Institute of Oceanology, Chinese Academy of Sciences
Article Information
- QIU Lixia, YU Zhiming, CAO Xihua, JI Hena, SONG Xiuxian
- The mechanism of a new type of modified clay controlling Phaeocystis globosa growth
- Journal of Oceanology and Limnology, 38(4): 1270-1282
- http://dx.doi.org/10.1007/s00343-020-0054-1
Article History
- Received Jan. 19, 2020
- accepted in principle Mar. 19, 2020
- accepted for publication May. 10, 2020
2 Laboratory of Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao 266071, China;
3 University of Chinese Academy of Sciences, Beijing 100049, China;
4 Center for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao 266071, China
Phaeocystis globosa is a harmful algal bloom (HAB) species of worldwide distribution and exists in single-cell and colony life forms. Among P. globosa blooms, Phaeocystis colonies are dominant (Qin et al., 2016). Phaeocystis colonies secrete a large amount of mucus (van Rijssel et al., 2000), which form a white foam on the sea surface, causing great harm to the aquaculture and tourism industries (Qi et al., 2002). Even worse, Phaeocystis blooms can threaten the safety of nuclear power stations because large colloidal colonies can block the filters of nuclear power plants (Qin et al., 2016; Cao et al., 2017). How to remove P. globosa in emergencies is important in the field of marine environmental protection.
Modified clay (MC) technology is currently one of the most common methods to mitigate HABs in China. MC can effectively remove algal bloom organisms and reduce the degree of eutrophication and improve water quality (Yu et al., 2017). However, previous research has been conducted mainly on HABs formed by single algal cells (as opposed to colonies), such as Aureococcus anophagefferens (Yu et al., 2004; Liu et al., 2016; Zhu et al., 2018), Skeletonema costatum (Lu et al., 2015; Qiu et al., 2017), Amphidinium carterae Hulburt (Liu et al., 2017), Prorocentrum donghaiense (Lu et al., 2017), Karenia mikimotoi (Liu et al., 2018) and Alexandrium tamarense (Zhang et al., 2018) cells. As mentioned above, P. globosa exists in two forms: single cells and colonies. When HABs occur, the colony consists of thousands or even tens of thousands of single cells. What are the effects of MC for P. globosa? In 2015, Cao et al. (2017) used MC technology to remove P. globosa algal blooms in Qinzhou Bay and achieved good results. Some research results in the laboratory also showed that the MC method was effective at removing single cells of P. globosa (Qiu et al., 2017). However, at present, there has been no research that has elaborated on the formation mechanism of the specific morphology and structure of P. globosa, nor has there been a targeted formula to improve the MC system to deal with this special HAB-causing species more effectively.
Based on previous research, this study used P. globosa as the research object, and the density of cells and colonies, polysaccharide content, reactive oxygen species (ROS) level, superoxide dismutase (SOD) activity, catalase (CAT) activity and malondialdehyde (MDA) content, photosynthesis system (photosynthetic pigment concentration, chlorophyll fluorescence kinetic parameters), etc., were measured. The potential mechanism by which oxidized composite modified clay (OXI-MC) inhibits the growth and colony formation of P. globosa was analyzed from both physiological and biochemical perspectives.
2 MATERIAL AND METHOD 2.1 Experimental algal speciesA strain of P. globosa (CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences) isolated from Beibu Bay was grown in L1 media (Guillard and Hargraves, 1993) at 20±1℃ under 65 μmol photons/(m2·s) and a 12꞉12 light:dark photoperiod. The density of the colonies was 14.45×104 ind./L, and the D10, D50, and D90 were 129.81, 312.63, and 592.57 μm, respectively.
The clay used in this experiment was kaolinite, obtained from Yankuang Beihai Kaolin Co., Ltd. (Guangxi, China). All reagents used in this experiment were of analytical grade. Polyaluminum chloride (PAC) was obtained from Tianjin Guangfu Fine Chemical (Tianjin, China), and potassium monopersulfate composite salt (PMS, the ingredient was 2KHSO5·KHSO4·K2SO4, henceforth expressed as the active ingredient KHSO5) was obtained from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). In our preliminary research, we used different materials (clay, KHSO5, KHSO5-MC, PAC-MC, and KHSO5/PAC-MC) to remove P. globosa and found that the removal efficiencies after 3 h were 19%, 59%, 66%, 30%, and 90%, respectively, under the same clay dose (0.05 g/L) or oxidant dose (0.005 g/L) (unpublished data). KHSO5/ PAC-MC (OXI-MC) that had the best effect on the elimination of P. globosa was used for subsequent research. The suspension of MC, with a clay content of 25 g/L, was prepared as described by Yu et al. (1994) and was prepared in advance before the removal experiment.
2.2 Experimental methods and experimental indicators 2.2.1 Experimental methodsDuring the experiment, cultures were maintained in 5-L beakers, and the concentrations of OXI-MC in the algal culture within each treatment were maintained at 0.05 g/L and 0.1 g/L (lower than the safe concentration (Wang et al., 2014a, b; Zhang et al., 2019). The removal experiments were run in triplicate. Samples were collected at 3, 12, 24, 48, and 96 h to measure the relevant parameters of biomass, polysaccharide content, the antioxidant system, and the photosynthesis system. In this experiment, an untreated group was used as control.
2.2.2 Biomass measurements and morphological observationsAt 3, 12, 24, 48, and 96 h, an appropriate amount of colonies was selected from both the control group and the experimental group and observed under a microscope (SZX16, Olympus, Japan) and a scanning electron microscope (SEM) (Hitachi, S-3400N, Japan). Images of the control and OXI-MC-exposed cultures were obtained by scanning electron microscopy. The procedures for SEM observations were implemented according to the methods described by Zhao et al. (2019).
A 10-mL algal sample from each group was taken for colony counting and size determination, a 1-mL counting frame was used to count the complete colonies 10 times, and the degree of colony fragmentation and pigmentation was observed and recorded. In order to observe the colony formation, draw the particle size distribution histogram of colonies according all the size determination in 10-mL algal sample. Single cells were released into the algal solution by repeatedly tapping the colony samples through a needle tube, placed in a counting box and then counted under a microscope (Shen et al., 2000).
2.2.3 Determination of ROS level, SOD activity, CAT activity and MDA contentThe algal cultures treated with OXI-MC were sampled at 3, 12, 24, 48, and 96 h; centrifuged at 10 000 r/min for 10 min at 4℃; and then stored at -80℃ for testing. The algal samples were subsequently sonicated for 10 min after the addition of 0.6-mL phosphate buffer solution (pH=7.8) and then centrifuged at 12 000×g for 15 min at 4℃. The supernatant was then collected and maintained at a low temperature. The experiment used ROS (E004), total SOD kit (A001), CAT kit (visible light method), and MDA kit (A003) to determine the ROS level, SOD activity, CAT activity and MDA content of the algae, respectively. The kits were purchased from Nanjing Jiancheng Company (Nanjing, China), and the experimental procedures were followed in strict accordance with the kit instructions.
