Institute of Oceanology, Chinese Academy of Sciences
Article Information
- QU Yang, XU Kuidong, LI Tao, WANG Maoyu, ZHONG Huan, CHEN Tianyu
- Deep-sea coral evidence for dissolved mercury evolution in the deep North Pacific Ocean over the last 700 years
- Journal of Oceanology and Limnology, 39(5): 1622-1633
- http://dx.doi.org/10.1007/s00343-021-0474-6
Article History
- Received Dec. 14, 2020
- accepted in principle Feb. 5, 2021
- accepted for publication May. 29, 2021
2 Laboratory of Marine Organism Taxonomy and Phylogeny, Institute of Oceanology, Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China;
3 State Key Laboratory of Pollution Control and Resources Reuse, School of the Environment, Nanjing University, Nanjing 210023, China;
4 Environmental and Life Sciences Program (EnLS), Trent University, Peterborough, Ontario K9L 0G2, Canada;
5 Center of Deep Sea Research, Institute of Oceanology, Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
Mercury (Hg) is a toxic trace metal that can bioaccumulate in the marine food webs and threatens human health through marine fish consumption. Over the last few centuries, anthropogenic emissions have increased the global atmospheric Hg deposition flux by 3 to 5-fold compared to pre-industrial time, and 15 to 23-fold compared to natural background (Amos et al., 2015; Beal et al., 2015; Enrico et al., 2017; Zhan et al., 2020). Modeling work agrees that the surface ocean Hg concentration evolves together with the atmosphere, while the deep ocean is less affected (Selin et al., 2008; Strode et al., 2010; Zhang et al., 2014a, b; Semeniuk and Dastoor, 2017; Archer and Blum, 2018; Kawai et al., 2020). However, the oceanic Hg cycle including the anthropogenic Hg perturbations in the deep ocean is much less investigated than the surface ocean.
A better understanding of oceanic Hg cycle will improve our ability in predicting future atmospheric Hg concentration, which is regulated by oceanatmosphere exchange, as well as developing sedimentary Hg as a proxy for paleoceanography and paleoclimate research. As the largest surficial reservoir of Hg, the deep ocean will take in a large amount of anthropogenic Hg in the future (Amos et al., 2013), and reemit it when water masses upwell to the surface. Knowing the dynamic of anthropogenic Hg penetrating into the deep ocean will help to constrain the fate of anthropogenic Hg and the legacy emission of Hg to the atmosphere (Amos et al., 2013). In addition, Hg concentrations in marine sediments have been used as a proxy of volcanic activity in recent years, e.g., Sanei et al. (2014) and Shen et al. (2020). However, Hg enrichment in sediments may also be affected by the depositional environment, such as varying primary productivity and redox conditions. A better understanding of oceanic Hg cycle will aid the development of this relatively new proxy. The common assumption of reversible adsorption of dissolved inorganic Hg (HgaqⅡ) on particulate organic matter (POM) generally used in modeling works is questioned by Lamborg et al. (2016), who proposed a mechanism of "regenerative scavenging" based on data from a cruise at North Atlantic. They proposed that the main process releasing HgⅡ from POM is remineralization, and HgⅡ hardly desorb from POM below the mixed layer. It has not been further tested whether reversible adsorption or regenerative scavenging is closer to actually mechanism. Conflicts also arise between models with different POM transport and degradation models, resulting in deep ocean Hg residence time ranging from hundreds to thousands of years (Kawai et al., 2020). There are few observations to constrain these uncertainties in modeling due to the low Hg concentration in seawater and the strict sample handling procedure (Lamborg et al., 2012). It has been shown that the seawater Hg profile has already been altered by anthropogenic Hg (Strode et al., 2010) and it is difficult to deconvolve the natural and anthropogenic Hg in the seawater profiles. Based on the correlation between dissolved phosphate and Hg, Lamborg et al. (2014) has inferred that, except for the locations where deep water forms, most of the deep ocean (South Atlantic, Tropical and Subtropical Pacific) has not been influenced by anthropogenic Hg, which is in contrast with the results of some modeling results (Selin et al., 2008; Strode et al., 2010; Zhang et al., 2014b; Archer and Blum, 2018). The uncertainties in modeling and modern observation call for the reconstruction of Hg concentration evolution of deep waters at least back to the era before the industrial revolution.
Deep-sea coral skeletons are promising archive of chemistry of the ocean (Robinson et al., 2014) as their food sources are sinking or suspended POM, which will carry the seawater Hg signal through adsorption desorption processes (Morel et al., 1998) or regenerative scavenging (Lamborg et al., 2016). The signatures of dissolved Hg will thus be registered in the organic matter of the coral skeleton layer by layer during coral growth. Lamborg et al. (2013) and Sun et al. (2016) reconstructed the Hg concentrations of near surface waters based on the reef corals near the continental shelf. They have identified perturbations by the local anthropogenic activities over the last 60 years and 200 years, respectively. Deep-sea proteinaceous corals live below the euphotic zone and mainly feed on POM (Griffin and Druffel, 1989; Sherwood et al., 2005). These long-lived deep-sea specimens would record the chemical signature of its food in its skeleton organic matter (Sherwood and Risk, 2007). Here, we report Hg concentrations of a 700-year deep sea proteinaceous coral recovered at the Caroline seamounts of the subtropical deep North Pacific. We attempt to reconstruct the ambient seawater HgaqⅡ concentration evolution over the last few hundred years. Our record extends well back to the period before distinct anthropogenic Hg emissions (Streets et al., 2017), and thus allows us to understand the natural background level of Hg and its potential perturbation by anthropogenic sources in the deep North Pacific.
