate (Ten-Hage et al., 2000; Fraga et al., 2008; David et al., 2019). Plate 1′ was located in the centre of the epitheca in C. tropicalis (Faust, 1995; MohammadNoor et al., 2013; Momigliano et al., 2013), C. canariensis (Fraga et al., 2008), and C. areolata (Ten-Hage et al., 2000) and C. guanchica (David et al., 2019), but was situated to the left of the epitheca in C. monotis, C. malayensis, C. palmyrensis, and C. santacroce (Leaw et al., 2010, 2016; Momigliano et al., 2013; David et al., 2014; Karafas et al., 2015)
The W/L ratio of plate 7′′ has been suggested as way to distinguish Coolia species (Leaw et al., 2010; Jeong et al., 2012; Ho and Nguyen, 2014). The following W/L ratios were observed: 1 for C. monotis (Fraga et al., 2008), 1.2 to 1.5 for C. malayensis (Leaw et al., 2010), 2 for C. areolata and C. canariensis, and 4 for C. tropicalis (Fraga et al., 2008). However, considerable variation in the W/L ratio of plate 7′′ was observed among strains from different geographic regions, ranging from 1.1 to 1.5 for C. monotis, 0.8 to 2.1 for C. malayensis, and 1.7– 2.2, 1.5–3.4, and 1.2–3.5 for C. canariensis from Biscay (Laza-Martinez et al., 2011), Jeju Island (Jeong et al., 2012) and Brazil (Nascimento et al. 2019), respectively. The W/L ratio of plate 7′′ was similar (W/L ratio ~1) among C. monotis complex (Karafas et al., 2015; Wakeman et al., 2015). However, it is still useful in differentiating species in C. monotis complex from species of C. tropicalis or C. canariensis (David et al., 2019).
Perforations within the large pores are commonly reported in Coolia, as we found in the Hainan C. malayensis strains. Jeong et al. (2012) observed perforations within the large pores in the Korean strains of C. canariensis and C. malayensis. However, this structure was not found in the Hainan C. canariensis strains. Additionally, Laza-Martinez et al. (2011) and Leaw et al. (2010) found perforations within the large pores in C. monotis and C. malayensis, respectively. Such structure could also be observed in C. guanchica (David et al., 2019) and C. palmyrensis (Karafas et al., 2015). Therefore, these perforations may be common features and, as such, would not be reliable for species identification.
Resolving the relationships among Coolia species requires the analysis of molecular data. The LSU rDNA phylogenies support C. monotis, C. malayensis, C. tropicalis, C. canariensis, C. santacroce, and C. palmyrensis as monophyletic lineages consistent with distinct species (Fraga et al., 2008; Leaw et al., 2010; Mohammad-Noor et al., 2013; Momigliano et al., 2013; Rhodes et al., 2014; Karafas et al., 2015; Leaw et al., 2016). Additionally, Molecular data in the previously literatures displayed the same branching patterns and main clades for Coolia species with C. monotis, C. malayensis, and C. santacroce as closely related species and C. palmyrensis as a basal lineage to those species (Leaw et al., 2010; Rhodes et al., 2014; Karafas et al., 2015; Wakeman et al., 2015).
Mohammad-Noor et al. (2013) reported that C. canariensis could be clustered into two clades in the LSU rDNA phylogeny, despite the strains originating from the same area. Two C. canariensis clades also formed based on 28S LSU rDNA sequences of 44 Coolia taxa (Wakeman et al., 2015). Nascimento et al. (2019) found that both morphological and phylogenetic data supported cryptic species existence in C. canariensis complex. The recently reported C. guanchica formed a well-supported sister clade with C. canariensis based on phylogenetic analyses and might be included in the C. canariensis complex (David et al., 2019). Nascimento et al. (2019) also suggested that additional molecular and morphology data of strains from different regions should be acquired to strength our knowledge of C. canariensis complex.
Estimates of the evolutionary divergence among Coolia depended on sequences analyzed by previous authors (Leaw et al., 2010; Momigliano et al., 2013; Karafas et al., 2015). Moderate values of 16.1%– 16.5% between C. malayensis and other Coolia species were observed by Wakeman et al. (2015), while a value of 3.42%–32.88% was observed in this work. Karafas et al. (2015) reported the smallest intraspecific distance of D1/D2 LSU rDNA to between C. monotis and C. santacroce. However, we found the lowest divergence of both LSU rDNA and ITS region was between C. monotis and C. malayensis. This agree with Leaw et al. (2010). Compensatory base changes between ITS2 secondary structures was considered as species level divergency though lowest divergence distances between C. monotis and C. malayensis within the genus (Leaw et al., 2010). Although Litaker et al. (2007) proposed genetic distances of 0–2.1% within dinoflagellate species; we suggest that the genetic divergence might be related to the species/isolates selected for analysis.