2.2.4 Determination of photosynthesis-related parameters(1) Determination of photosynthetic pigments
The algal cultures were sampled after OXI-MC treatment at 3, 12, 24, 48, and 96 h. Afterward, 100 mL of prefiltered algal solution was collected, and the sample was filtered through a 0.45-μm GF/F glass fiber filter. The filtrate was then wrapped in aluminum foil and stored at -20℃ for the determination of chlorophyll a (Chl a). The collected Chl a samples were extracted with 90% acetone for 24 h in the dark under low-temperature conditions. The fluorescence of the supernatant before and after acidification was then measured by using a Trilogy fluorometer (Turner Desige, Ltd., USA). The concentration of Chl a in the sample was calculated according to the methods of Parsons et al. (1984).
(2) Determination of fast chlorophyll fluorescence parameters
The Chl a fluorescence transients (OJIP) (10 μs to 1 s) of the algae were measured using a Handy PEA fluorometer (Hansatech, UK) after a 10-min darkadaptation treatment. The OJIP test was used to determine the photosynthesis parameters with Biolyzer software (Stirbet and Govindjee, 2011; Gururani et al., 2015).
Fv /Fm is the maximal photochemical efficiency of photosystem Ⅱ (PSII) under dark adaptation and is also referred to as the energy capture efficiency of the open PSII reaction center (RC). The calculation formula is as follows:
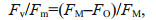 (1)
(1)where Fv is the maximum variable fluorescence, FM is the maximum fluorescence, FO is the minimum fluorescence.
ФPSII is the actual photochemical efficiency of PSII, which reflects the actual photochemical efficiency when the PSII RC is partially closed under light. The calculation formula is as follows:
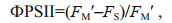 (2)
(2)where FM′ is the minimum fluorescence under light, FS is the steady-state fluorescence.
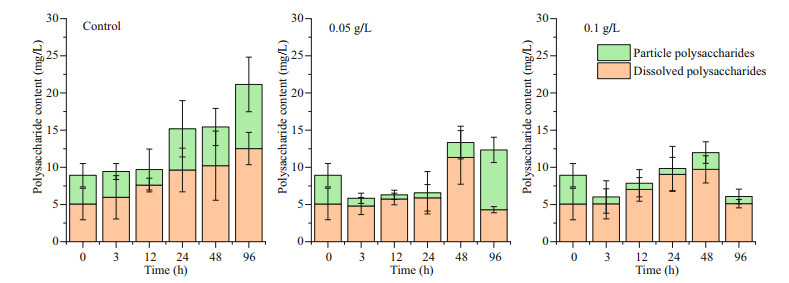
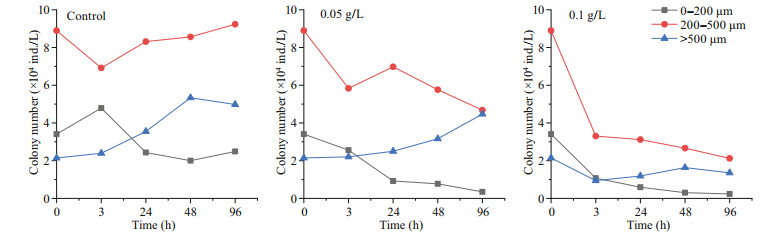
3 RESULT 3.1 Effects of oxidative composite modified clay on the growth of P. globosa 3.1.1 Effects of modified clay on the growth indicators of P. globosaRemoval experiments with P. globosa revealed growth limitations after the addition of OXI-MC, primarily manifested as the number of cells and colonies of P. globosa decreasing, and the higher the concentration of OXI-MC was, the stronger the inhibitory effect on the growth of P. globosa (Fig. 1). During the experiment, the number of colonies smaller than 200 μm in the control group were 3.41×104, 4.79×104, 2.43×104, 2.00×104, and 2.49×104 ind./L at 0, 3, 24, 48, and 96 h, respectively, roughly showing a gradual decrease with time, but a considerable number was also maintained. The number of colonies between 200–500 μm ranged 6.92×104–9.23×104 ind./L. The number of colonies larger than 500 μm at 0, 3, 24, 48, and 96 h was 2.14×104, 2.39×104, 3.55×104, 5.34×104, and 4.98×104 ind./L, respectively, which showed a general trend of a gradual increasing with time. After the addition of OXI-MC, the total number of colonies decreased, and the number of colonies smaller than 200 μm gradually decreased with time. The number of colonies smaller than 200 μm in the 0.05 g/L group was 2.56×104, 0.92×104, 0.77×104, and 0.35×104 ind./L at 3, 24, 48, and 96 h, respectively; in the 0.1 g/L group, the values were 1.08×104, 0.59×104, 0.30×104, and 0.23×104 ind./L, respectively (Fig. 2). The P. globosa particle polysaccharide content of the control group generally increased with the growth of the colonies, and the particle polysaccharide content in the control group was 3.87, 3.46, 2.09, 5.54, 5.21, and 8.63 mg/L at 0, 3, 12, 24, 48, and 96 h, respectively. After the addition of OXI-MC, the particle polysaccharide content decreased significantly, especially in the 0.1 g/L OXI-MC group. The particle polysaccharide content remained at a relatively low level and was only 0.97 mg/L at 96 h, which was 25% of that of the control group (Fig. 3).