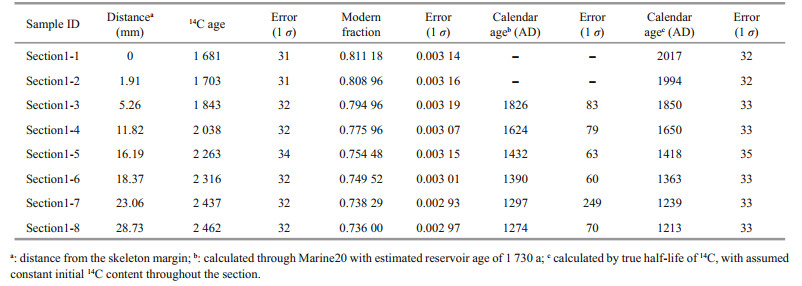
2 MATERIAL AND METHOD 2.1 Sample collection and preparationThe proteinaceous coral specimen was collected alive by remotely operated vehicle (ROV) Faxian (Discovery in Chinese) during the cruise of R/V Kexue (Science in Chinese) on August 24, 2017 on the Caroline Seamounts (140°10′55″E, 10°31′22″N, 1 249 m) and identified as Primnoa sp. The coral used in this study is inferred to be a long-lived proteinaceous coral due to its large size, which contains abundant organic matter. The basal part of this coral skeleton was cut and used in our study (Fig. 1). It can be seen that the growth layers of coral skeleton change from the calcite-dominating base (white) into the organic rich trunk (black) (Andrews et al., 2002). The specimen was air-dried and sectioned into two neighboring slabs near the skeletal base with the lower section named Section-1 and the upper one named Section-2. Another two sections sampled from the bottom of the branch were also obtained and named as Branch-2 and Branch-4, respectively. The positions of these four sections are shown in Fig. 1. All the four sections were scraped from the outer edge to the center with a tungsten steel knife to get high temporal resolution samples. The distance between the sampling site and the skeleton margin was measured by a Vernier caliper with precision of 0.02 mm. The outermost part of the Section-1 is almost pure calcite with no detectable organic matter (Fig. 1, the white part of Section-1), making its Hg concentration not detectable by DMA-80. As a result, the record of Section-1 does not continue to 2017 AD. A sample from the white part of the base specimen (Fig. 1) was also taken to represent the Hg concentration in the organic-barren coral skeleton.

|
| Fig.1 Images of the deep-sea coral Primnoa sp. of this study The red lines on the coral sample illustrate how the cross sections (shown in the right) were cut. Section-1 and Section-2 are neighboring sections at the base of the trunk. Branch-2 and Branch-4 are sections of the two branches. The sampling areas of each section are highlighted red. |
Section-1 was radiocarbon dated based on calcite which is the main component of the skeleton (about 80%, see below) and the deep water reservoir age at this site is well known from the GLODAP database. The radiocarbon dating was conducted at Bristol Radiocarbon Accelerator Mass Spectrometry Facility. Each of the powder sample (about 20 mg) was leached by 0.1-mol/L HCl to remove the adsorbed CO2 (Adkins et al., 2002). The residue was then oven dried and went through the standard carbon isotope measurement as described in detail by Knowles et al. (2019). In brief, the samples were dissolved in concentrated phosphoric acid in a pre-vacuumed tube. The CO2 generated was graphitized following the hydrogen reduction method (Vogel et al., 1984). Three carbon isotopes of 12C, 13C, and 14C were measured simultaneously, and the radiocarbon data were reported as the fraction modern (Fm) after normalized to a δ13C value of -25‰. A radiocarbondead deep-sea coral sample whose age is about 145 ka (Fm=0.002 3±0.001 1 (n=23, 2SD)) was measured to correct the blank of each sample (Li et al., 2020).
2.3 Mercury concentration measurementThe Hg concentrations of the coral skeleton were measured by DMA-80 (Milestone) at the Center for Marine Geochemistry Research, State Key Laboratory for Mineral Deposit Research, Nanjing University. The combustion temperature was set to 650 ℃, which has been proved to completely release the Hg in coral skeleton (Lamborg et al., 2013). The DMA-80 was calibrated with a high purity Hg standard (HighPurity Standards, HPS) which was diluted to 5 ng/g. Calibrated pipettes were used to transfer specific amounts of diluted standard into quartz boats (used for holding liquid samples). The calibration curve contains 10 points ranging from 0.018 ng to 1 ng (R2=0.999 9). The procedure blank was checked before and after every measurement. The accuracy of the calibration curve was tested using the reference materials, with measured Hg concentration of 3.4 ng/g for JG-2 (reference value 3.3–3.8 ng/g) and 2.7 ng/g for JB-3 (reference value 2.4–3.1 ng/g), respectively. An in-house standard was prepared to monitor the long-term reproducibility of our low-concentration measurements by mixing 0.938 79-g GSS-9 (certified reference material, reference Hg concentration of 32 ng/g) with 14.435 81-g Kieselguhr (diatomaceous earth, Aladdin). The Kieselguhr shows no detectable Hg concentration probably because it experienced high temperature burning during production. The mixture was milled for 1.5 h with an agate mortar to ensure homogeneity. The calculated Hg concentration of our in-house standard is 2.1 ng/g, in consistent with the measured Hg concentration of 1.9±0.2 ng/g (1SD).
Test measurements were run to see whether the existing of the calcite will protect the Hg from releasing. A piece of the coral skeleton was cut and milled with an agate mortar to powder. Six subsamples were weighed into quartz boats and three of them were processed with excess 4 mol/L acetate acid to remove calcite. After the reaction cease, all the subsamples were put into a drying oven set at 45 ℃. When the samples were totally dry out, the subsamples were measured by DMA-80. The average Hg concentrations of original samples and samples without calcite are 2.9 ng/g and 3.0 ng/g, respectively. Considering an relative standard deviation (RSD) of 5% of the DMA-80 measurement, these results are consistent within measurement errors, which provides additional support that the direct measurement of coral skeleton using DMA-80 is reliable.