Coolia species are highly morphologically similar and exhibit high phylogenetic diversity. Further studies are needed to clarify the taxonomic ambiguity of Coolia. Moreover, C. tropicalis produces cooliatoxin (Holmes et al., 1995; Mohammad-Noor et al., 2013) and C. malayensis is also toxic (MohammadNoor et al., 2013; Rhodes et al., 2014). Consequently, toxicity and species distributions of Coolia are important for understanding the structure of the marine food web in Chinese coastal waters.
5 CONCLUSIONMarine benthic dinoflagellates in the genus Coolia are reported in both tropical and temperate regions. Three Coolia species, including Coolia canariensis, C. tropicalis, and C. malayensis were observed in the coastal waters of Hainan Island. In phylogenetic analyses based on the LSU rDNA and ITS regions (ITS1-5.8S-ITS2), Hainan strains of C. canariensis, C. tropicalis, and C. malayensis clustered within the clades of these species with other isolates from different areas. Our study reveals the morphological and genetic diversity of Coolia species from Hainan Island, South China Sea, which provides a detailed understanding of Coolia species of this area.
6 DATA AVAILABILITY STATEMENTThe data that support the findings of this study are available on request from the corresponding author.
7 ACKNOWLEDGMENTWe thank Dr. Hualong WANG for his help with sampling.
David H, Laza-Martínez A, Miguel I, Orive E. 2014. Broad distribution of Coolia monotis and restricted distribution of Coolia cf.canariensis (Dinophyceae) on the Atlantic coast of the Iberian Peninsula. Phycologia, 53(4): 342-352.
DOI:10.2216/13-214.1 |
David H, Laza-Martínez A, Rodríguez F, Fraga S, Orive E. 2020. Coolia guanchica sp. nov. (Dinophyceae) a new epibenthic dinoflagellate from the Canary Islands (NE Atlantic Ocean). European Journal of Phycology, 55(1): 76-88.
DOI:10.1080/09670262.2019.1651400 |
Dolapsakis N P, Kilpatrick M W, Economou-Amilli A, Tafas T. 2006. Morphology and rDNA phylogeny of a Mediterranean Coolia monotis (Dinophyceae) strain from Greece. Scientia Marina, 70(1): 67-76.
DOI:10.3989/scimar.2006.70n167 |
Faust M A. 1992. Observations on the morphology and sexual reproduction of Coolia monotis (Dinophyceae). Journal of Phycology, 28(1): 94-104.
DOI:10.1111/j.0022-3646.1992.00094.x |
Faust MA. 1995. Observation of sand-dwelling toxic dinoflagellates (Dinophyceae) from widely differing sites, including two new species. Journal of Phycology, 31(6): 996-1003.
DOI:10.1111/j.0022-3646.1995.00996.x |
Fraga S, Penna A, Bianconi I, Paz B, Zapata M. 2008. Coolia canariensis sp. nov. (Dinophyceae), a new nontoxic epiphytic benthic dinoflagellate from the Canary Islands. Journal of Phycology, 44(4): 1060-1070.
DOI:10.1111/j.1529-8817.2008.00555.x |
Fukuyo Y. 1981. Taxonomical study on benthic dinoflagellates collected in coral reefs. Nippon Suisan Gakkaishi, 47(8): 967-978.
DOI:10.2331/suisan.47.967 |
GEOHAB. 2012. global Ecology and Oceanography of Harmful Algal Blooms, GEOHAB Core Research Project: HABs in Benthic Systems. In: Berdalet E, Tester P, Zingone A eds. IOC of UNESCO and SCOR, Paris and Newark, 64pp.
|
Guillard R R L, Hargraves P E. 1993. Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia, 32(3): 234-236.
DOI:10.2216/i0031-8884-32-3-234.1 |
Ho T V, Nguyen L N. 2014. Morphology and distribution of the three epiphytic dinoflagellate species Coolia monotis, C. tropicalis, and C. canariensis (Ostreopsidaceae, Gonyaulacales, Dinophyceae) from Vietnamese coastal waters. Ocean Science Journal, 49(3): 211-221.