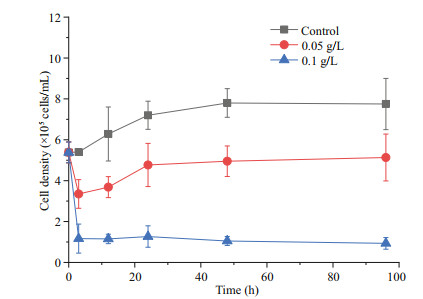
|
| Fig.1 Effects of OXI-MC on the growth of P. globose |
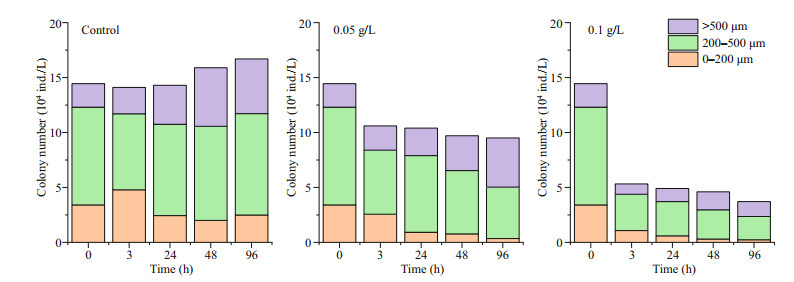
|
| Fig.2 Particle size distribution of colonies after the addition of OXI-MC |
|
| Fig.3 Effects of OXI-MC on the polysaccharide content in P. globose |
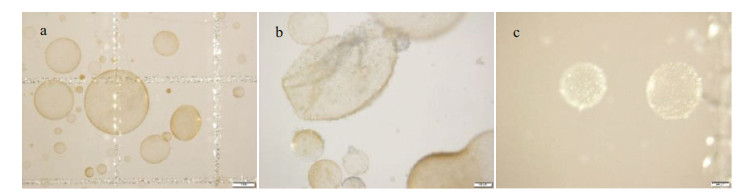
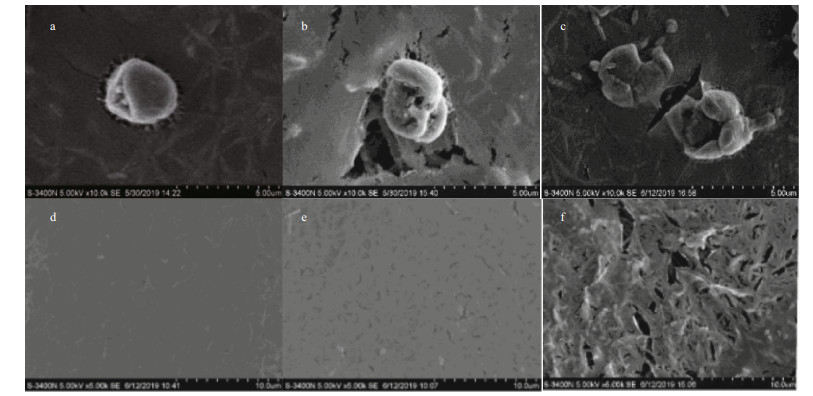
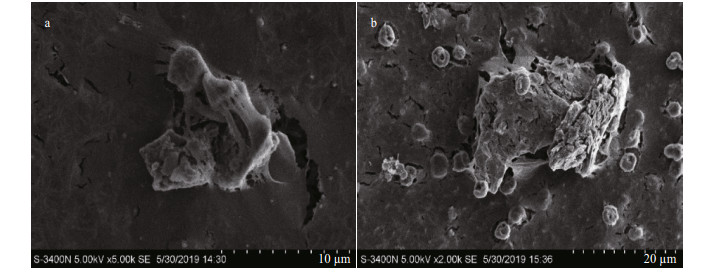
It was observed via microscopy that the Phaeocystis colonies in the control group were round and full and displayed a brownish-yellow color (Fig. 4a). After the addition of OXI-MC, the clay particles adhered to the surface of the colony and formed a colony-MC floc, causing the colonies to shrink, rupture (Fig. 4b) and decolor to white or become transparent (Fig. 4c). There were no living algal cells among the discolored portion, and the polysaccharide glial membrane became thin. SEM images also showed that the algal cells in the control group were relatively complete and that the polysaccharide glial membranes were smooth and complete. After the OXI-MC was added, the single cells ruptured, shrank, and became morphologically deformed, and the polysaccharide glial membranes ruptured. The higher the amount of OXI-MC was, the higher the degree of destruction of the polysaccharide glial membrane and single cells (Fig. 5). For instance, at a concentration of 0.1 g/L, a large number of holes could be observed in the polysaccharide glial membrane (Fig. 5f). Moreover, the clay particles adhered to the surface of the colonies and penetrated the colonies, which caused the polysaccharide glial membranes to rupture; the effect of clay particles was more effective on the cells after the polysaccharide glial membranes rupture (Fig. 6). The SEM images also indicated that exposure to OXIMC led to increased growth inhibition, reduced cell division and proliferation, and aggravated cell damage and rupture.
|
| Fig.4 Different morphologies of P. globosa colonies under the microscope a. complete colony; b. ruptured and shrunken colony; c. discolored colony. |
|
| Fig.5 Effects of OXI-MC on P. globosa cells and the polysaccharide glial membrane under SEM a. complete cell in control group; b. ruptured cell at a concentration of 0.05 g/L; c. ruptured cell at a concentration of 0.1 g/L; d. polysaccharide glial membranes in control group; e. polysaccharide glial membranes at a concentration of 0.05 g/L; f. polysaccharide glial membranes at a concentration of 0.1 g/L. |
|
| Fig.6 OXI-MC penetration of the polysaccharide glial membrane of P. globosa colonies under SEM a. the inner surface of colony; b. the outer surface of colony. |
The ROS level, SOD activity, CAT activity and MDA content per unit of P. globosa cells increased after the addition of OXI-MC, and the oxidative stress of the 0.1 g/L OXI-MC group was greater than that of the 0.05 g/L OXI-MC group, indicating that the higher the content of the OXI-MC was, the more ROS that accumulated in the cells and the stronger the growth inhibition of P. globosa (Fig. 7). At 3 h after the addition of 0.05 g/L OXI-MC, there were less increases in the ROS level, CAT activity and MDA content in this group (equal to 1.33, 1.68, and 1.61 times of the levels of the control group, respectively), while the SOD activity significantly increased (equal to 3.35 times that of the control group). The ROS level, SOD activity, CAT activity, and MDA content in the 0.1 g/L group significantly increased; the levels were 6.54, 3.91, 11.16, and 2.85 times of the levels in the control group, respectively, and were maintained at a high level throughout the experiment. These results indicated that the excessive intracellular ROS in the cells is the main factor inhibiting the growth of P. globosa cells. Excess ROS were shown to trigger a series of damaging processes in algal cells, leading to the dysfunction of many fundamental organelles and structures (Guan et al., 2015).