The scraped samples were ground by agate mortar before measurement to ensure complete burning. About 20-mg sample was accurately weighed by a six-digit balance and added to the nickel boat to introduce 0.02- to 0.2-ng Hg into DMA-80. Samples after the DMA-80 measurement were weighed again to calculate the loss on ignition (LOI).
3 RESULT AND DISCUSSION 3.1 Growth rate and age modelThe coral was collected alive in 2017 AD, so that the outermost layer of the skeleton should have the same calendar age. The Δ14C (calculated as Δ14C=(Fm×exp(1950–2017)–1)×1000‰) of the outermost layer is -195 (corresponding to 14C age of 1 730 a), which is consistent with 14C content of the modern seawater dissolved inorganic carbon (DIC) that has not been affected by bomb-14C at the sampling depth (GLODAP database (Key et al., 2004)).
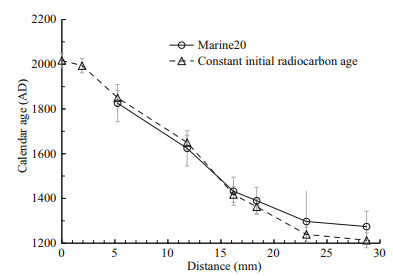
The calendar ages of each sample were calculated by two methods (Table 1). Note that every sample actually represents an age range of 1–3 years inferred from the calculated growth rate and thickness of each sample. For simplicity, we used the youngest age of the time span to represent the calendar age of each sample, which will not affect our results. We first calculated the calendar age using Calib 8.20 (Stuiver et al., 2021) with calibration curve of Marine20 and a reservoir age of 1 730 a (with global ocean age of 550 a and local reservoir age correction of 1 180 a). For another set of calendar age, the Fm was calculated into a 14C age with true half-life (5 730 a), and the age difference between each sample and the outermost sample was regarded as the growth age of that sample. The calendar age of each sample was then calculated by 2017 AD minus the growth age. The first method assumes that the 14C ages of seawater at the sampling site vary in parallel to the atmosphere but corrected by adding the reservoir ages. However, because the seawater at 1 249 m of the deep subtropical North Pacific represents the mixture of different water masses, the short-term variability in atmosphere 14C activity might be smoothed out (DeVries and Primeau, 2010). The second method assumes that the initial 14C ages at the sampling site did not change through the past 700 years because of mixing of different water masses that cancels out the small 14C variations in the atmosphere. We thus chose to use the calendar ages calculated by the second method for simplicity. However, as shown in Fig. 2, the two sets of calendar age are consistent with each other within the overall errors, thus will not lead to any significant difference in age model of this coral.
|
| Fig.2 Radiocarbon age models of Section-1 The x-axis represents the distance from the skeleton margin. The calendar ages are calculated either with Marine20 (Heaton et al., 2020) or with constant initial radiocarbon age. The comparison of these two methods is discussed in the main text. |
The radiocarbon dated Section-1 shows continuous growth with nearly constant growth rate in the outer layer, as shown by the linear pattern in Fig. 2. The growth rate of the coral in this study (0.036 mm/a) is among the lowest ones of this order (Roberts et al., 2009) probably because of the low organic carbon flux in this area (Kawahata et al., 1998; Kawahata, 2002). Although previous researches showed that growth rate plays an important role in Hg bioconcentration in muscle of other animals, the growth rate of this coral is relatively stable, thus we surmise growth rate will not affect the record. The growth rate was then used to calculate the sample age of Section-2 as these two sections are neighboring ones. The age of the near surface samples in Branch-2 and Branch-4 were also calculated with the same growth rate to give a rough indication of the ages, which might be subject to potential age errors. These two sections are measured to explore the internal Hg concentration variability of this coral sample.
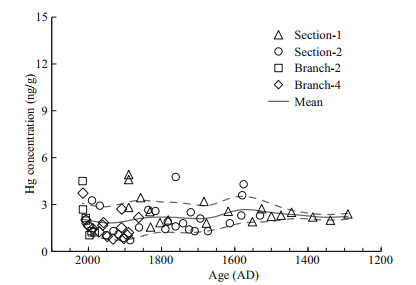
3.2 Mercury concentrations in coral skeletonThe Hg concentrations of the coral skeleton range from 0.7 to 4.9 ng/g (Table 2; Fig. 3), which is much higher than the presumably lattice-bound Hg concentrations in aragonite coral skeleton of about 0.1 ng/g for non-polluted period and about 1.6 ng/g during local source emission (Lamborg et al., 2013; Sun et al., 2016). As Hg has a high affinity to organic matter, we suggest the much higher Hg concentration in our sample compared to the aragonite coral skeleton is the result of high organic matter content in our sample. This is confirmed by the measurement of the coral skeleton base with little organics, giving Hg concentration less than 0.2 ng/g. As a result, normalizing the Hg concentration to organic matter content is a useful way to eliminate the influence of potential variations of organic matter content.
|
| Fig.3 Mercury concentrations of bulk coral skeleton samples Mercury concentrations range from 0.7 to 4.9 ng/g. The data are smoothed with a Gaussian filter with a span of 50 years (±2σ uncertainties, grey dashed curves). |
As the sample size is quite limited, we use the weight difference of each sample before and after DMA-80 measurement (i.e., loss on ignition, LOI) as an estimation of coral skeleton organic matter content, rather than measuring the total organic matter of each sample separately (Boyle, 2004). The decomposition temperature of DMA-80, at which Hg in the sample is released from the solid, is set at 650 ℃, well below the decomposition temperature of calcite. The weight difference before and after the measurement should thus be caused by organic matter combustion and the release of adsorbed water. Deep-sea proteinaceous coral secretes the organic matter while building up its skeleton with calcite (Lewis et al., 1992; Risk et al., 2002) so that Hg/LOI in the skeleton is applied here as an indicator of Hg concentration in the coral skeleton organic matter.