DOI:10.1007/s12601-014-0021-x |
Holmes M J, Lewis R J, Jones A, Hoy A W W. 1995. Cooliatoxin, the first toxin from Coolia monotis (Dinophyceae). Natural Toxins, 3(5): 355-362.
DOI:10.1002/nt.2620030506 |
Hoppenrath M, Murray S A, Chomérat N, Horiguchi T. 2014. Marine Benthic Dinoflagellates—Unveiling Their Worldwide Biodiversity. Kleine Senckenberg-Reihe, Band 54, Schweizerbart, Stuttgart, Germany, 276pp.
|
Jeong H J, Yih W, Kang N S, Lee S Y, Yoon E Y, Yoo Y D, Kim H S, Kim J H. 2012. First report of the epiphytic benthic dinoflagellates Coolia canariensis and Coolia malayensis in the waters off Jeju Island, Korea: morphology and rDNA sequences. The Journal of Eukaryotic Microbiology, 59(2): 114-133.
DOI:10.1111/j.1550-7408.2012.00610.x |
Karafas S J, Tomas C R. 2015. Further observations on the genetics and morphometrics of Coolia santacroce (Dinophyceae). Algae, 30(4): 275-280.
DOI:10.4490/algae.2015.30.4.275 |
Karafas S, York R, Tomas C. 2015. Morphological and genetic analysis of the Coolia monotis species complex with the introduction of two new species, Coolia santacroce sp. nov. and Coolia palmyrensis sp. nov. (Dinophyceae). Harmful Algae, 46: 18-33.
DOI:10.1016/j.hal.2015.05.002 |
Kofoid G A. 1909. On Peridinium steinii Jöngensen, with a note on the nomenclature of the skeleton of the Peridinidae. Archiv Protistenkunde, 16: 25-47.
|
Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Molecular Biology and Evolution, 35(6): 1547-1549.
DOI:10.1093/molbev/msy096 |
Laza-Martinez A, Orive E, Miguel I. 2011. Morphological and genetic characterization of benthic dinoflagellates of the genera Coolia, Ostreopsis and Prorocentrum from the south-eastern Bay of Biscay. European Journal of Phycology, 46(1): 45-65.
DOI:10.1080/09670262.2010.550387 |
Leaw C P, Lim P T, Cheng K W, Ng B K, Usup G. 2010. Morphology and molecular characterization of a new species of thecate benthic dinoflagellate, Coolia malayensis sp. nov. (Dinophyceae). Journal of Phycology, 46(1): 162-171.
DOI:10.1111/j.1529-8817.2009.00778.x |
Leaw C P, Tan T H, Lim H C, Teng S T, Yong H L, Smith K F, Rhodes L, Wolf M, Holland W C, Vandersea M W, Litaker R W, Tester P A, Gu H F, Usup G, Lim P T. 2016. New scenario for speciation in the benthic dinoflagellate genus Coolia (Dinophyceae). Harmful Algae, 55: 137-149.
DOI:10.1016/j.hal.2016.02.010 |
Leung P T Y, Yan M, Yiu S K F, Lam V T T, Ip J C H, Au M W Y, Chen C Y, Wai T C, Lam P K S. 2017. Molecular phylogeny and toxicity of harmful benthic dinoflagellates Coolia (Ostreopsidaceae, Dinophyceae) in a sub-tropical marine ecosystem: the first record from Hong Kong. Marine Pollution Bulletin, 124(2): 878-889.
DOI:10.1016/j.marpolbul.2017.01.017 |
Lewis N I, Wolny J L, Achenbach J C, Ellis L, Pitula J S, Rafuse C, Rosales D S, McCarron P. 2018. Identification, growth and toxicity assessment of Coolia Meunier (Dinophyceae) from Nova Scotia, Canada. Harmful Algae, 75: 45-56.
DOI:10.1016/j.hal.2018.04.001 |
Litaker R W, Vandersea M W, Kibler S R, Reece K S, Stokes N A, Lutzoni F M, Yonish B A, West M A, Black M N D, Tester P A. 2007. Recognizing dinoflagellate species using ITS rDNA sequences. Journal of Phycology, 43(2): 344-355.