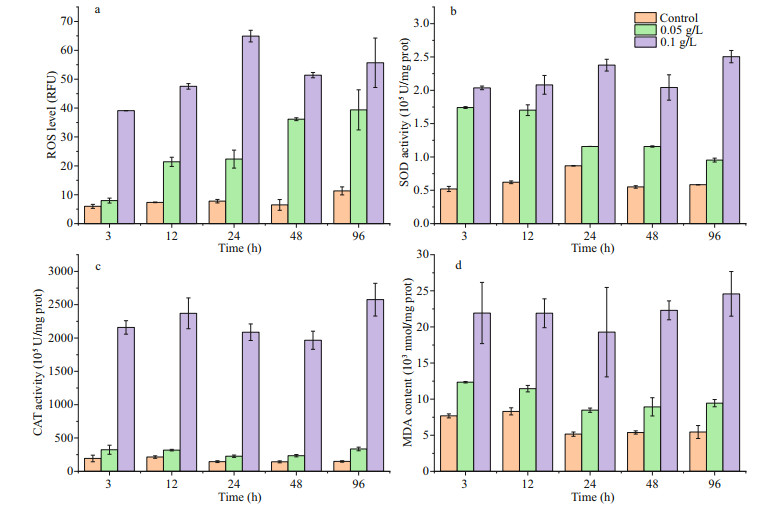
|
| Fig.7 Effects of OXI-MC on ROS level (a), SOD activity (b), CAT activity (c), and MDA concentration (d) in P. globose |
After the addition of OXI-MC, the Chl a content of P. globosa decreased. The content of Chl a in the control group was 59.4 μg/L at 3 h, while the content of Chl a in the 0.05 g/L group and the 0.1 g/L group was 37.9 and 7.0 μg/L, respectively, which were 63.8% and 11.8% of the control group level, respectively (Fig. 8). Chlorophyll fluorescence kinetic parameters showed that both the Fv/Fm and the ФPSII decreased. During the experiment, the Fv/Fm of the control group fluctuated from 0.72 to 0.77, while the fluctuation ranges of the 0.05 g/L group and the 0.1 g/L group were 0.69–0.73 and 0.42–0.50, respectively. The ФPSII of the control group fluctuated from 0.72–0.79, while the fluctuation ranges of the 0.05 g/L group and 0.1 g/L group were 0.67–0.73 and 0.42–0.61, respectively (Fig. 9).
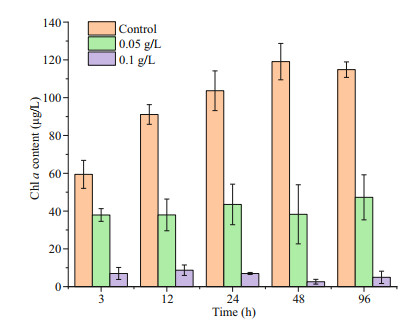
|
| Fig.8 Effects of OXI-MC on the Chl a content in P. globose |
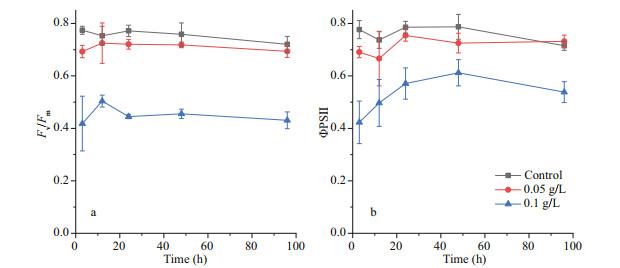
|
| Fig.9 Effects of OXI-MC on the the Fv/Fm (a) and the ФPSII (b) of P. globose |
After the addition of OXI-MC, the numbers of cells and colonies of P. globosa significantly decreased; additionally, the levels were unable to return to the level of the control group, and the growth was inhibited significantly. The higher the concentration of the OXI-MC was, the stronger the inhibitory effect on the growth of P. globosa (Fig. 10). During the experiment, the number of colonies larger than 500 μm in the control group gradually increased. In the system, the diameter of the colonies gradually increased, implying the transition from small colonies to large colonies. The number of colonies from 200 to 500 μm decreased slightly after the addition of OXIMC; later, the number gradually increased and was maintained at a high level during the experiment. The number of colonies smaller than 200 μm tended to increase first but then decrease, but a considerable number was still maintained. New small colonies continued to form in control group. Previous studies have also shown that free single cells without flagella are released from mature colonies, after which the cells resume movement; subsequently, the flagella are lost and adhere to the surface of the matrix, releasing the mucus matrix and forming new colonies (Zhang et al., 2020). After the addition of 0.05 g/L OXI-MC, the number of colonies smaller than 200 μm and the number of colonies between 200 and 500 μm tended to decrease, and so did the total number of colonies. On the other hand, the number of colonies larger than 500 μm gradually increased; but this increase was smaller than that of the control group. Huang et al. (2012) reported that, compared with relatively small colonies, large colonies were more easily to be broken. However, after the addition of 0.05 g/L OXIMC, the number of colonies larger than 500 μm tended to increase gradually. This may be because 0.05 g/L OXI-MC could effectively disrupt some large colonies but poorly affected the transformation process by which small colonies become large colonies, manifested as the number of eliminated colonies being lower than the number of newly formed large colonies. Therefore, the number of colonies larger than 500 μm still increased slightly. The results show that 0.05 g/L OXI-MC could effectively remove small colonies and medium-sized colonies smaller than 500 μm and inhibit their formation, but the inhibitory effect on the formation of large colonies was relatively weak. After the addition of 0.1 g/L OXI-MC, the number of colonies smaller than 200 μm were significantly reduced and continued to decrease during the experiment; this number was only 0.24×104 ind./L until 96 h. It was difficult to form new colonies in the experimental system. The number of 500 μm colonies in the 0.1 g/L OXI-MC group decreased sharply from 0–3 h and then gradually decreased. Moreover, the number of colonies larger than 500 μm tended to first decrease but then increased; however, the increase was small, and the number of colonies larger than 500 μm was significantly lower than the number in the control group. This showed that 0.1 g/L OXI-MC not only could rapidly disrupt colonies with different particle sizes but also could effectively inhibit the formation of small colonies. In this experiment, there was a significant positive correlation between the amount of particle polysaccharides and the number of colonies (Fig. 11). The process of organic matter metabolism, especially the biosynthesis and degradation of glycosaminoglycans, played an important role in the formation and extinction of colonies. At different stages of P. globosa colony formation, the biosynthesis of glycosaminoglycans increased significantly, and the degradation process was significantly downregulated; therefore, glycosaminoglycans accumulated in large quantities (Zhang et al., 2020). Glycosaminoglycan is the essential substrate of P. globosa colonies, so the particle polysaccharide content increases during colony formation. Therefore, after the addition of OXI-MC, the number of colonies significantly decreased, and the content of particle polysaccharides significantly decreased. The particle polysaccharide contents at 3 h in the 0.05 g/L and 0.1 g/L groups were 1.04 mg/L and 0.92 mg/L, respectively, which were 30% and 27% of the control levels, respectively. In the 0.1 g/L OXI-MC group, the particle polysaccharide content remained at a low level throughout the experiment, and the size composition of the colonies was essentially consistent with the particle polysaccharide content. The three main mechanisms for the production of dissolved polysaccharides are (1) release after cell lysis, such as release due to viral infection, zooplankton herbivory, and indulgence; (2) bacterial lysis of extracellular polysaccharides (Smith et al., 1995); and (3) direct release as a physiological process of intact cells (Nagata, 2000). After the addition of the MC, there many phenomena occur, such as cell rupture, bacterial degradation (Smith et al., 1995), and photosynthesis, which may explain why the dissolved polysaccharide content fluctuated during the experiment.