At the modern time, atmospheric deposition is the main source of Hg to the open ocean. The oxidized form of Hg (HgⅡ) is soluble in seawater and can go through a series of transformations. The dissolved inorganic Hg (HgaqⅡ) can be methylated to form organic Hg species, including methylmercury (MMHg) and dimethylmercury (DMHg) (Mason and Fitzgerald, 1991). Although MMHg can be bioaccumulated, its concentration the seawater is low in most of the pelagic oceans without strong upwelling (Cossa et al., 2011; Munson et al., 2015; Bratkič et al., 2016; Agather et al., 2019; Bowman et al., 2020). The HgaqⅡ can also be adsorbed to particulate organic matter (POM), forming HgPⅡ. As POM sinks to deep ocean, HgⅡ is transferred to the deep water dissolved pool through either POM degradation or desorption (Sunderland et al., 2009; Lamborg et al., 2016; Cossa et al., 2018). The exchange of HgⅡ between POM and ambient seawater might be governed by reversible processes (Morel et al., 1998). For example, Hg/POM in the open ocean water column can be calculated by dissolved Hg concentration (deep-water value is about 1–2 pmol/L) and the partition coefficient of Hg on POM (Kd, estimated to be 105 to 106 L/kg (Zhang et al., 2014a)), giving values range 20–400 ng/g. This value is far from saturated adsorption capacity as more than 1 000 ng/g Hg in sedimentary organic matter (Hg/POM) has been reported in marine system (Hare et al., 2010). Modeling works show that preanthropogenic seawater Hg concentration increases with depth mainly due to the partitioning of HgⅡ between POM and ambient seawater as well as the deep HgⅡ accumulation along the oceanic conveyer belt (Strode et al., 2010; Zhang et al., 2014a). However, in the modern seawater, a maximum dissolved Hg concentration has been observed at the thermocline presumably due to anthropogenic Hg pollution (Gill and Fitzgerald, 1988; Sunderland and Mason, 2007).
In our coral sample, the food sources might include fresh sinking POM as shown in previous studies (Griffin and Druffel, 1989; Sherwood et al., 2005), but may also involve organic matter produced within the water column by microbial carbon pump in the dark water column (Herndl and Reinthaler, 2013; Jiao et al., 2014) or suspended organic carbon from the sediments. Regardless of the exact food sources, a higher dissolved Hg concentration in the deep water is expected to support higher Hg/POM being utilized during the coral growth. In turn, if the skeletal organics preserved the food Hg concentration evolution as it did for other chemical information (Sherwood et al., 2014; McMahon et al., 2015), the Hg/LOI of the coral skeleton might provide a proxy of past seawater Hg concentration variability. We envisage that the organics preserved in our proteinaceous coral skeleton may record Hg evolution of the ambient North Pacific deep water during the time of coral growth if the exchange of HgⅡ between POM and ambient seawater is governed by reversible adsorption as suggested in model studies (Morel et al., 1998). In addition, the sampling site is near to Mariana Trench with active hydrothermal activities. The nearest reported hydrothermal activities are a few hundred to a few thousand kilometers away with a water depth mostly blow 2.5 km from the Mariana trough (http://www.interridge.org). Because it is still unclear whether hydrothermal activities would contribute a large amount of Hg to the deep ocean (Bowman et al., 2015, 2016), hydrothermal activities would not be expected to be an important source at our studied site due to the limited diapycnal upwelling in the Pacific Ocean (e.g., refer to the dissolved Fe dispersal from the hydrothermal vents of East Pacific Rise (Resing et al., 2015)). There are also shallower hydrothermal activities in the Mariana back-arc basin, but its influence on our studied region would be restricted by the geographical barrier of the arc system.
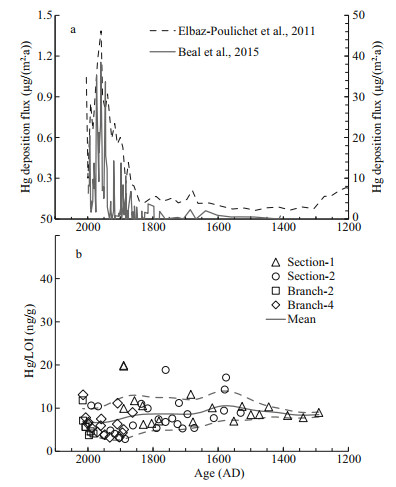
In the long term, the coral skeleton Hg/LOI ratio keeps fairly stable during the past 700 years (Fig. 4b), compared to the ~10-folds enrichment of Hg deposition flux since the 1700s recorded in the ice cores (Beal et al., 2015) and a Mediterranean sediment core (Elbaz-Poulichet et al., 2011) (Fig. 4a). The coral skeleton Hg/LOI of our sample, however, shows large short-term variations, especially since the 1600s as the sampling density increases (Fig. 4b). It is not yet clear what has caused such short-term variations, but it is most likely related to the inhomogeneity of Hg distribution among the organics in the skeleton and/or changing food source with slightly different Hg/POM. During the degradation of POM, the quality and quantity of Hg-binding functional groups change continuously, contributing to variable Hg concentrations in different types of POM. In any case, such short-term variability is very different from the pattern of varing atmosphere deposition flux (ElbazPoulichet et al., 2011; Beal et al., 2015), suggesting that there is no causal link between them. The stable long-term Hg/LOI indicates that the seawater HgaqⅡ around the coral at 1 249 m should have been stable during the past 700 years in agreement with the enrichment factor of less than 2 based on previous modeled results (Strode et al., 2010; Zhang et al., 2014b) and little enrichment of the tropical and subtropical Pacific deep-waters below 1 000 m as inferred from modern seawater profiles (Lamborg et al., 2014).