DOI:10.1111/j.1529-8817.2007.00320.x |
Meunier A. 1919. Microplankton de la mer Flamande. III. Les Péridiniens. Mémoires du Musée Royal d'Histoire Naturelle de Belgique. Bruxelles: Hayez, imprimeur de l'Académie royale de Belgique, 8: 1-116.
|
Mohammad-Noor N, Moestrup Ø, Lundholm N, Fraga S, Adam A, Holmes M J, Saleh E. 2013. Autecology and phylogeny of Coolia tropicalis and Coolia malayensis (Dinophyceae), with emphasis on taxonomy of C. tropicalis based on light microscopy, scanning electron microscopy and LSU rDNA. Journal of Phycology, 49(3): 536-545.
DOI:10.1111/jpy.12062 |
Momigliano P, Sparrow L, Blair D, Heimann K. 2013. The diversity of Coolia spp. (Dinophyceae Ostreopsidaceae) in the Central Great Barrier Reef Region. PLoS One, 8(10).
DOI:10.1371/journal.pone.0079278 |
Nascimento S M, Da Silva RAF, Oliveira F, Fraga S, Salgueiro F. 2019. Morphology and molecular phylogeny of Coolia tropicalis, Coolia malayensis and a new lineage of the Coolia canariensis species complex (Dinophyceae) isolated from Brazil. European Journal of Phycology, 54(3): 484-496.
DOI:10.1080/09670262.2019.1599449 |
Nunn G B, Theisen B F, Christensen B, Arctander P. 1996. Simplicity-correlated size growth of the nuclear 28S ribosomal RNA D3 expansion segment in the crustacean order Isopoda. Journal of Molecular Evolution, 42(2): 211-223.
DOI:10.1007/BF02198847 |
Nylander JAA. 2004. MrModeltest v2. Program distributed by the author. Evolutionary Biology Center, Uppsala University.
|
Penna A, Vila M, Fraga S, Giacobbe M G, Andreoni F, Riobó P, Vernesi C. 2005. Characterization of Ostreopsis and Coolia (Dinophyceae) isolates in the western Mediterranean Sea based on morphology, toxicity and internal transcribed spacer 5.8S rDNA sequences. Journal of Phycology, 41(1): 212-225.
DOI:10.1111/j.1529-8817.2005.04011.X |
Rhodes L, Smith K, Papiol G G, Adamson J, Harwood T, Munday R. 2014. Epiphytic dinoflagellates in sub-tropical New Zealand, in particular the genus Coolia Meunier. Harmful Algae, 34: 36-41.
DOI:10.1016/j.hal.2014.02.004 |
Ronquist F, Huelsenbeck J P. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19(12): 1572-1574.
DOI:10.1093/bioinformatics/btg180 |
Scholin C A, Herzog M, Sogin M, Anderson D A. 1994. Identification of group- and strain- specific genetic markers for globally distributed Alexandrium (Dinophyceae). II. Sequence analysis of a fragment of the LSU rRNA gene. Journal of Phycology, 30(6): 999-1011.
DOI:10.1111/j.0022-3646.1994.00999.x |
Tawong W, Nishimura T, Sakanari H, Sato S, Yamaguchi H, Adachi M. 2015. Characterization of Gambierdiscus and Coolia (Dinophyceae) isolates from Thailand based on morphology and phylogeny. Phycological Research, 63(2): 125-133.
DOI:10.1111/pre.12074 |
Ten-Hage L, Turquet J, Quod J P, Couté A. 2000. Coolia areolata sp. nov. (Dinophyceae), a new sand-dwelling dinoflagellate from the southwestern Indian Ocean. Phycologia, 39(5): 377-383.
|
Thompson J D, Higgins D G, Gibson T J. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research, 22(22): 4673-4680.
DOI:10.1093/nar/22.22.4673 |
Wakeman K C, Yamaguchi A, Roy M C, Jenke-Kodama H. 2015. Morphology, phylogeny and novel chemical compounds from Coolia malayensis (Dinophyceae) from Okinawa, Japan. Harmful Algae, 44: 8-19.
DOI:10.1016/j.hal.2015.02.009 |
Zhang H, Li Y, Cen J Y, Wang H L, Cui L, Dong Y L, Lu S H. 2015. Morphotypes of Prorocentrum lima (Dinophyceae) from Hainan Island, South China Sea: morphological and molecular characterization. Phycologia, 54(5): 503-516.
DOI:10.2216/15-8.1 |
 2021, Vol. 39
2021, Vol. 39