|
| Fig.10 Variation trend of the number of colonies in different particle sizes after OXI-MC was added |
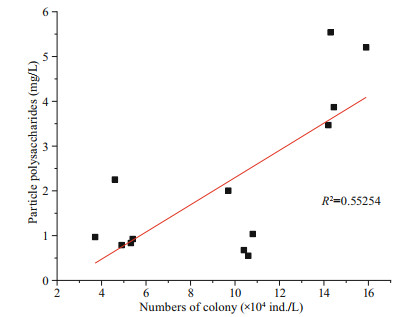
|
| Fig.11 Correlation of the numbers of colonies and the contents of particle polysaccharides (P < 0.01) |
The removal of P. globosa by OXI-MC is a relatively complicated process that can exert many effects on microalgal cells, thereby inhibiting their growth and disrupting normal physiological and biochemical activities. ROS are common byproducts of aerobic metabolism and are produced predominantly in the chloroplasts and mitochondria of algal cells as a control response to abiotic stress, defense against pathogen invasion, and programmed cell death (Mittler et al., 2004). Plants produce reactive oxygen during normal metabolism, and the rate of production increases during stress. Oxidative stress has several obvious characteristics: (1) the production of ROS increases; (2) uncontrolled oxidation occurs because the rate of ROS production exceeds the rate of normal metabolism; (3) cellular components are destroyed by oxidation due to the destruction rate exceeding the repair rate; and (4) cell components that were destroyed lose function, which can result in cell death (Foyer and Noctor, 2005). The most important biochemical characteristics of ROS are their ability to react with biomolecules and to diffuse away from the site of production. In plants, ROS have double effects: as a byproduct of metabolism, ROS have toxic and destructive effects at high concentrations, and as a signaling molecule at low concentrations, ROS can initiate development and responses to environmental stress. The results indicate that the addition of OXIMC reduced the biomass of P. globosa (decreased the chlorophyll content, decreased the number of single cells, and decreased the number of colonies) and increased the ROS level, SOD activity, CAT activity and MDA content, suggesting that excessive accumulation of ROS in cells may be the main intrinsic factor that inhibits the growth of residual P. globosa. Liu et al.(2017, 2018) studied the effects of MC on A. carterae Hulburt and K. mikimotoi and found that the residual microalgae could enhance the antioxidant enzyme activity and increase the MDA content, indicating that a large amount of ROS accumulated in the cells and ROS were the main reason for algal cell death, which is consistent with our result. Excessive amounts of ROS affect a series of biochemical processes in algal cells, leading to functional disturbances and obstacles of basic organelles and structures within the cells. MDA is one of the most important products of membrane lipid peroxidation, and an increase in MDA content can indirectly reflect damage to the membrane system and oxidative stress in cells (Mittler et al., 2004). In this paper, the MDA content in P. globosa cells in the experimental group increased significantly within a short period, indicating that the microalgal cells produced more ROS after OXI-MC treatment and the algal cells were under continuous oxidative stress. The lipids within the cell membranes were oxidized to MDA, so the membrane permeability was altered, and the algal cells eventually die due to dysfunction. The SEM results also proved this. After the addition of OXI-MC, it was observed that the proportion of algal cells that were disrupted and deformed increased, and the polysaccharide glial membrane was more severely disrupted. The OXI-MC directly caused the polysaccharide membrane of the P. globosa colonies to rupture within a short period and the cells of P. globosa to be released, and this irreversible damage continued to inhibit the growth of P. globosa.
Environmental factors often affect important physiological functions of algal cells. Fv/Fm and ФPSII are two important parameters for assessing photosynthesis efficiency and capacity. These parameters of algal cells would vary in an environmental stress. Domingues et al. (2012) studied the response of Phaeodactylum tricornutum to high light, and the results showed that the Fv/Fm of the algal cells decreased and that the photosynthesis activity decreased significantly. After treatment with OXI-MC in this study, the chlorophyll content of P. globosa decreased significantly. In addition, the damage to residual microalgal photosynthesis system increased with the increase of the concentration of OXI-MC. Photosynthetic pigments are the key components of the photosynthesis system and are responsible for light capture and photosynthesis. The reduction in chlorophyll content also proved oxidative damage occurred. The chloroplast is the main location for the production of ROS in the cell, and it is one of the locations most vulnerable to oxidative damage (Asada, 2006). Therefore, we speculated that the chloroplasts suffered oxidative damage. Moreover, the maximum photochemical efficiency of PSII and the actual photochemical efficiency of PSII under dark adaptation were reduced, indicating that the OXI-MC severely damaged the photosynthesis system of P. globosa. OXI-MC damaged the photosynthesis apparatus, causing an imbalance in light energy absorption and utilization and blocking electron transfer, which may lead to the formation of ROS in chloroplasts. In addition, the damage to residual microalgal chlorophyll increased with an increase in the concentration of OXI-MC, which was consistent with the previous changes in microalgal growth inhibition and ROS accumulation.
Previous studies have also shown that excessive ROS can cause photoinhibition of algal cells by destroying pigments and proteins and by affecting the expression of photosynthesis-related genes and proteins, and the damaged photosynthesis system cannot be repaired, resulting in algal cell dysfunction and eventually death of the algal cells (Ma et al., 2012; Chen et al., 2014; Guan et al., 2015; Gururani et al., 2015). In this paper, the decrease of chlorophyll in P. globosa cells and the decrease in photosynthesis activity of P. globosa may be caused by oxidative stress from the OXI-MC. Superoxide anion radical (·O2-), which originates mainly from the Mehler's reaction of photosystem I, can be rapidly transformed into H2O2 under the catalysis of SOD. H2O2 can deactivate some enzymes (such as those involved in the reduction cycle of photosynthesis carbon, Cu/Zn SOD, and tyrosine phosphatase) by oxidizing thiol groups, affecting plant photosynthesis activity (Alvarez et al., 1998). Photosynthesis is the source of energy and basic driving force, so a lack of light energy will severely restrict photosynthesis. Plants perceive the quality, quantity, and method of incident light through unique photosensitive receptors. Therefore, changes in light quantity and quality can cause escape response of plants to shade. Shade will cause plants to undergo a series of adjustments to adapt to low-light environments, including a reduced maximum electron transfer rate and minimum saturated irradiance and a reduced soluble sugar content in their tissues (Jiang et al., 2013). Monosaccharides are the first products of photosynthesis and form the precursors for the biosynthesis of all organic molecules, whereas oligosaccharides are intermediates in polysaccharide synthesis. During the catabolism of stored glucans, monosaccharides are formed as intermediates (Granum and Myklestad, 1999). Long-term photoinhibition will affect algal cell survival and eventually cause algal cell death. With respect to P. globosa, photosynthesis diminished after the addition of OXI-MC, and the formation of monosaccharides and subsequent oligosaccharides and polysaccharides decreased, causing insufficient polysaccharides needed for colony formation.