|
| Fig.4 Mercury records of the deep-sea coral, ice core as well as Mediterranean lagoon sediments over the past seven centuries a. Hg deposition flux of the Mediterranean lagoon sediments (~1-m water depth, right y-axis, Elbaz-Poulichet et al., 2011) and a Canadian ice core (left y-axis, Beal et al., 2015), both show the anthropogenic Hg enrichment factor of more than 10 compared to the natural background; b. Hg/LOI (representing the Hg to organic matter ratio) of the deep-sea coral in this study. The smoothed mean value and error curves are calculated with the same method as in Fig. 3. |
All else being equal, the atmospheric Hg deposition flux to the surface ocean should have the same trend with the terrestrial records, as the residence time of Hg in the surface waters is only about a year (Zhang et al., 2014a; Semeniuk and Dastoor, 2017; Kawai et al., 2020). Taking HgaqⅡ reduction and the subsequent elemental Hg evasion at the sea surface as the first order control on dissolved Hg concentration, the surface water Hg will also have a ~10-fold enrichment compared to the natural background. The POM produced in the euphotic zone carries this signal as it sinks out of the mixed layer. However, such enriched signal will be diluted by HgaqⅡ exchange in the deep ocean. At the thermocline, the remineralization of organic matter converts HgPⅡ to HgaqⅡ, resulting in a maximum HgaqⅡ in water column at a depth of about 500 m (Strode et al., 2010; Zhang et al., 2014b). Below this depth, both organic matter flux and the degradation rate decrease, making the Hg released by desorption and organic matter degradation limited compared to the relatively large pre-anthropogenic HgaqⅡ pool. It is worth noting that another polluted and particle reactive trace metal, Pb, has been shown to be already dominant by anthropogenic sources in the deep North Pacific (Wu et al., 2010). As a result, our data support that the residence time of Hg could be much higher than Pb (~100 years above 2 000 m in the subtropical North Pacific (Henderson and MaierReimer, 2002). In fact, the residence time of Hg may have typical values of a few thousand years in the ocean as suggested by modeling studies (Zhang et al., 2014a; Semeniuk and Dastoor, 2017).
The fluxes and pathways of methylmercury cycling are clearly different from HgⅡ in the water column and sediments (Shi et al., 2018; Zhang et al., 2020). For example, compared with HgaqⅡ transported by POM, MMHg forming in the upper water column is mainly transported to the deep ocean trench by fish carrion (Blum et al., 2020; Sun et al., 2020) and probably other POM in large size class, preventing the alteration of surface Hg signatures by deep water column processes during sinking. Our study thus represents the first attempt to constrain the concentration evolution of the dissolved HgaqⅡ, which is the dominant aquatic Hg species, in deep waters of the ocean. More records from different depths and ocean basins are apparently needed to reliably deconvolve the natural oceanic Hg cycles from the anthropogenic impact.
4 CONCLUSIONMercury concentrations in the proteinaceous deepsea coral skeleton is reported in this study as a proxy to trace the evolution of dissolved Hg concentration in the deep North Pacific over the last 700 years. Our data show that the dissolved Hg pool of deep water in this oligotrophic region have not been significantly affected by anthropogenic emission yet. Nevertheless, without detailed understanding on the interaction between Hg and marine particles as well as Hg incorporation into the coral tissues, our interpretation on the coral Hg record remains preliminary and thus awaits future validation. Proteinaceous deep-water coral contains considerable amount of organic matter in its skeleton (more than 20% in this study), and as a result, has an order of magnitude higher Hg concentration than scleractinian coral skeleton (Lamborg et al., 2013; Sun et al., 2016). This level of Hg concentration can be readily measured without complex Hg pre-concentration and introduction of procedural blank, suggesting proteinaceous corals could be a promising archive to trace Hg sources at different locations of the ocean. More reconstructions on open ocean Hg records will be required to reliably understand oceanic Hg cycling and to test existing understandings from modeling works.
5 DATA AVAILABILITY STATEMENTAll data generated or analyzed during this study are included in this article.
6 ACKNOWLEDGMENTWe thank the three anonymous reviewers for their constructive comments that helped improving the manuscript.
Adkins J F, Griffin S, Kashgarian M, Cheng H, Druffel E R M, Boyle E A, Edwards R L, Shen C C. 2002. Radiocarbon dating of deep-sea corals. Radiocarbon, 44(2): 567-580.
DOI:10.1017/S0033822200031921 |
Agather A M, Bowman K L, Lamborg C H, Hammerschmidt C R. 2019. Distribution of mercury species in the Western Arctic Ocean (U.S. GEOTRACES GN01). Marine Chemistry, 216: 103686.
DOI:10.1016/j.marchem.2019.103686 |
Amos H M, Jacob D J, Streets D G, Sunderland E M. 2013. Legacy impacts of all-time anthropogenic emissions on the global mercury cycle. Global Biogeochemical Cycles, 27(2): 410-421.
DOI:10.1002/gbc.20040 |
Amos H M, Sonke J E, Obrist D, Robins N, Hagan N, Horowitz H M, Mason R P, Witt M, Hedgecock I M, Corbitt E S, Sunderland E M. 2015. Observational and modeling constraints on global anthropogenic enrichment of mercury. Environmental Science & Technology, 49(7): 4 036-4 047.
|
Andrews A H, Cordes E E, Mahoney M M, Munk K, Coale K H, Cailliet G M, Heifetz J. 2002. Age, growth and radiometric age validation of a deep-sea, habitat-forming gorgonian (Primnoa resedaeformis) from the Gulf of Alaska. Hydrobiologia, 471(1): 101-110.
|
Archer D E, Blum J D. 2018. A model of mercury cycling and isotopic fractionation in the ocean. Biogeosciences, 15(20): 6 297-6 313.