4.3 The mechanism by which modified clay controls P. globosaThe principle of high-efficiency treatment of HABs by MC is based mainly on the conversion of the naturally negative electrical property of the clay surface to a positive one, which could increase the number of positive charges on the surface of clay particles, strengthen the bridging effects between clay particles and algal cells, and finally result in a significant increase in flocculation and subsequent removal efficiency of HAB organisms (Yu et al., 2017). Liu et al.(2017, 2018) studied the effects of MC on the growth of residual A. carterae Hulburt and K. mikimotoi from physiological and biochemical perspectives. The results showed that the MC can affect the oxidative stress of residual algal cells, and the accumulation of ROS is supposed to be the main internal factor in the growth inhibition of residual algae. After the addition of MC, excessive ROS accumulated in the residual algae, as indicated by the increased SOD activity, CAT activity, and MDA content. Additionally, the pigment contents and net photosynthesis rate decreased after the treatments, indicating that photosynthesis efficiency severely decreased. Moreover, the number of active RCs decreased, the oxygen-evolving complex (OEC) activity was impaired, and the electron transport chain (ETC) was blocked, inevitably inducing the accumulation of ROS at PSII. The accumulation of ROS is supposed to be the main internal factor in the growth inhibition of residual algae. Zhu et al.(2018, 2019) studied the effects of MC on residual A. anophagefferens cells from a molecular biological perspective and found that MC caused a change in gene expression in the residual algal cells, and programmed cell death even occurred, which disrupted physiological processes and inhibited the growth of residual cells.
In contrast to methods used in our previous studies, we used OXI-MC to remove P. globosa for the first time in this study. How does KHSO5 function as a new component of MC? In previous experiments, different materials (clay, KHSO5, KHSO5-MC, PACMC, and KHSO5/PAC-MC) were used to remove P. globosa while maintaining the clay content (0.05 g/L) or the oxidant content (0.005 g/L) under the same conditions, and the removal efficiencies after 3 h were 19%, 59%, 66%, 30%, and 90%, respectively (unpublished data). We speculated that the different components of OXI-MC, including clay, PAC and KHSO5, played a synergistic role in removing P. globosa, whereas KHSO5 played a major role in the process. Potassium monopersulfate composite salt (PMS) is a new type of peroxide disinfectant used in sewage treatment and drinking water disinfection, and is also a commonly used water quality improver in aquaculture, which has no adverse effects on humans and the environment (Sharma, 2002). When dissolved in water, PMS can release a variety of active substances, including small-molecule free radicals and neoecological oxygen (Paetzold and Davidson, 2011), which can disrupt the barrier membrane permeability of microorganisms (Waites et al., 1976). PMS can be activated by metal ions such as calcium and iron ions within nucleic acids to generate free radicals, which can break the phosphodiester bonds of DNA and interfere with DNA and RNA synthesis (Demple et al., 1983). PMS can oxidize pathogens, causing bacterial proteins to denature and solidify, thereby killing pathogenic microorganisms (Anipsitakis et al., 2008). PMS has a strong killing effect on a variety of pathogenic microorganisms, such as bacteria, fungi, and viruses (Su and D'Souza, 2012). Liu et al. (2006) also reported that oxidants caused changes in the structure of chlorophyll and proteins and damaged the algal cell membrane system, after which the algal cells eventually died. The presence of KHSO5 could aggravate the degree of destruction of P. globosa cells and accelerate both the rupture of the outer glial membrane and the degradation of polysaccharides, thus effectively inhibiting the growth, division, and colony formation of algal cells. Therefore, OXI-MC not only can quickly break up a large number of colonies of P. globosa and remove most of the cells but also can effectively inhibit the growth of residual microalgae and the formation of colonies.
Unlike other microalgae, P. globosa is protected by a layer of polysaccharide glial membrane. What is the mechanism by which OXI-MC controls P. globosa? Via microscopy and SEM observations, it was discovered that after the OXI-MC addition, the clay particles adhered to the surface of the colonies or inserted in the colonies under the action of gravity and electrostatic attraction, resulting in membrane rupturing into fragments of various sizes and P. globosa cells being released from the colonies. OXI-MC may operate more effectively on P. globosa cells after released. The presence of a polysaccharide glial membrane could create a competitive advantage for the growth of P. globosa in the sea, but OXI-MC could disrupt polysaccharide colloid membranes and weaken their competitive advantage. After OXI-MC addition, most of the colonies were broken and decomposed gradually. In addition, the OXI-MC in this system also mainly inhibited the growth of residual P. globosa by causing physical damage and oxidative stress and by disrupting photosynthesis, which are essentially consistent with the above understanding. After the clay was modified by PAC and KHSO5, the surface of the clay particles was positively charged (Qiu et al., 2017), while the P. globosa cells and the outer glial membrane of the colonies are negatively charged (Van Boekel, 1992). Due to electrical neutralization, the effective collision of OXI-MC particles with colonies and single cells increased, and the flocculation efficiency improved. Algal cells that have not been flocculated effectively would also be physically damaged during the collision. In short, the OXI-MC may cause continuous damage to the residual P. globosa cells via physical methods such as collision damage and attenuating light, thereby effectively inhibiting the growth and colony formation of P. globosa. The parameters of the antioxidant system also revealed that the algal cells had been under a high level of oxidative stress during the experiment. Excessive ROS would affect a series of biochemical processes in P. globosa cells, leading to both the dysfunction and disorder of basic organelles and structures in the cells. After the addition of the OXI-MC, the photosynthesis of P. globosa diminished, inhibiting the formation of new colonies due to physical damage, shading, and physiological and biochemical changes.
OXI-MC could remove the most of algal cells and broke the colonies of P. globosa by collision, flocculation, and releasing active substances, as well as effectively inhibit the growth and colony formation of residual P. globosa by causing oxidative stress, reducing photosynthesis activity, accelerating the degradation of polysaccharides, and inhibiting the formation of polysaccharides, preventing a second outbreak of HABs. This study provides a preliminary reference for the control and mitigation of P. globosa blooms in an emergency.