DOI:10.5194/bg-15-6297-2018 |
Beal S A, Osterberg E C, Zdanowicz C M, Fisher D A. 2015. Ice core perspective on mercury pollution during the past 600 years. Environmental Science & Technology, 49(13): 7 641-7 647.
|
Blum J D, Drazen J C, Johnson M W, Popp B N, Motta L C, Jamieson A J. 2020. Mercury isotopes identify nearsurface marine mercury in deep-sea trench biota. Proceedings of the National Academy of Sciences of the United States of America, 117(47): 29 292-29 298.
DOI:10.1073/pnas.2012773117 |
Bowman K L, Hammerschmidt C R, Lamborg C H, Swarr G. 2015. Mercury in the North Atlantic Ocean: the U. S. GEOTRACES zonal and meridional sections. Deep Sea Research Part Ⅱ: Topical Studies in Oceanography, 116: 251-261.
|
Bowman K L, Hammerschmidt C R, Lamborg C H, Swarr G J, Agather A M. 2016. Distribution of mercury species across a zonal section of the eastern tropical South Pacific Ocean(U. S. GEOTRACES GP16). Marine Chemistry, 186: 156-166.
DOI:10.1016/j.marchem.2016.09.005 |
Bowman K L, Lamborg C H, Agather A M. 2020. A global perspective on mercury cycling in the ocean. Science of the Total Environment, 710: 136166.
DOI:10.1016/j.scitotenv.2019.136166 |
Boyle J. 2004. A comparison of two methods for estimating the organic matter content of sediments. Journal of Paleolimnology, 31(1): 125-127.
DOI:10.1023/B:JOPL.0000013354.67645.df |
Bratkič A, Vahčič M, Kotnik J, Vazner K O, Begu E, Woodward E M S, Horvat M. 2016. Mercury presence and speciation in the South Atlantic Ocean along the 40°S transect. Global Biogeochemical Cycles, 30(2): 105-119.
DOI:10.1002/2015GB005275 |
Cossa D, Heimbürger L E, Lannuzel D, Rintoul S R, Butler E C V, Bowie A R, Averty B, Watson R J, Remenyi T. 2011. Mercury in the Southern Ocean. Geochimica et Cosmochimica Acta, 75(14): 4 037-4 052.
DOI:10.1016/j.gca.2011.05.001 |
Cossa D, Heimbürger L E, Pérez F F, García-Ibáñez M I, Sonke J E, Planquette H, Lherminier P, Boutorh J, Cheize M, Barraqueta J L M, Shelley R, Sarthou G. 2018. Mercury distribution and transport in the North Atlantic Ocean along the GEOTRACES-GA01 transect. Biogeosciences, 15(8): 2 309-2 323.
DOI:10.5194/bg-15-2309-2018 |
DeVries T, Primeau F. 2010. An improved method for estimating water-mass ventilation age from radiocarbon data. Earth and Planetary Science Letters, 295(3-4): 367-378.
DOI:10.1016/j.epsl.2010.04.011 |
Elbaz-Poulichet F, Dezileau L, Freydier R, Cossa D, Sabatier P. 2011. A 3500-year record of Hg and Pb contamination in a Mediterranean sedimentary archive (the Pierre Blanche Lagoon, France). Environmental Science & Technology, 45(20): 8 642-8 647.
|
Enrico M, Le Roux G, Heimbürger L E, Van Beek P, Souhaut M, Chmeleff J, Sonke J E. 2017. Holocene atmospheric mercury levels reconstructed from peat bog mercury stable isotopes. Environmental Science & Technology, 51(11): 5 899-5 906.
|
Gill G A, Fitzgerald W F. 1988. Vertical mercury distributions in the oceans. Geochimica et Cosmochimica Acta, 52(6): 1 719-1 728.
DOI:10.1016/0016-7037(88)90240-2 |
Griffin S, Druffel E R M. 1989. Sources of carbon to deep-sea corals. Radiocarbon, 31(3): 533-543.
DOI:10.1017/S0033822200012121 |
Hare A A, Stern G A, Kuzyk Z Z A, Macdonald R W, Johannessen S C, Wang F Y. 2010. Natural and anthropogenic mercury distribution in marine sediments from Hudson Bay, Canada. Environmental Science & Technology, 44(15): 5 805-5 811.
|
Heaton T J, Köhler P, Butzin M, Bard E, Reimer R W, Austin W E N, Ramsey C B, Grootes P M, Hughen K A, Kromer B, Reimer P J, Adkins J, Burke A, Cook M S, Olsen J, Skinner L C. 2020. Marine20-the marine radiocarbon age calibration curve (0-55, 000 cal BP). Radiocarbon, 62(4): 779-820.
DOI:10.1017/RDC.2020.68 |
Henderson G M, Maier-Reimer E. 2002. Advection and removal of 210Pb and stable Pb isotopes in the oceans: a general circulation model study. Geochimica et Cosmochimica Acta, 66(2): 257-272.
DOI:10.1016/S0016-7037(01)00779-7 |
Herndl G J, Reinthaler T. 2013. Microbial control of the dark end of the biological pump. Nature Geoscience, 6(9): 718-724.
DOI:10.1038/ngeo1921 |
Jiao N, Robinson C, Azam F, Thomas H, Baltar F, Dang H, Hardman-Mountford N J, Johnson M, Kirchman D L, Koch B P, Legendre L, Li C, Liu J, Luo T, Luo Y W, Mitra A, Romanou A, Tang K, Wang X, Zhang C, Zhang R. 2014. Mechanisms of microbial carbon sequestration in the ocean-future research directions. Biogeosciences, 11(19): 5 285-5 306.
DOI:10.5194/bg-11-5285-2014 |
Kawahata H. 2002. Suspended and settling particles in the Pacific. Deep Sea Research Part Ⅱ: Topical Studies in Oceanography, 49(24-25): 5 647-5 664.
DOI:10.1016/S0967-0645(02)00216-3 |
Kawahata H, Yamamuro M, Ohta H. 1998. Seasonal and vertical variations of sinking particle fluxes in the West Caroline Basin Variations saisonnières et verticales des flux de particules dans le bassin des Carolines. Oceanologica Acta, 21(4): 521-532.
DOI:10.1016/S0399-1784(98)80035-2 |
Kawai T, Sakurai T, Suzuki N. 2020. Application of a new dynamic 3-D model to investigate human impacts on the fate of mercury in the global ocean. Environmental Modelling & Software, 124: 104599.
|
Key R M, Kozyr A, Sabine C L, Lee K, Wanninkhof R, Bullister J L, Feely R A, Millero F J, Mordy C, Peng T H. 2004. A global ocean carbon climatology: results from Global Data Analysis Project (GLODAP). Global Biogeochemical Cycles, 18(4): GB4031.
|
Knowles T D J, Monaghan P S, Evershed R P. 2019. Radiocarbon sample preparation procedures and the first status report from the Bristol radiocarbon AMS (BRAMS) facility. Radiocarbon, 61(5): 1 541-1 550.
DOI:10.1017/RDC.2019.28 |
Lamborg C H, Hammerschmidt C R, Bowman K L. 2016. An examination of the role of particles in oceanic mercury cycling. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 374(2081): 20150297.
DOI:10.1098/rsta.2015.0297 |
Lamborg C H, Hammerschmidt C R, Bowman K L, Swarr G J, Munson K M, Ohnemus D C, Lam P J, Heimbürger L E, Rijkenberg M J A, Saito M A. 2014. A global ocean inventory of anthropogenic mercury based on water column measurements. Nature, 512(7512): 65-68.
DOI:10.1038/nature13563 |
Lamborg C H, Hammerschmidt C R, Gill G A, Mason R P, Gichuki S. 2012. An intercomparison of procedures for the determination of total mercury in seawater and recommendations regarding mercury speciation during GEOTRACES cruises. Limnology and Oceanography: Methods, 10(2): 90-100.
DOI:10.4319/lom.2012.10.90 |
Lamborg C H, Swarr G, Hughen K, Jones R J, Birdwhistell S, Furby K, Murty S A, Prouty N, Tseng C M. 2013. Determination of low-level mercury in coralline aragonite by calcination-isotope dilution-inductively coupled plasma-mass spectrometry and its application to Diploria specimens from Castle Harbour, Bermuda. Geochimica et Cosmochimica Acta, 109: 27-37.
DOI:10.1016/j.gca.2013.01.026 |
Lewis J C, Barnowski T F, Telesnicki G J. 1992. Characteristics of carbonates of Gorgonian axes (Coelenterata, Octocorallia). The Biological Bulletin, 183(2): 278-296.
DOI:10.2307/1542215 |
Li T, Robinson L F, Chen T Y, Wang X C T, Burke A, Rae J W B, Pegrum-Haram A, Knowles T D J, Li G J, Chen J, Ng H C, Prokopenko M, Rowland G H, Samperiz A, Stewart J A, Southon J, Spooner P T. 2020. Rapid shifts in circulation and biogeochemistry of the Southern Ocean during deglacial carbon cycle events. Science Advances, 6(42): eabb3807,.
DOI:10.1126/sciadv.abb3807 |
Mason R P, Fitzgerald W F. 1991. Mercury speciation in open ocean waters. Water Air & Soil Pollution, 56(1): 779-789.
|
McMahon K W, McCarthy M D, Sherwood O A, Larsen T, Guilderson T P. 2015. Millennial-scale plankton regime shifts in the subtropical North Pacific Ocean. Science, 350(6267): 1 530-1 533.
DOI:10.1126/science.aaa9942 |
Morel F M M, Kraepiel A M L, Amyot M. 1998. The chemical cycle and bioaccumulation of mercury. Annual Review of Ecology and Systematics, 29: 543-566.
DOI:10.1146/annurev.ecolsys.29.1.543 |
Munson K M, Lamborg C H, Swarr G J, Saito M A. 2015. Mercury species concentrations and fluxes in the Central Tropical Pacific Ocean. Global Biogeochemical Cycles, 29(5): 656-676.
DOI:10.1002/2015GB005120 |
Resing J A, Sedwick P N, German C R, Jenkins W J, Moffett J W, Sohst B M, Tagliabue A. 2015. Basin-scale transport of hydrothermal dissolved metals across the South Pacific Ocean. Nature, 523(7559): 200-203.
DOI:10.1038/nature14577 |
Risk M J, Heikoop J M, Snow M G, Beukens R. 2002. Lifespans and growth patterns of two deep-sea corals: primnoa resedaeformis and Desmophyllum cristagalli. Hydrobiologia, 471(1): 125-131.
|
Roberts J M, Wheeler A, Freiwald A, Cairns S. 2009. Biology. Cold-Water Corals. Cambridge University Press, Cambridge. p. 67-107.
|
Robinson L F, Adkins J F, Frank N, Gagnon A C, Prouty N G, Roark E B, van de Flierdt T. 2014. The geochemistry of deep-sea coral skeletons: a review of vital effects and applications for palaeoceanography. Deep Sea Research Part Ⅱ: Topical Studies in Oceanography, 99: 184-198.