5 CONCLUSIONIn this paper, the effects of OXI-MC on P. globosa were studied from different perspectives. The results showed that OXI-MC not only could effectively remove P. globosa but also could inhibit the growth of residual algal cells and the formation of new colonies. Compared with those of the control, the SOD activity, CAT activity, and MDA content of the residual algae significantly increased, indicating that OXI-MC stimulated the accumulation of ROS in the algal cells and caused oxidative stress. This paper further evaluated the effect of OXI-MC on the photosynthesis of the residual microalgae and showed that Fv/Fm and ФPSII decreased, and the photosynthesis system of P. globosa was affected. The results of our study clarified that OXI-MC could remove the most of algal cells and broke the colonies of P. globosa by collision, flocculation, and releasing active substances, as well as effectively inhibit the growth and colony formation of residual P. globosa by causing oxidative stress, reducing photosynthesis activity, accelerating the degradation of polysaccharides, and inhibiting the formation of colonies.
6 DADA AVAILABILITY STATEMENTThe authors declare that all data generated or analyzed during this study are included in this published article.
7 ACKNOWLEDGMENTThe authors sincerely thank the assistance of Dr. ZHANG Peipei, SHEN Huihui, REN Xiangzheng, and WU Ting in this experiment.
Alvarez M E, Pennell R I, Meijer P, Ishikawa A, Dixon R A, Lamb C. 1998. Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell, 92(6): 773-784.
DOI:10.1016/S0092-8674(00)81405-1 |
Anipsitakis G P, Tufano T P, Dionysiou D D. 2008. Chemical and microbial decontamination of pool water using activated potassium peroxymonosulfate. Water Research, 42(12): 2 899-2 910.
DOI:10.1016/j.watres.2008.03.002 |
Asada K. 2006. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiology, 141(2): 391-396.
DOI:10.1104/pp.106.082040 |
Cao X H, Yu Z M, Qiu L X. 2017. Field experiment and emergent application of modified clays for Phaeocystis globosa blooms mitigation. Oceanologia et Limnologia Sinica, 48(4): 753-759.
(in Chinese with English abstract) |
Chen S G, Strasser R J, Qiang S. 2014. In vivo assessment of effect of phytotoxin tenuazonic acid on PSⅡ reaction centers. Plant Physiology and Biochemistry, 84: 10-21.
DOI:10.1016/j.plaphy.2014.09.004 |
Demple B, Halbrook J, Linn S. 1983. Escherichia coli xth mutants are hypersensitive to hydrogen peroxide. Journal of Bacteriology, 153(2): 1 079-1 082.
DOI:10.1128/JB.153.2.1079-1082.1983 |
Domingues N, Matos A R, Marques da Silva J, Cartaxana P. 2012. Response of the diatom Phaeodactylum tricornutum to photooxidative stress resulting from high light exposure. PLoS One, 7(6): e38162.
DOI:10.1371/journal.pone.0038162 |
Foyer C H, Noctor G. 2005. Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. The Plant Cell, 17(7): 1 866-1 875.
DOI:10.1105/tpc.105.033589 |
Granum E, Myklestad S M. 1999. Effects of NH4+ assimilation on dark carbon fixation and β-1, 3-glucan metabolism in the marine diatom Skeletonema costatum (Bacillariophyceae). Journal of Phycology, 35(6): 1191-1 199.
DOI:10.1046/j.1529-8817.1999.3561191.x |
Guan C W, Guo X Y, Li Y, Zhang H J, Lei X Q, Cai G J, Guo J J, Yu Z M, Zheng T L. 2015. Photoinhibition of Phaeocystis globosa resulting from oxidative stress induced by a marine algicidal bacterium Bacillus sp. LP-10. Scientific Reports, 5(1): 17002.
DOI:10.1038/srep17002 |
Guillard R R L, Hargraves P E. 1993. Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia, 32(3): 234-236.
DOI:10.2216/i0031-8884-32-3-234.1 |
Gururani M A, Venkatesh J, Tran L S P. 2015. Regulation of photosynthesis during abiotic stress-induced photoinhibition. Molecular Plant, 8(9): 1 304-1 320.
DOI:10.1016/j.molp.2015.05.005 |
Huang T W, Wang X D, Wang Y. 2012. Growth, architecture and cell distribution in Phaeocystis globosa colonies. Chinese Bulletin of Botany, 47(5): 508-514.
(in Chinese with English abstract) |
Jiang Z J, Huang X P, Zhang J P. 2013. Dynamics of nonstructural carbohydrates in seagrass Thalassia hemprichii and its response to shading. Acta Oceanologica Sinica, 32(8): 61-67.
DOI:10.1007/s13131-013-0342-0 |
Liu J S, Yang W D, Zhang H, Wu Y. 2006. Effects of chlorine dioxide on contents of chlorophyll a, proteins and DNA in Phaeocystis globosa. Journal of Tropical and Subtropical Botany, 14(5): 427-432.
(in Chinese with English abstract) |
Liu S Y, Yu Z M, Song X X, Cao X H. 2017. Effects of modified clay on the physiological and photosynthetic activities of Amphidinium carterae Hulburt. Harmful Algae, 70: 64-72.
DOI:10.1016/j.hal.2017.10.007 |
Liu S Y, Yu Z M, Song X X, Cao X H. 2018. Physiological and photosynthetic responses of Karenia mikimotoi to the modified clay mitigation method. Marine Pollution Bulletin, 133: 491-499.
DOI:10.1016/j.marpolbul.2018.05.044 |
Liu Y, Cao X H, Yu Z M, Song X X, Qiu L X. 2016. Controlling harmful algae blooms using aluminum-modified clay. Marine Pollution Bulletin, 103(1-2): 211-219.
DOI:10.1016/j.marpolbul.2015.12.017 |
Lu G Y, Song X X, Yu Z M, Cao X H, Yuan Y Q. 2015. Effects of modified clay flocculation on major nutrients and diatom aggregation during Skeletonema costatum blooms in the laboratory. Chinese Journal of Oceanology and Limnology, 33(4): 1 007-1 019.
DOI:10.1007/s00343-015-4162-2 |
Lu G Y, Song X X, Yu Z M, Cao X H. 2017. Application of PAC-modified kaolin to mitigate Prorocentrum donghaiense: effects on cell removal and phosphorus cycling in a laboratory setting. Journal of Applied Phycology, 29(2): 917-928.
DOI:10.1007/s10811-016-0992-3 |
Ma B H, Gao L, Zhang H X, Cui J, Shen Z G. 2012. Aluminuminduced oxidative stress and changes in antioxidant defenses in the roots of rice varieties differing in Al tolerance. Plant Cell Reports, 31(4): 687-696.
DOI:10.1007/s00299-011-1187-7 |
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. 2004. Reactive oxygen gene network of plants. Trends in Plant Science, 9(10): 490-498.
DOI:10.1016/j.tplants.2004.08.009 |
Nagata T. 2000. Production mechanisms of dissolved organic matter. In: Kirchmann DL ed. Microbial Ecology of the Oceans. Wiley-Liss, New York, p.121-152.
|
Paetzold S C, Davidson J. 2011. Aquaculture fouling: efficacy of potassium monopersulphonate triple salt based disinfectant (Virkon® Aquatic) against Ciona intestinalis. Biofouling, 27(6): 655-665.
DOI:10.1080/08927014.2011.594503 |
Parsons T. 1984. A Manual of chemical & biological methods for seawater analysis. Pergamon Press, 31: 158-161.
|
Qi Y Z, Xu N, Wang Y, Lv S H, Chen J F. 2002. Progress of studies on red tide in China-studies on Phaeocystis globosa red tide and its DMS (DMSP) production. China Basic Science, (4): 23-28.
(in Chinese with English abstract) |
Qin X L, Lai J X, Chen B, Jiang F J, Xu M B. 2016. Molecular identification of Phaeocystis from Beibu Gulf based on 18S rDNA sequences. Journal of Tropical and Subtropical Botany, 24(2): 176-181.
(in Chinese with English abstract) |
Qiu L X, Yu Z M, Cao X H, Song X X, Liu Y, Zhong Y. 2017. Removal efficiencies for Phaeocystis globosa and Prorocentrum donghaiense with modified clay. Oceanologia et Limnologia Sinica, 48(5): 982-989.
(in Chinese with English abstract) |
Sharma V K. 2002. Potassium ferrate (Ⅵ): an environmentally friendly oxidant. Advances in Environmental Research, 6(2): 143-156.
DOI:10.1016/S1093-0191(01)00119-8 |
Shen P P, Wang Y, Qi Y Z, Xie L C, Lv S H, Hodgkiss I J. 2000. Growth characteristics and life cycle of Phaeocystis globosa Scherffel. Acta Hydrobiologica Sinca, 24(6): 635-643.
(in Chinese with English abstract) |
Smith D C, Steward G F, Long R A, Azam F. 1995. Bacterial mediation of carbon fluxes during a diatom bloom in a mesocosm. Deep Sea Research Part Ⅱ: Topical Studies in Oceanography, 42(1): 75-97.
DOI:10.1016/0967-0645(95)00005-B |
Stirbet A, Govindjee. 2011. On the relation between the Kautsky effect (chlorophyll a fluorescence induction) and photosystem Ⅱ: basics and applications of the OJIP fluorescence transient. Journal of Photochemistry and Photobiology B: Biology, 104(1-2): 236-257.
DOI:10.1016/j.jphotobiol.2010.12.010 |
Su X W, D'Souza D H. 2012. Inactivation of human norovirus surrogates by benzalkonium chloride, potassium peroxymonosulfate, tannic acid, and Gallic acid. Foodborne Pathogens and Disease, 9(9): 829-834.
DOI:10.1089/fpd.2012.1155 |
Van Boekel W H M. 1992. Phaeocystis colony mucus components and the importance of calcium ions for colony stability. Marine Ecology Progress Series, 87: 301-305.
DOI:10.3354/meps087301 |
van Rijssel M, Janse I, Noordkamp D J B, Gieskes W W C. 2000. An inventory of factors that affect polysaccharide production by Phaeocystis globosa. Journal of Sea Research, 43(3-4): 297-306.
DOI:10.1016/S1385-1101(00)00013-7 |
Waites W M, Wyatt L R, King N R, Bayliss C E. 1976. Changes in spores of Clostridium bifermentans caused by treatment with hydrogen peroxide and cations. Journal of General Microbiology, 93(2): 388-396.
DOI:10.1099/00221287-93-2-388 |
Wang Z F, Yu Z M, Song X X, Cao X H, Liu K. 2014b. Impact of modified clay on the growth of the infant Apostichopus japonicas Selenka in HABs controling. Oceanologia et Limnologia Sinica, 45(2): 233-238.
(in Chinese with English abstract) |
Wang Z F, Yu Z M, Song X X, Cao X H. 2014a. Effects of modified clay on the infant of Patinopecten yessoensis for HABs control. Marine Environmental Science, 33(6): 817-821, 836.
(in Chinese with English abstract) |
Yu Z M, Sengco M R, Anderson D M. 2004. Flocculation and removal of the brown tide organism, Aureococcus anophagefferens (Chrysophyceae), using clays. Journal of Applied Phycology, 16(2): 101-110.
DOI:10.1023/B:JAPH.0000044775.33548.38 |
Yu Z M, Song X X, Cao X H, Liu Y. 2017. Mitigation of harmful algal blooms using modified clays: theory, mechanisms, and applications. Harmful Algae, 69: 48-64.
DOI:10.1016/j.hal.2017.09.004 |
Yu Z M, Zou J Z, Ma X N. 1994. Application of clays to removal of red tide organisms Ⅱ. Coagulation of different species of red tide organisms with montmorillonite and effect of clay pretreatment. Chinese Journal of Oceanology and Limnology, 12(4): 316-324.
DOI:10.1007/BF02850491 |
Zhang S F, Zhang K, Cheng H M, Lin L, Wang D Z. 2020. Comparative transcriptomics reveals colony formation mechanism of a harmful algal bloom species Phaeocystis globosa. Science of the Total Environment, 719: 137454.
DOI:10.1016/j.scitotenv.2020.137454 |
Zhang Y, Song X X, Yu Z M, Zhang P P, Cao X H, Yuan Y Q. 2019. Impact assessment of modified clay on embryolarval stages of turbot Scophthalmus maximus L. Journal of Oceanology and Limnology, 37(3): 1 051-1 061.
DOI:10.1007/s00343-019-8043-y |
Zhang Y, Yu Z M, Song X X, Yuan Y Q, Cao X H. 2018. Effects of modified clay used for the control of harmful algal blooms on Alexandrium pacificum cysts. Harmful Algae, 72: 36-45.
DOI:10.1016/j.hal.2017.12.001 |
Zhao T, Tan L J, Huang W Q, Wang J T. 2019. The interactions between micro polyvinyl chloride (mPVC) and marine dinoflagellate Karenia mikimotoi: the inhibition of growth, chlorophyll and photosynthetic efficiency. Environmental Pollution, 247: 883-889.
DOI:10.1016/j.envpol.2019.01.114 |
Zhu J N, Yu Z M, He L Y, Cao X H, Ji H N, Song X X. 2019. Physiological response dynamics of the brown tide organism Aureococcus anophagefferens treated with modified clay. Harmful Algae, 86: 1-9.
DOI:10.1016/j.hal.2019.04.009 |
Zhu J N, Yu Z M, He L Y, Cao X H, Liu S Y, Song X X. 2018. Molecular mechanism of modified clay controlling the brown tide organism Aureococcus anophagefferens revealed by transcriptome analysis. Environmental Science & Technology, 52(12): 7 006-7 014.
|
 2020, Vol. 38
2020, Vol. 38