DOI:10.1016/j.dsr2.2013.06.005 |
Sanei H, Outridge P M, Stern G A, Macdonald R W. 2014. Classification of mercury-labile organic matter relationships in lake sediments. Chemical Geology, 373: 87-92.
DOI:10.1016/j.chemgeo.2014.02.029 |
Selin N E, Jacob D J, Yantosca R M, Strode S, Jaeglé L, Sunderland E M. 2008. Global 3-D land-oceanatmosphere model for mercury: present-day versus preindustrial cycles and anthropogenic enrichment factors for deposition. Global Biogeochemical Cycles, 22(2): GB2011.
|
Semeniuk K, Dastoor A. 2017. Development of a global ocean mercury model with a methylation cycle: outstanding issues. Global Biogeochemical Cycles, 31(2): 400-433.
|
Shen J, Feng Q L, Algeo T J, Liu J L, Zhou C Y, Wei W, Liu J S, Them II T R, Gill B C, Chen J B. 2020. Sedimentary host phases of mercury (Hg) and implications for use of Hg as a volcanic proxy. Earth and Planetary Science Letters, 543: 116333.
DOI:10.1016/j.epsl.2020.116333 |
Sherwood O A, Guilderson T P, Batista F C, Schiff J T, McCarthy M D. 2014. Increasing subtropical North Pacific Ocean nitrogen fixation since the Little Ice Age. Nature, 505(7481): 78-81.
DOI:10.1038/nature12784 |
Sherwood O A, Heikoop J M, Scott D B, Risk M J, Guilderson T P, McKinney R A. 2005. Stable isotopic composition of deep-sea gorgonian corals Primnoa spp. : a new archive of surface processes. Marine Ecology Progress Series, 301: 135-148.
DOI:10.3354/meps301135 |
Sherwood O A, Risk M J. 2007. Chapter twelve deep-sea corals: new insights to paleoceanography. Developments in Marine Geology, 1: 491-522.
|
Shi X M, Mason R P, Charette M A, Mazrui N M, Cai P H. 2018. Mercury flux from salt marsh sediments: insights from a comparison between 224Ra/228Th disequilibrium and core incubation methods. Geochimica et Cosmochimica Acta, 222: 569-583.
DOI:10.1016/j.gca.2017.10.033 |
Streets D G, Horowitz H M, Jacob D J, Lu Z F, Levin L, Schure A F H T, Sunderland E M. 2017. Total mercury released to the environment by human activities. Environmental Science & Technology, 51(11): 5 969-5 977.
|
Strode S, Jaeglé L, Emerson S. 2010. Vertical transport of anthropogenic mercury in the ocean. Global Biogeochemical Cycles, 24(4): GB4014.
|
Stuiver M, Reimer P J, Reimer R W. 2021. CALIB 8.2[WWW program] at http://calib.org. Accessed on 2021-3-21.
|
Sun R Y, Hintelmann H, Liu Y, Li X H, Dimock B. 2016. Two centuries of coral skeletons from the northern South China Sea record mercury emissions from modern Chinese wars. Environmental Science & Technology, 50(11): 5 481-5 488.
|
Sun R Y, Yuan J J, Sonke J E, Zhang Y X, Zhang T, Zheng W, Chen S, Meng M, Chen J B, Liu Y, Peng X T, Liu C Q. 2020. Methylmercury produced in upper oceans accumulates in deep Mariana Trench fauna. Nature Communications, 11(1): 3 389.
DOI:10.1038/s41467-020-17045-3 |
Sunderland E M, Krabbenhoft D P, Moreau J W, Strode S A, Landing W M. 2009. Mercury sources, distribution, and bioavailability in the North Pacific Ocean: insights from data and models. Global Biogeochemical Cycles, 23(2): GB2010.
|
Sunderland E M, Mason R P. 2007. Human impacts on open ocean mercury concentrations. Global Biogeochemical Cycles, 21(4): GB4022.
|
Vogel J S, Southon J R, Nelson D E, Brown T A. 1984. Performance of catalytically condensed carbon for use in accelerator mass spectrometry. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 5(2): 289-293.
DOI:10.1016/0168-583X(84)90529-9 |
Wu J F, Rember R, Jin M B, Boyle E A, Flegal A R. 2010. Isotopic evidence for the source of lead in the North Pacific abyssal water. Geochimica et Cosmochimica Acta, 74(16): 4 629-4 638.
DOI:10.1016/j.gca.2010.05.017 |
Zhan T, Zhou X, Cheng W H, He X Q, Tu L Y, Liu X Y, Ge J Y, Xie Y Y, Zhang J, Ma Y F, Li E, Qiao Y S. 2020. Atmospheric mercury accumulation rate in northeastern China during the past 800 years as recorded by the sediments of Tianchi Crater Lake. Environmental Science and Pollution Research, 27(1): 571-578.
DOI:10.1007/s11356-019-06927-9 |
Zhang Y X, Jaeglé L, Thompson L. 2014a. Natural biogeochemical cycle of mercury in a global three-dimensional ocean tracer model. Global Biogeochemical Cycles, 28(5): 553-570.
DOI:10.1002/2014GB004814 |
Zhang Y X, Jaeglé L, Thompson L, Streets D G. 2014b. Six centuries of changing oceanic mercury. Global Biogeochemical Cycles, 28(11): 1 251-1 261.
DOI:10.1002/2014GB004939 |
Zhang Y X, Soerensen A L, Schartup A T, Sunderland E M. 2020. A global model for methylmercury formation and uptake at the base of marine food webs. Global Biogeochemical Cycles, 34(2): e2019GB006348.
|
 2021, Vol. 39
2021, Vol. 39